Repetitive Sequence Transcription in Breast Cancer
Abstract
1. Introduction
1.1. Breast Cancer Classification
1.2. Non-Coding RNA in Mammals
1.3. Repetitive DNA Sequence Classification
1.4. Repetitive DNA Sequence and Cancer
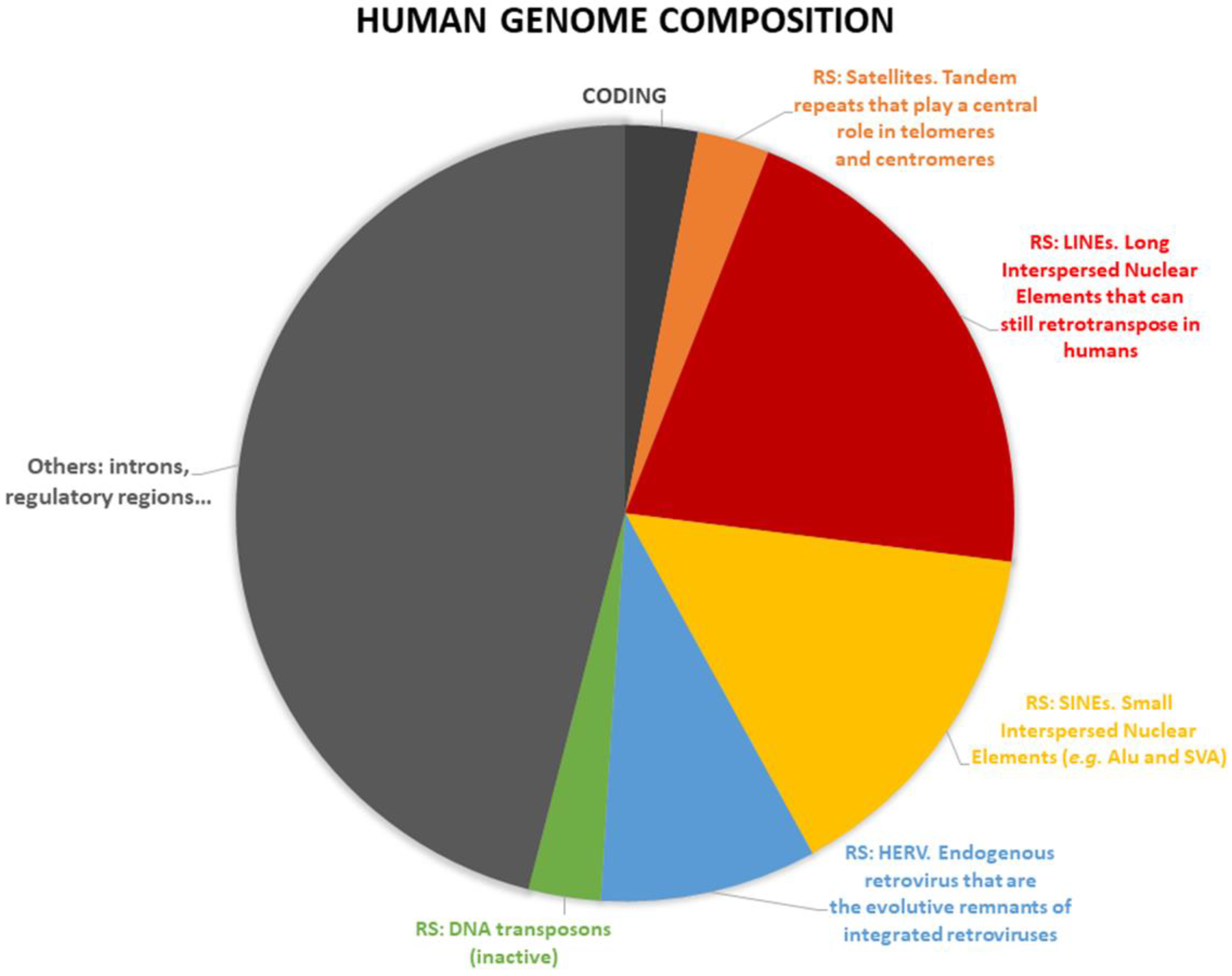
1.5. Main Aim
2. Materials and Methods
2.1. Identifying and Quantifying the Repetitive Sequence Expression
2.2. Analysis of Coding Gene Expression
2.3. Statistical Analyses
2.4. Dataset Used
3. Results
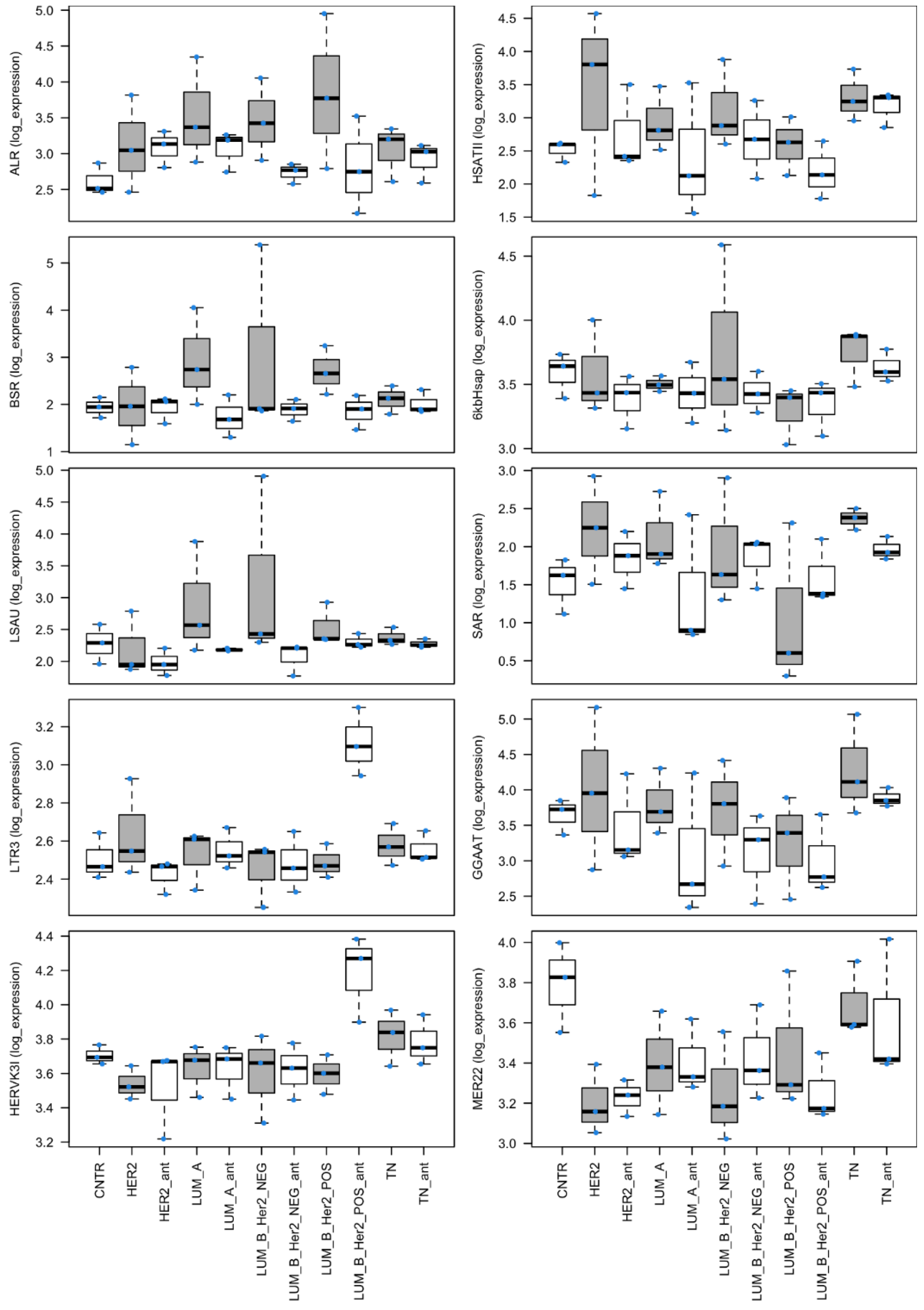
3.1. Analysis of the Expression of Repetitive Sequences in Cancer Specimens
3.2. Analysis of Expression Background
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and Current Knowledge of Breast Cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, A.; Jain, P.; Duranti, E.; Margolskee, E.; Arancio, W.; Facchetti, F.; Alobeid, B.; Santanelli di Pompeo, F.; Mansukhani, M.; Bhagat, G. Targeted next Generation Sequencing of Breast Implant-Associated Anaplastic Large Cell Lymphoma Reveals Mutations in JAK/STAT Signalling Pathway Genes, TP53 and DNMT3A. Br. J. Haematol. 2018, 180, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Kong, D.; Chen, Q.; Ping, Y.; Pang, D. Oncogenic Long Noncoding RNA Landscape in Breast Cancer. Mol. Cancer 2017, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Wang, Q.; Ning, S.; Wu, J.; Zhang, X.; Chen, Y.; Xu, S. Landscape of Tumor Suppressor Long Noncoding RNAs in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 79. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J. Strategies for Subtypes-Dealing with the Diversity of Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J.; Albain, K.S.; André, F.; Bergh, J.; et al. Personalizing the Treatment of Women with Early Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, A.; Riedel, F.; Gondos, A.; Sinn, P.; Schirmacher, P.; Marmé, F.; Jäger, D.; Kauczor, H.U.; Stieber, A.; Lindel, K.; et al. Prognosis of Breast Cancer Molecular Subtypes in Routine Clinical Care: A Large Prospective Cohort Study. BMC Cancer 2016, 16, 734. [Google Scholar] [CrossRef]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical Implications of the Intrinsic Molecular Subtypes of Breast Cancer. Breast 2015, 24, S26–S35. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.B.; Amaral, P.P.; Schlesinger, F.J.; Dinger, M.E.; Taft, R.J.; Rinn, J.L.; Ponting, C.P.; Stadler, P.F.; Morris, K.V.; Morillon, A.; et al. The Reality of Pervasive Transcription. PLoS Biol. 2011, 9, e1000625. [Google Scholar] [CrossRef] [PubMed]
- Arancio, W.; Coronnello, C. Repetitive Sequences in Aging. Aging 2021, 13, 10816–10817. [Google Scholar] [CrossRef] [PubMed]
- Hirschberger, S.; Hinske, L.C.; Kreth, S. MiRNAs: Dynamic Regulators of Immune Cell Functions in Inflammation and Cancer. Cancer Lett. 2018, 431, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cui, X.; Guan, H. MicroRNAs: Pivotal Regulators in Acute Myeloid Leukemia. Ann. Hematol. 2020, 99, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ke, F.; Chen, T.; Zhou, Q.; Weng, L.; Tan, J.; Shen, W.; Li, L.; Zhou, J.; Xu, C.; et al. MicroRNAs That Regulate PTEN as Potential Biomarkers in Colorectal Cancer: A Systematic Review. J. Cancer Res. Clin. Oncol. 2020, 146, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Arancio, W.; Calogero Amato, M.; Magliozzo, M.; Pizzolanti, G.; Vesco, R.; Giordano, C. Serum MiRNAs in Women Affected by Hyperandrogenic Polycystic Ovary Syndrome: The Potential Role of MiR-155 as a Biomarker for Monitoring the Estroprogestinic Treatment. Gynecol. Endocrinol. 2018, 34, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, A.; Gorji-bahri, G. MicroRNA: Promising Roles in Cancer Therapy. Curr. Pharm. Biotechnol. 2020, 21, 1186–1203. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Ding, B.; Fu, P. Pseudogene-Derived LncRNAs and Their MiRNA Sponging Mechanism in Human Cancer. Front. Cell Dev. Biol. 2020, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Arancio, W.; Carina, V.; Pizzolanti, G.; Tomasello, L.; Pitrone, M.; Baiamonte, C.; Amato, M.C.; Giordano, C. Anaplastic Thyroid Carcinoma: A CeRNA Analysis Pointed to a Crosstalk between SOX2, TP53, and MicroRNA Biogenesis. Int. J. Endocrinol. 2015, 2015, 439370. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Pandolfi, P.P. PTEN CeRNA Networks in Human Cancer. Methods 2015, 77–78, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Arancio, W.; Genovese, S.I.; Bongiovanni, L.; Tripodo, C. A CeRNA Approach May Unveil Unexpected Contributors to Deletion Syndromes, the Model of 5q-Syndrome. Oncoscience 2015, 2, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Arancio, W.; Giordano, C.; Pizzolanti, G. A CeRNA Analysis on LMNA Gene Focusing on the Hutchinson-Gilford Progeria Syndrome. J. Clin. Bioinform. 2013, 3, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arancio, W. A Bioinformatics Analysis of Lamin-A Regulatory Network: A Perspective on Epigenetic Involvement in Hutchinson-Gilford Progeria Syndrome. Rejuvenation Res. 2012, 15, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Arancio, W. CeRNA Analysis of SARS-CoV-2. Arch. Virol. 2021, 166, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Bertolazzi, G.; Cipollina, C.; Benos, P.V.; Tumminello, M.; Coronnello, C. MiR-1207-5p Can Contribute to Dysregulation of Inflammatory Response in COVID-19 via Targeting SARS-CoV-2 RNA. Front. Cell. Infect. Microbiol. 2020, 10, 586592. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, G.J.; Kimura, Y.; Daub, C.O.; Wani, S.; Plessy, C.; Irvine, K.M.; Schroder, K.; Cloonan, N.; Steptoe, A.L.; Lassmann, T.; et al. The Regulated Retrotransposon Transcriptome of Mammalian Cells. Nat. Genet. 2009, 41, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.A.; Wang, L.; Rishishwar, L.; Wang, J.; McDonald, J.F.; Jordan, I.K. Patterns of Transposable Element Expression and Insertion in Cancer. Front. Mol. Biosci. 2016, 3, 76. [Google Scholar] [CrossRef]
- Sciamanna, I.; De Luca, C.; Spadafora, C. The Reverse Transcriptase Encoded by LINE-1 Retrotransposons in the Genesis, Progression, and Therapy of Cancer. Front. Chem. 2016, 4, 6. [Google Scholar] [CrossRef]
- Ohms, S.; Lee, S.H.; Rangasamy, D. LINE-1 Retrotransposons and Let-7 MiRNA: Partners in the Pathogenesis of Cancer? Front. Genet. 2014, 5, 338. [Google Scholar] [CrossRef]
- Tubio, J.M.C.; Li, Y.; Ju, Y.S.; Martincorena, I.; Cooke, S.L.; Tojo, M.; Gundem, G.; Pipinikas, C.P.; Zamora, J.; Raine, K.; et al. Extensive Transduction of Nonrepetitive DNA Mediated by L1 Retrotransposition in Cancer Genomes. Science 2014, 345, 1251343. [Google Scholar] [CrossRef] [PubMed]
- Di Ruocco, F.; Basso, V.; Rivoire, M.; Mehlen, P.; Ambati, J.; De Falco, S.; Tarallo, V. Alu RNA Accumulation Induces Epithelial-to-Mesenchymal Transition by Modulating MiR-566 and Is Associated with Cancer Progression. Oncogene 2018, 37, 627–637. [Google Scholar] [CrossRef]
- Padeken, J.; Zeller, P.; Gasser, S.M. Repeat DNA in Genome Organization and Stability. Curr. Opin. Genet. Dev. 2015, 31, 12–19. [Google Scholar] [CrossRef]
- Treangen, T.J.; Salzberg, S.L. Repetitive DNA and Next-Generation Sequencing: Computational Challenges and Solutions. Nat. Rev. Genet. 2012, 13, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Coufal, N.G.; Garcia-Perez, J.L.; Peng, G.E.; Yeo, G.W.; Mu, Y.; Lovci, M.T.; Morell, M.; O’Shea, K.S.; Moran, J.V.; Gage, F.H. L1 Retrotransposition in Human Neural Progenitor Cells. Nature 2009, 460, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Arancio, W. Progerin Expression Induces a Significant Downregulation of Transcription from Human Repetitive Sequences in IPSC-Derived Dopaminergic Neurons. GeroScience 2019, 41, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hoong, N.; Aslanian, A.; Hara, T.; Benner, C.; Heinz, S.; Miga, K.H.; Ke, E.; Verma, S.; Soroczynski, J.; et al. Heterochromatin-Encoded Satellite RNAs Induce Breast Cancer. Mol. Cell 2018, 70, 842–853.e7. [Google Scholar] [CrossRef]
- Kakizawa, N.; Suzuki, K.; Abe, I.; Endo, Y.; Tamaki, S.; Ishikawa, H.; Watanabe, F.; Ichida, K.; Saito, M.; Futsuhara, K.; et al. High Relative Levels of Satellite Alpha Transcripts Predict Increased Risk of Bilateral Breast Cancer and Multiple Primary Cancer in Patients with Breast Cancer and Lacking BRCA-Related Clinical Features. Oncol. Rep. 2019, 42, 857–865. [Google Scholar] [CrossRef]
- Patnala, R.; Lee, S.H.; Dahlstrom, J.E.; Ohms, S.; Chen, L.; Dheen, S.T.; Rangasamy, D. Inhibition of LINE-1 Retrotransposon-Encoded Reverse Transcriptase Modulates the Expression of Cell Differentiation Genes in Breast Cancer Cells. Breast Cancer Res. Treat. 2014, 143, 239–253. [Google Scholar] [CrossRef]
- Park, S.Y.; Seo, A.N.; Jung, H.Y.; Gwak, J.M.; Jung, N.; Cho, N.Y.; Kang, G.H. Alu and LINE-1 Hypomethylation Is Associated with HER2 Enriched Subtype of Breast Cancer. PLoS ONE 2014, 9, e100429. [Google Scholar] [CrossRef]
- Van Hoesel, A.Q.; Van De Velde, C.J.H.; Kuppen, P.J.K.; Liefers, G.J.; Putter, H.; Sato, Y.; Elashoff, D.A.; Turner, R.R.; Shamonki, J.M.; De Kruijf, E.M.; et al. Hypomethylation of LINE-1 in Primary Tumor Has Poor Prognosis in Young Breast Cancer Patients: A Retrospective Cohort Study. Breast Cancer Res. Treat. 2012, 134, 1103–1114. [Google Scholar] [CrossRef]
- Harris, C.R.; Normart, R.; Yang, Q.; Stevenson, E.; Haffty, B.G.; Ganesan, S.; Cordon-Cardo, C.; Levine, A.J.; Tang, L.H. Association of Nuclear Localization of a Long Interspersed Nuclear Element-1 Protein in Breast Tumors with Poor Prognostic Outcomes. Genes and Cancer 2010, 1, 115–124. [Google Scholar] [CrossRef]
- Gualtieri, A.; Andreola, F.; Sciamanna, I.; Sinibaldi-Vallebona, P.; Serafino, A.; Spadafora, C. Increased Expression and Copy Number Amplification of LINE-1 and SINE B1 Retrotransposable Elements in Murine Mammary Carcinoma Progression. Oncotarget 2013, 4, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dahlstrom, J.E.; Chandra, A.; Board, P.; Rangasamy, D. Prognostic Value of LINE-1 Retrotransposon Expression and Its Subcellular Localization in Breast Cancer. Breast Cancer Res. Treat. 2012, 136, 129–142. [Google Scholar] [CrossRef]
- Miret, N.; Zappia, C.D.; Altamirano, G.; Pontillo, C.; Zárate, L.; Gómez, A.; Lasagna, M.; Cocca, C.; Kass, L.; Monczor, F.; et al. AhR Ligands Reactivate LINE-1 Retrotransposon in Triple-Negative Breast Cancer Cells MDA-MB-231 and Non-Tumorigenic Mammary Epithelial Cells NMuMG. Biochem. Pharmacol. 2020, 175, 113904. [Google Scholar] [CrossRef] [PubMed]
- Miglio, U.; Berrino, E.; Panero, M.; Ferrero, G.; Coscujuela Tarrero, L.; Miano, V.; Dell’Aglio, C.; Sarotto, I.; Annaratone, L.; Marchiò, C.; et al. The Expression of LINE1-MET Chimeric Transcript Identifies a Subgroup of Aggressive Breast Cancers. Int. J. Cancer 2018, 143, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Bratthauer, G.L.; Cardiff, R.D.; Fanning, T.G. Expression of LINE-1 Retrotransposons in Human Breast Cancer. Cancer 1994, 73, 2333–2336. [Google Scholar] [CrossRef]
- Wang, Y.; Bernhardy, A.J.; Nacson, J.; Krais, J.J.; Tan, Y.F.; Nicolas, E.; Radke, M.R.; Handorf, E.; Llop-Guevara, A.; Balmaña, J.; et al. BRCA1 Intronic Alu Elements Drive Gene Rearrangements and PARP Inhibitor Resistance. Nat. Commun. 2019, 10, 5661. [Google Scholar] [CrossRef] [PubMed]
- Staaf, J.; Glodzik, D.; Bosch, A.; Vallon-Christersson, J.; Reuterswärd, C.; Häkkinen, J.; Degasperi, A.; Amarante, T.D.; Saal, L.H.; Hegardt, C.; et al. Whole-Genome Sequencing of Triple-Negative Breast Cancers in a Population-Based Clinical Study. Nat. Med. 2019, 25, 1526–1533. [Google Scholar] [CrossRef]
- Felicio, P.S.; Alemar, B.; Coelho, A.S.; Berardinelli, G.N.; Melendez, M.E.; Lengert, A.V.H.; Miche lli, R.D.; Reis, R.M.; Fernandes, G.C.; Ewald, I.P.; et al. Screening and Characterization of BRCA2 c.156_157insAlu in Brazil: Results from 1380 Individuals from the South and Southeast. Cancer Genet. 2018, 228–229, 93–97. [Google Scholar] [CrossRef]
- Rizza, R.; Hackmann, K.; Paris, I.; Minucci, A.; De Leo, R.; Schrock, E.; Urbani, A.; Capoluongo, E.; Gelli, G.; Concolino, P. Novel BRCA1 Large Genomic Rearrangements in Italian Breast/Ovarian Cancer Patients. Mol. Diagnosis Ther. 2019, 23, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Gallegos-Arreola, M.P.; Figuera, L.E.; Flores-Ramos, L.G.; Puebla-Pérez, A.M.; Zúñiga-González, G.M. Association of the Alu Insertion Polymorphism in the Progesterone Receptor Gene with Breast Cancer in a Mexican Population. Arch. Med. Sci. 2015, 11, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.M.; Brandão, R.D.; Cavaco, B.M.; Eugénio, J.; Bento, S.; Nave, M.; Rodrigues, P.; Fernandes, A.; Vaz, F. Screening for a BRCA2 Rearrangement in High-Risk Breast/Ovarian Cancer Families: Evidence for a Founder Effect and Analysis of the Associated Phenotypes. J. Clin. Oncol. 2007, 25, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, W.; Liu, Y.; Tan, X.; Li, X.; Zou, Q.; Xiao, Z.; Xu, H.; Wang, Y.; Yang, X. Function of HNRNPC in Breast Cancer Cells by Controlling the DsRNA-induced Interferon Response. EMBO J. 2018, 37, e99017. [Google Scholar] [CrossRef] [PubMed]
- Grabski, D.F.; Hu, Y.; Sharma, M.; Rasmussen, S.K. Close to the Bedside: A Systematic Review of Endogenous Retroviruses and Their Impact in Oncology. J. Surg. Res. 2019, 240, 145–155. [Google Scholar] [CrossRef]
- Zhao, J.; Rycaj, K.; Geng, S.; Li, M.; Plummer, J.B.; Yin, B.; Liu, H.; Xu, X.; Zhang, Y.; Yan, Y.; et al. Expression of Human Endogenous Retrovirus Type K Envelope Protein Is a Novel Candidate Prognostic Marker for Human Breast Cancer. Genes Cancer 2011, 2, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Radvanyi, L.; Rycaj, K.; Plummer, J.B.; Yan, P.; Sastry, K.J.; Piyathilake, C.J.; Hunt, K.K.; Johanning, G.L. Human Endogenous Retrovirus K Triggers an Antigen-Specific Immune Response in Breast Cancer Patients. Cancer Res. 2008, 68, 5869–5877. [Google Scholar] [CrossRef]
- Golan, M.; Hizi, A.; Resau, J.H.; Yaal-Hahoshen, N.; Reichman, H.; Keydar, I.; Tsarfaty, I. Human Endogenous Retrovirus (HERV-K) Reverse Transcriptase as a Breast Cancer Prognostic Marker. Neoplasia 2008, 10, 521–533. [Google Scholar] [CrossRef]
- Wang-Johanning, F.; Frost, A.R.; Jian, B.; Epp, L.; Lu, D.W.; Johanning, G.L. Quantitation of HERV-K Env Gene Expression and Splicing in Human Breast Cancer. Oncogene 2003, 22, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Frost, A.R.; Johanning, G.L.; Khazaeli, M.B.; LoBuglio, A.F.; Shaw, D.R.; Strong, T.V. Expression of Human Endogenous Retrovirus K Envelope Transcripts in Human Breast Cancer. Clin. Cancer Res. 2001, 7, 1553–1560. [Google Scholar] [PubMed]
- Matteucci, C.; Balestrieri, E.; Argaw-Denboba, A.; Sinibaldi-Vallebona, P. Human Endogenous Retroviruses Role in Cancer Cell Stemness. Semin. Cancer Biol. 2018, 53, 17–30. [Google Scholar] [CrossRef]
- Salmons, B.; Lawson, J.S.; Günzburg, W.H. Recent Developments Linking Retroviruses to Human Breast Cancer: Infectious Agent, Enemy within or Both? J. Gen. Virol. 2014, 95, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Davis, J.; Eugenio, R.A.; Liu, Y. Female Sex Hormones Activate Human Endogenous Retrovirus Type K Through the OCT4 Transcription Factor in T47D Breast Cancer Cells. AIDS Res. Hum. Retrovir. 2019, 35, 348–356. [Google Scholar] [CrossRef]
- Johanning, G.L.; Malouf, G.G.; Zheng, X.; Esteva, F.J.; Weinstein, J.N.; Wang-Johanning, F.; Su, X. Expression of Human Endogenous Retrovirus-K Is Strongly Associated with the Basal-like Breast Cancer Phenotype. Sci. Rep. 2017, 7, 41960. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xu, X.E.; Jiang, Y.Z.; Liu, Y.R.; Sun, W.; Guo, Y.J.; Ren, Y.X.; Zuo, W.J.; Hu, X.; Huang, S.L.; et al. The Endogenous Retrovirus-Derived Long Noncoding RNA TROJAN Promotes Triple-Negative Breast Cancer Progression via ZMYND8 Degradation. Sci. Adv. 2019, 5, eaat9820. [Google Scholar] [CrossRef] [PubMed]
- Lemaître, C.; Tsang, J.; Bireau, C.; Heidmann, T.; Dewannieux, M. A Human Endogenous Retrovirus-Derived Gene That Can Contribute to Oncogenesis by Activating the ERK Pathway and Inducing Migration and Invasion. PLoS Pathog. 2017, 13, e1006451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, M.; Wei, Y.; Lin, K.; Lu, Y.; Shen, J.; Johanning, G.L.; Wang-Johanning, F. Activation of HERV-K Env Protein Is Essential for Tumorigenesis and Metastasis of Breast Cancer Cells. Oncotarget 2016, 7, 84093–84117. [Google Scholar] [CrossRef] [PubMed]
- Wang-Johanning, F.; Li, M.; Esteva, F.J.; Hess, K.R.; Yin, B.; Rycaj, K.; Plummer, J.B.; Garza, J.G.; Ambs, S.; Johanning, G.L. Human Endogenous Retrovirus Type K Antibodies and MRNA as Serum Biomarkers of Early-Stage Breast Cancer. Int. J. Cancer 2014, 134, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.C.; Upton, K.R. Human Transposons Are an Abundant Supply of Transcription Factor Binding Sites and Promoter Activities in Breast Cancer Cell Lines. Mob. DNA 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Presneau, N.; Laplace-Marieze, V.; Sylvain, V.; Lortholary, A.; Hardouin, A.; Bernard-Gallon, D.; Bignon, Y.J. New Mechanism of BRCA-1 Mutation by Deletion/Insertion at the Same Nucleotide Position in Three Unrelated French Breast/Ovarian Cancer Families. Hum. Genet. 1998, 103, 334–339. [Google Scholar] [CrossRef]
- Jalili, V.; Afgan, E.; Gu, Q.; Clements, D.; Blankenberg, D.; Goecks, J.; Taylor, J.; Nekrutenko, A. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2020 Update. Nucleic Acids Res. 2020, 48, W395–W402. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; Van Den Beek, M.; Bouvier, D.; Ech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W. Benjamini–Hochberg Method. In Encyclopedia of Systems Biology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Available online: from https://www.ncbi.nlm.nih.gov/sra/SRX1135937 to https://www.ncbi.nlm.nih.gov/sra/SRX1135969; (accessed on 19 July 2022).
- Hoyt, S.J.; Storer, J.M.; Hartley, G.A.; Grady, P.G.S.; Gershman, A.; de Lima, L.G.; Limouse, C.; Halabian, R.; Wojenski, L.; Rodriguez, M.; et al. From Telomere to Telomere: The Transcriptional and Epigenetic State of Human Repeat Elements. Science 2022, 376, eabk3112. [Google Scholar] [CrossRef]
- Fatyol, K.; Illes, K.; Diamond, D.C.; Janish, C.; Szalay, A.A. Mer22-Related Sequence Elements Form Pericentric Repetitive DNA Families in Primates. Mol. Gen. Genet. 2000, 262, 931–939. [Google Scholar] [CrossRef]
- Hancks, D.C.; Kazazian, H.H. SVA Retrotransposons: Evolution and Genetic Instability. Semin. Cancer Biol. 2010, 20, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Black, E.M.; Giunta, S. Repetitive Fragile Sites: Centromere Satellite DNA as a Source of Genome Instability in Human Diseases. Genes 2018, 9, 615. [Google Scholar] [CrossRef]
- Ferreira, D.; Meles, S.; Escudeiro, A.; Mendes-da-Silva, A.; Adega, F.; Chaves, R. Satellite Non-Coding RNAs: The Emerging Players in Cells, Cellular Pathways and Cancer. Chromosom. Res. 2015, 23, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Suzuki, K.; Fukui, T.; Takayama, Y.; Kakizawa, N.; Watanabe, F.; Ishikawa, H.; Muto, Y.; Kato, T.; Saito, M.; et al. Overexpression of Satellite Alpha Transcripts Leads to Chromosomal Instability via Segregation Errors at Specific Chromosomes. Int. J. Oncol. 2018, 52, 1685–1693. [Google Scholar] [CrossRef]
- Nogalski, M.T.; Solovyov, A.; Kulkarni, A.S.; Desai, N.; Oberstein, A.; Levine, A.J.; Ting, D.T.; Shenk, T.; Greenbaum, B.D. A Tumor-Specific Endogenous Repetitive Element Is Induced by Herpesviruses. Nat. Commun. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Yandım, C.; Karakülah, G. Dysregulated Expression of Repetitive DNA in ER+/HER2- Breast Cancer. Cancer Genet. 2019, 239, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Rošić, S.; Köhler, F.; Erhardt, S. Repetitive Centromeric Satellite RNA Is Essential for Kinetochore Formation and Cell Division. J. Cell Biol. 2014, 207, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, L.; Saunus, J.M.; McCart Reed, A.E.; Lakhani, S.R. Breast Cancer Heterogeneity in Primary and Metastatic Disease. Adv Exp Med Biol. 2019, 1152, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Predic Ki67 Is a Promising Molecular Target in the Diagnosis of Cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Tremblay, D.C.; Alexander, G.; Moseley, S.; Chadwick, B.P. Expression, Tandem Repeat Copy Number Variation and Stability of Four Macrosatellite Arrays in the Human Genome. BMC Genom. 2010, 11, 632. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Kim, Y.S.; Yasuda, T.; Ohmachi, Y.; Yokouchi, H.; Monden, M.; Emi, M.; Konishi, N.; Nogami, M.; Okumura, K.; et al. A Novel Sperm-Specific Hypomethylation Sequence Is a Demethylation Hotspot in Human Hepatocellular Carcinomas. Gene 1999, 237, 15–20. [Google Scholar] [CrossRef]
- Suzuki, K.; Suzuki, I.; Leodolter, A.; Alonso, S.; Horiuchi, S.; Yamashita, K.; Perucho, M. Global DNA Demethylation in Gastrointestinal Cancer Is Age Dependent and Precedes Genomic Damage. Cancer Cell 2006, 9, 199–207. [Google Scholar] [CrossRef]
- Igarashi, S.; Suzuki, H.; Niinuma, T.; Shimizu, H.; Nojima, M.; Iwaki, H.; Nobuoka, T.; Nishida, T.; Miyazaki, Y.; Takamaru, H.; et al. A Novel Correlation between LINE-1 Hypomethylation and the Malignancy of Gastrointestinal Stromal Tumors. Clin. Cancer Res. 2010, 16, 5114–5123. [Google Scholar] [CrossRef]
- Samuelsson, J.K.; Dumbovic, G.; Polo, C.; Moreta, C.; Alibés, A.; Ruiz-Larroya, T.; Giménez-Bonafé, P.; Alonso, S.; Forcales, S.V.; Perucho, M. Helicase Lymphoid-Specific Enzyme Contributes to the Maintenance of Methylation of Sst1 Pericentromeric Repeats That Are Frequently Demethylated in Colon Cancer and Associate with Genomic Damage. Epigenomes 2017, 1, 2. [Google Scholar] [CrossRef]
| GeneID | Base Mean | log2(FC) | StdErr | Wald-Stats | p-Value | P-adj |
|---|---|---|---|---|---|---|
| Cancer vs. normal | ||||||
| BSR | 597.99 | 1.62 | 0.28 | 5.80 | 6.57 × 10−9 | 1.93 × 10−4 |
| ALR | 5004.62 | 1.52 | 0.28 | 5.44 | 5.24 × 10−8 | 7.70 × 10−4 |
| LSAU | 484.17 | 1.36 | 0.28 | 4.91 | 9.31 × 10−7 | 8.20 × 10−3 |
| ALRb | 1358.44 | 1.03 | 0.27 | 3.81 | 1.37 × 10−4 | 1.44 × 10−1 |
| ALR1 | 4308.91 | 0.99 | 0.27 | 3.73 | 1.90 × 10−4 | 1.48 × 10−1 |
| GGAAT | 11,375.95 | 0.91 | 0.28 | 3.28 | 1.05 × 10−3 | 3.35 × 10−1 |
| HSATII | 2167.60 | 0.91 | 0.28 | 3.27 | 1.08 × 10−3 | 3.35 × 10−1 |
| PABL_BI | 121.89 | 0.46 | 0.16 | 2.89 | 3.86 × 10−3 | 5.15 × 10−1 |
| SAR | 137.70 | 0.75 | 0.28 | 2.69 | 7.08 × 10−3 | 6.09 × 10−1 |
| LTR22B2 | 517.80 | −0.32 | 0.13 | −2.52 | 1.17 × 10−2 | 7.00 × 10−1 |
| LTR72 | 226.51 | −0.30 | 0.12 | −2.41 | 1.58 × 10−2 | 7.74 × 10−1 |
| LTR12C | 39,355.33 | −0.51 | 0.22 | −2.36 | 1.84 × 10−2 | 8.01 × 10−1 |
| MER9B | 648.51 | −0.26 | 0.12 | −2.27 | 2.33 × 10−2 | 8.33 × 10−1 |
| LTR7B | 2761.48 | −0.35 | 0.16 | −2.21 | 2.70 × 10−2 | 8.50 × 10−1 |
| LTR7C | 483.73 | −0.25 | 0.12 | −2.11 | 3.46 × 10−2 | 8.83 × 10−1 |
| LTR35 | 156.02 | −0.21 | 0.10 | −2.10 | 3.61 × 10−2 | 8.86 × 10−1 |
| ALR_ | 10,312.15 | 0.49 | 0.24 | 2.00 | 4.51 × 10−2 | 9.16 × 10−1 |
| HER2 vs. ant | ||||||
| ZAPHOD | 160.72 | −0.86 | 0.41 | −2.07 | 3.81 × 10−2 | 1.00 |
| HSATII | 6250.25 | 1.10 | 0.56 | 1.96 | 4.98 × 10−2 | 1.00 |
| LumA vs. ant | ||||||
| ALR | 5513.55 | 1.11 | 0.44 | 2.52 | 1.16 × 10−2 | 1.00 |
| LTR38B | 472.48 | −1.04 | 0.45 | −2.32 | 2.04 × 10−2 | 1.00 |
| LumB_Her2Neg vs. ant | ||||||
| ALR | 2804.92 | 1.75 | 0.48 | 3.64 | 2.74 × 10−4 | 1.29 × 10−1 |
| ALR1 | 3720.86 | 1.36 | 0.46 | 2.96 | 3.07 × 10−3 | 3.40 × 10−1 |
| ALRb | 1210.79 | 1.13 | 0.45 | 2.52 | 1.19 × 10−2 | 6.05 × 10−1 |
| MER57C1 | 196.12 | 1.00 | 0.43 | 2.31 | 2.08 × 10−2 | 7.23 × 10−1 |
| 6kbHsap | 7701.13 | 1.06 | 0.49 | 2.18 | 2.94 × 10−2 | 8.01 × 10−1 |
| LumB_Her2Pos vs. ant | ||||||
| LTR3 | 853.33 | −1.87 | 0.45 | −4.14 | 3.45 × 10−5 | 1.33 × 10−2 |
| HERVK3I | 10,531.29 | −1.76 | 0.47 | −3.72 | 1.99 × 10−4 | 4.53 × 10−2 |
| BSR | 436.11 | 2.09 | 0.60 | 3.49 | 4.92 × 10−4 | 8.70 × 10−2 |
| ALR1 | 20,223.11 | 2.06 | 0.64 | 3.21 | 1.32 × 10−3 | 1.56 × 10−1 |
| ALR | 14,122.78 | 1.97 | 0.64 | 3.06 | 2.24 × 10−3 | 2.10 × 10−1 |
| LTR1B0 | 5449.61 | −1.94 | 0.64 | −3.02 | 2.50 × 10−3 | 2.23 × 10−1 |
| LTR12C | 61,931.23 | −1.35 | 0.55 | −2.45 | 1.45 × 10−2 | 5.91 × 10−1 |
| MER122 | 128.05 | 1.31 | 0.64 | 2.06 | 3.97 × 10−2 | 9.10 × 10−1 |
| ALR_ | 13,301.52 | 1.18 | 0.59 | 1.99 | 4.61 × 10−2 | 9.55 × 10−1 |
| TN vs. ant | ||||||
| SAR | 171.13 | 0.84 | 0.28 | 3.01 | 2.63 × 10−3 | 2.41 × 10−1 |
| LTR72B | 423.96 | −0.56 | 0.22 | −2.57 | 1.03 × 10−2 | 4.98 × 10−1 |
| MER87B | 580.38 | 0.43 | 0.19 | 2.23 | 2.59 × 10−2 | 7.70 × 10−1 |
| GSAT | 291.10 | 0.58 | 0.29 | 1.98 | 4.72 × 10−2 | 9.7 × 10−1 |
| GeneID | Base Mean | log2(FC) | StdErr | Wald-Stats | p-Value | P-adj |
|---|---|---|---|---|---|---|
| MER22 | 3245.14 | −1.03 | 0.35 | −2.95 | 3.18 × 10−3 | 6.42 × 10−1 |
| PABL_BI | 101.04 | 0.67 | 0.24 | 2.82 | 4.78 × 10−3 | 6.42 × 10−1 |
| PTR5 | 394.09 | −1.10 | 0.41 | −2.67 | 7.67 × 10−3 | 6.42 × 10−1 |
| TAR1 | 184.59 | −0.66 | 0.26 | −2.53 | 1.15 × 10−2 | 6.42 × 10−1 |
| LTR7C | 516.36 | −0.55 | 0.23 | −2.44 | 1.45 × 10−2 | 6.42 × 10−1 |
| GSAT | 178.69 | −1.22 | 0.51 | −2.37 | 1.79 × 10−2 | 6.42 × 10−1 |
| LTR46 | 249.63 | −0.69 | 0.31 | −2.18 | 2.94 × 10−2 | 6.42 × 10−1 |
| LTR7Y | 1511.10 | −0.76 | 0.35 | −2.16 | 3.05 × 10−2 | 6.42 × 10−1 |
| LTR1E | 413.79 | −0.59 | 0.28 | −2.15 | 3.19 × 10−2 | 6.42 × 10−1 |
| LTR7A | 2067.24 | −0.77 | 0.36 | −2.14 | 3.20 × 10−2 | 6.42 × 10−1 |
| LTR22B2 | 563.94 | −0.50 | 0.23 | −2.14 | 3.27 × 10−2 | 6.42 × 10−1 |
| LTR44 | 116.12 | 0.81 | 0.39 | 2.08 | 3.79 × 10−2 | 6.42 × 10−1 |
| LTR27C | 315.21 | −0.59 | 0.28 | −2.08 | 3.79 × 10−2 | 6.42 × 10−1 |
| ALR | 1028.61 | 1.00 | 0.48 | 2.07 | 3.83 × 10−2 | 6.42 × 10−1 |
| HARLEQUINLTR | 3541.69 | −0.45 | 0.22 | −2.07 | 3.84 × 10−2 | 6.42 × 10−1 |
| MER51C | 493.52 | −0.45 | 0.22 | −2.03 | 4.19 × 10−2 | 6.42 × 10−1 |
| SVA_A | 65,198.32 | −0.57 | 0.28 | −2.02 | 4.32 × 10−2 | 6.42 × 10−1 |
| MER54B | 165.03 | −0.57 | 0.28 | −2.00 | 4.54 × 10−2 | 6.42 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arancio, W.; Coronnello, C. Repetitive Sequence Transcription in Breast Cancer. Cells 2022, 11, 2522. https://doi.org/10.3390/cells11162522
Arancio W, Coronnello C. Repetitive Sequence Transcription in Breast Cancer. Cells. 2022; 11(16):2522. https://doi.org/10.3390/cells11162522
Chicago/Turabian StyleArancio, Walter, and Claudia Coronnello. 2022. "Repetitive Sequence Transcription in Breast Cancer" Cells 11, no. 16: 2522. https://doi.org/10.3390/cells11162522
APA StyleArancio, W., & Coronnello, C. (2022). Repetitive Sequence Transcription in Breast Cancer. Cells, 11(16), 2522. https://doi.org/10.3390/cells11162522






