Diving into the Evolutionary History of HSC70-Linked Selective Autophagy Pathways: Endosomal Microautophagy and Chaperone-Mediated Autophagy
Abstract
:1. Introduction
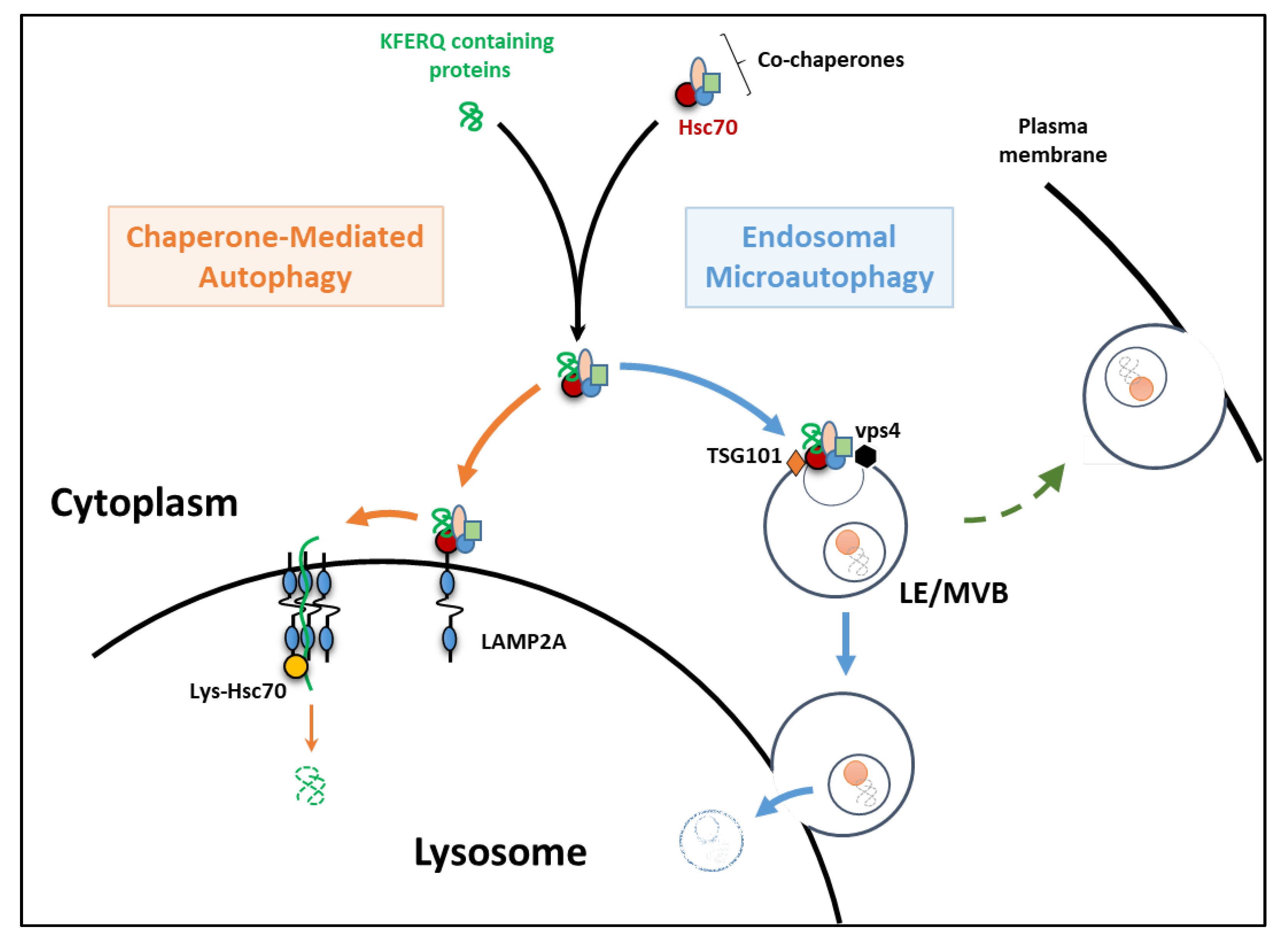
2. The Essentials of eMI and CMA
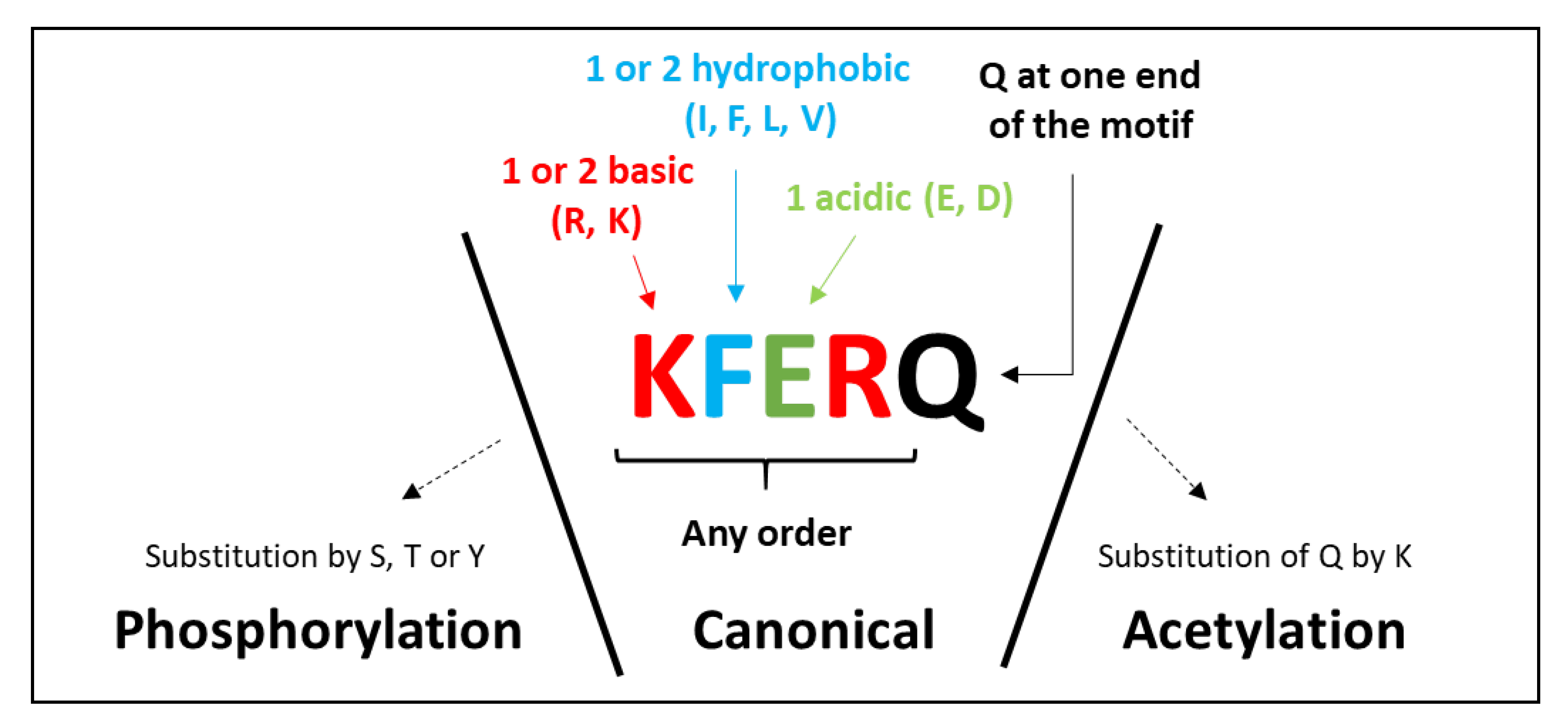
2.1. The KFERQ Motif
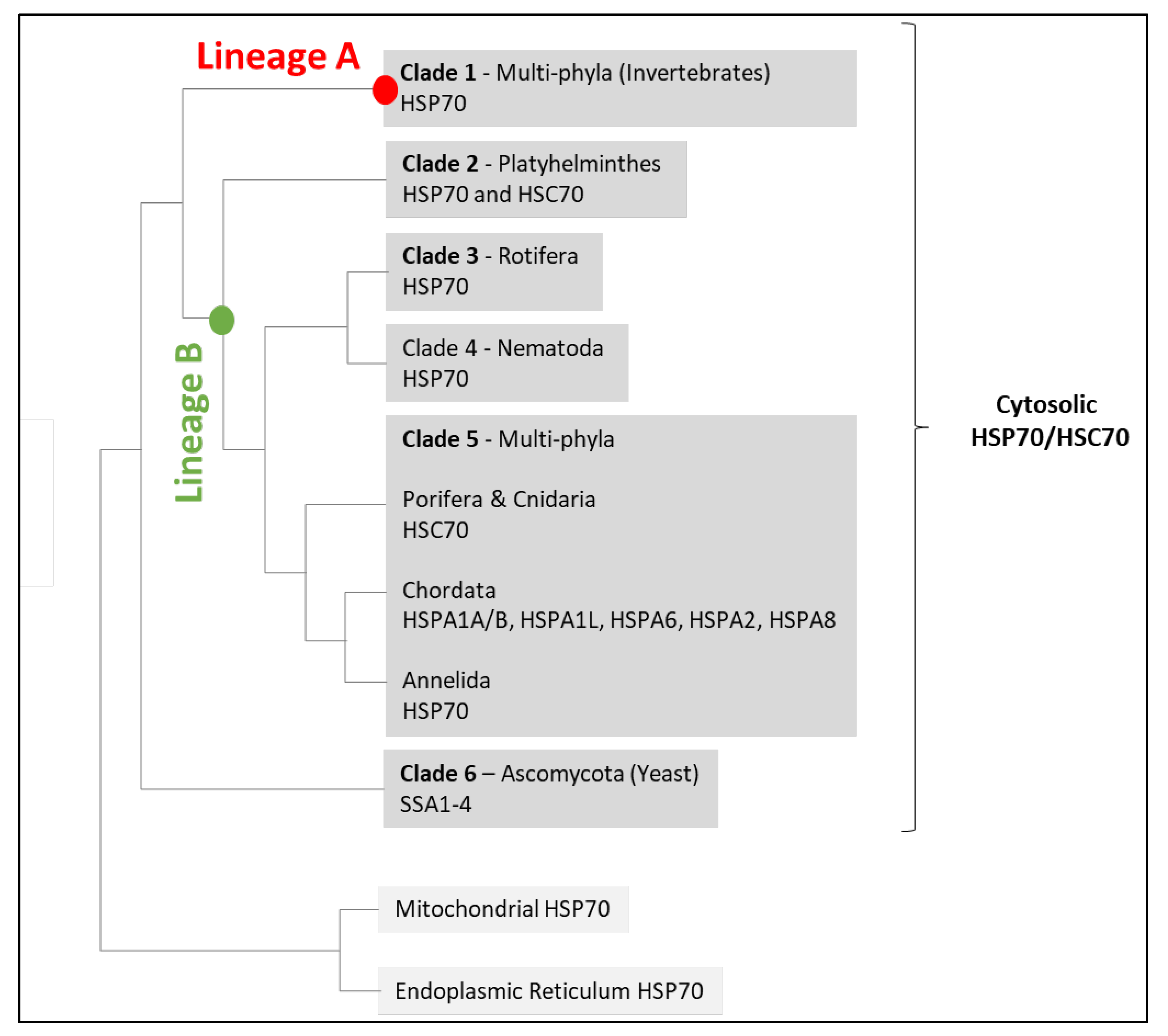
2.2. HSC70
3. Endosomal Microautophagy
4. Chaperone-Mediated Autophagy
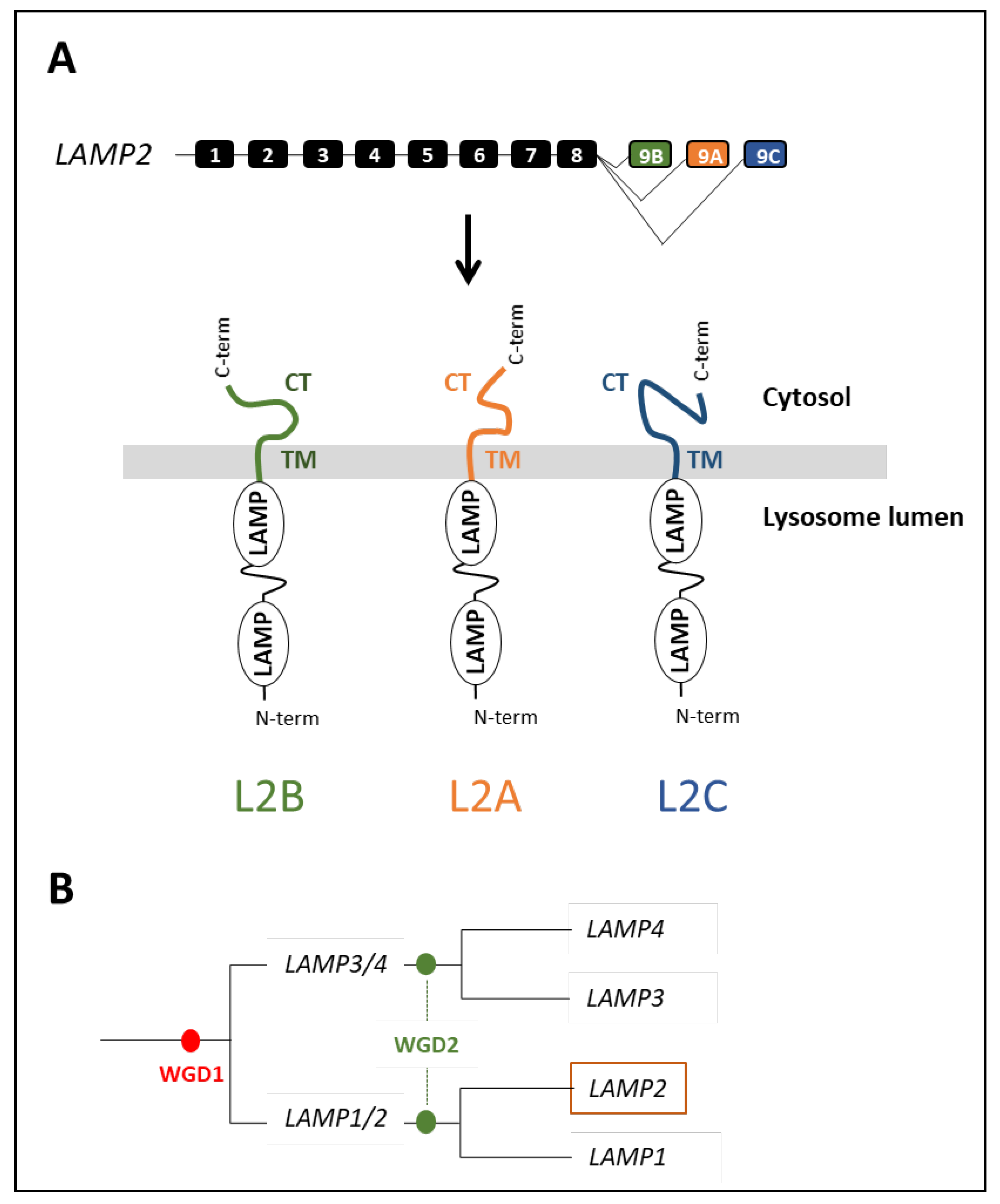
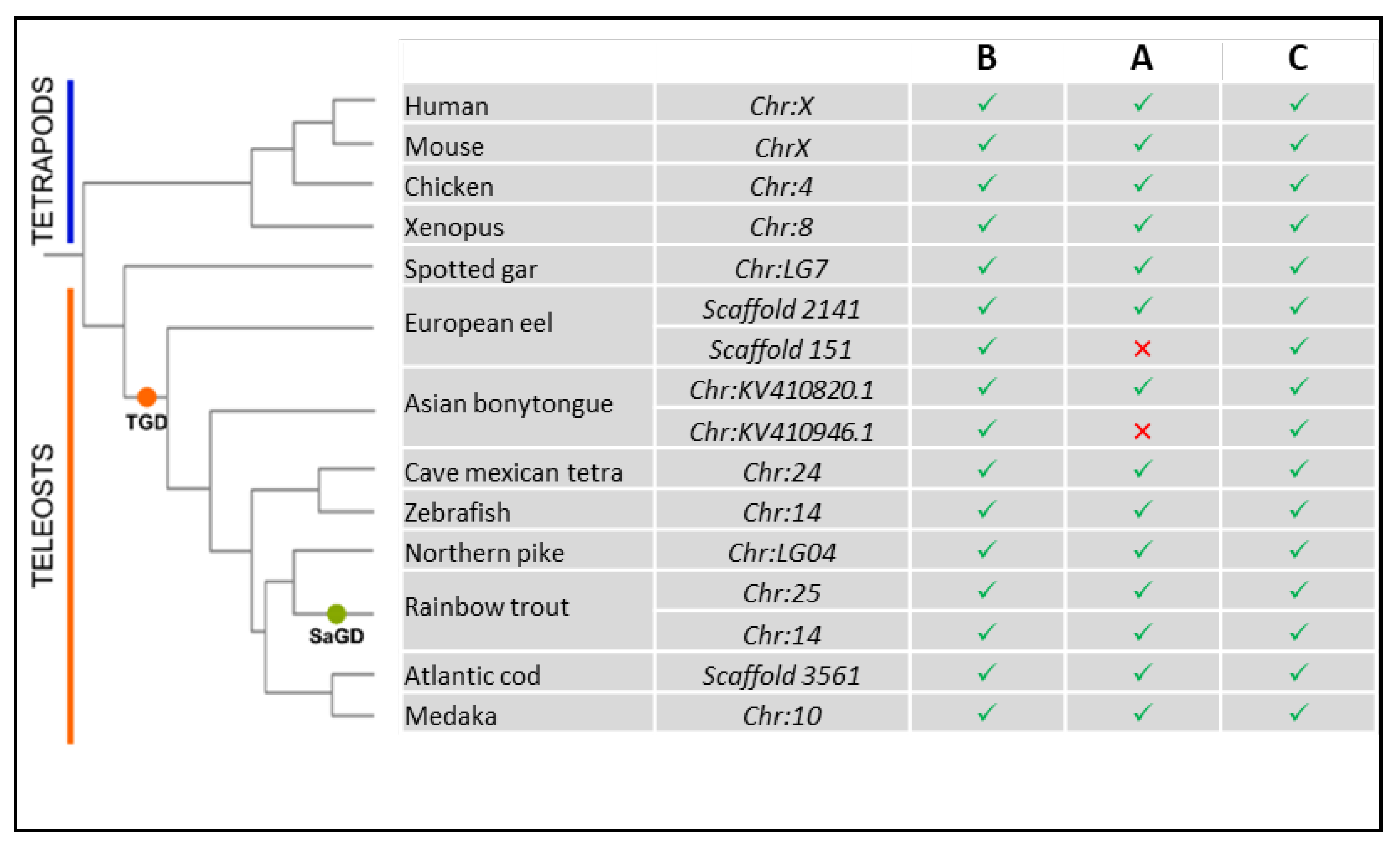
4.1. Origin and Evolution of LAMP2
4.2. Structure Evolution of LAMP2A across Vertebrates
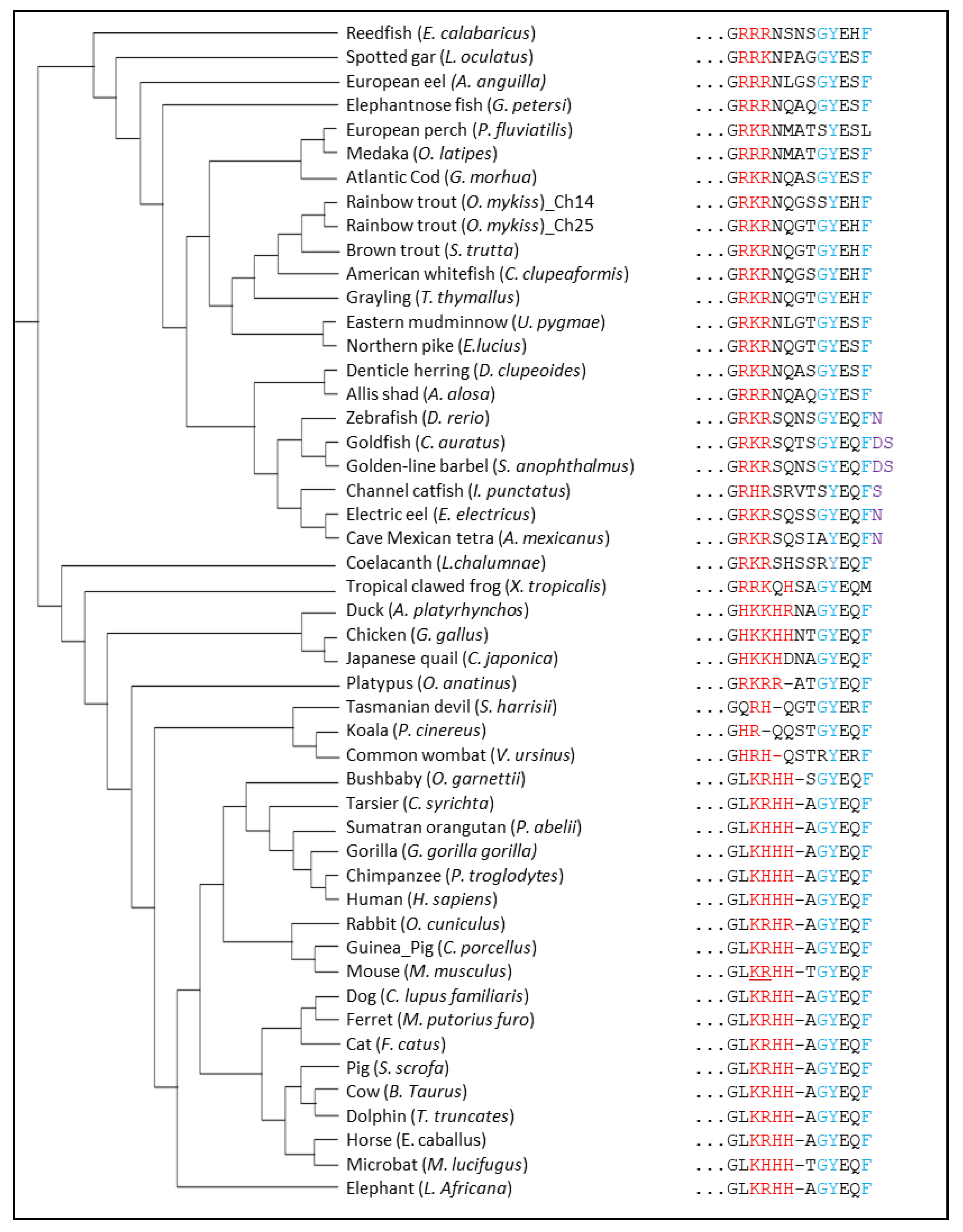
4.2.1. The GYXXϕ Motif
4.2.2. Positively-Charged Amino Acids
4.2.3. Glycine Residues in the Transmembrane Region
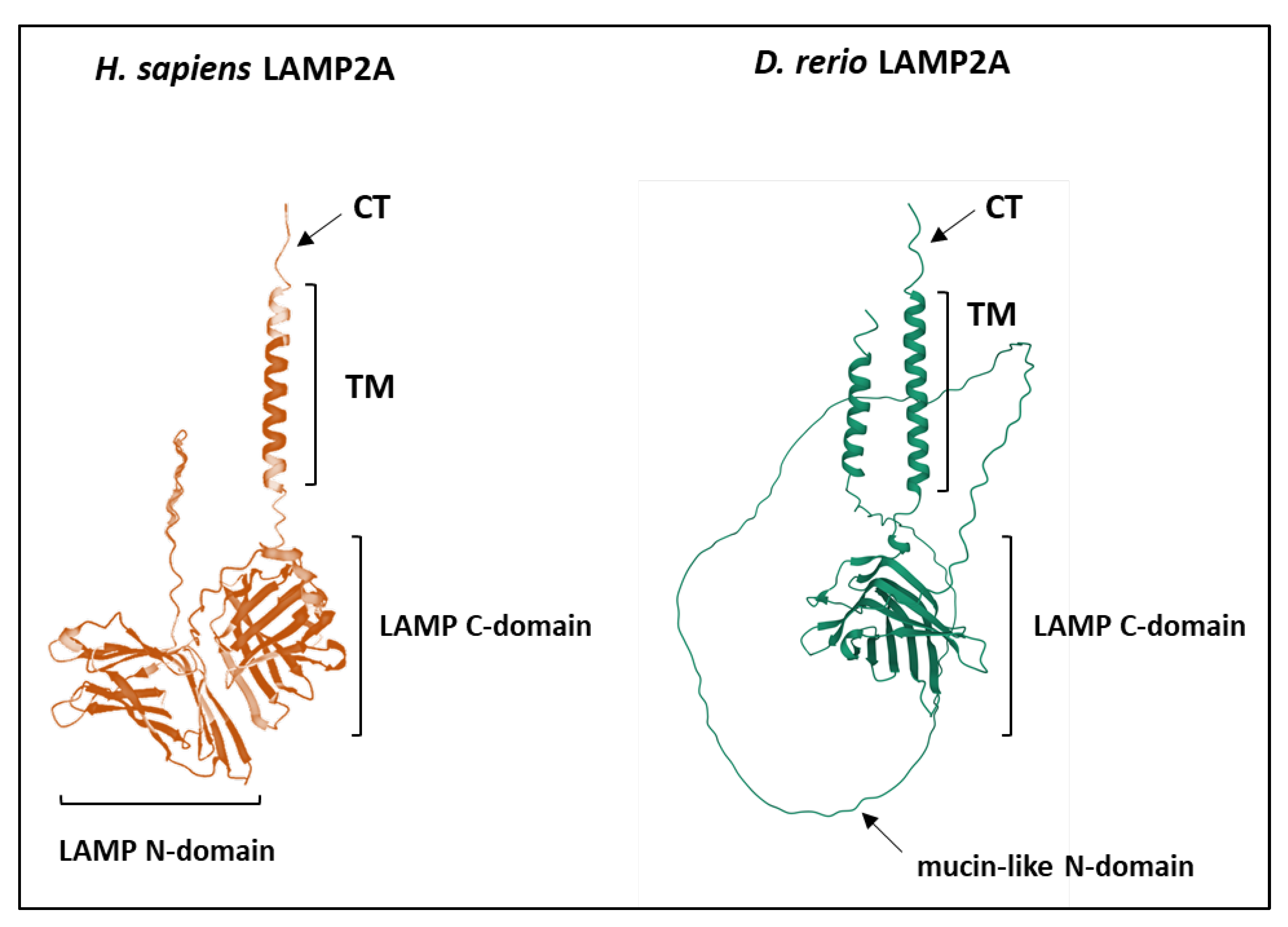
4.2.4. The LAMP Domains
5. Concluding Remarks and Pending Questions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiang, H.L.; Dice, J.F. Peptide Sequences That Target Proteins for Enhanced Degradation during Serum Withdrawal. J. Biol. Chem. 1988, 263, 6797–6805. [Google Scholar] [CrossRef]
- Chiang, H.L.; Terlecky, S.R.; Plant, C.P.; Dice, J.F. A Role for a 70-Kilodalton Heat Shock Protein in Lysosomal Degradation of Intracellular Proteins. Science 1989, 246, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Agarraberes, F.A.; Dice, J.F. A Molecular Chaperone Complex at the Lysosomal Membrane Is Required for Protein Translocation. J. Cell Sci. 2001, 114, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Dice, J.F. A Receptor for the Selective Uptake and Degradation of Proteins by Lysosomes. Science 1996, 273, 501–503. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Kaushik, S.; Varticovski, L.; Cuervo, A.M. The Chaperone-Mediated Autophagy Receptor Organizes in Dynamic Protein Complexes at the Lysosomal Membrane. Mol. Cell. Biol. 2008, 28, 5747–5763. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, U.; Sridhar, S.; Kaushik, S.; Kiffin, R.; Cuervo, A.M. Identification of Regulators of Chaperone-Mediated Autophagy. Mol. Cell 2010, 39, 535–547. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.; Cuervo, A.M. The Coming of Age of Chaperone-Mediated Autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 365–381. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of Cytosolic Proteins by Late Endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Tekirdag, K.; Cuervo, A.M. Chaperone-Mediated Autophagy and Endosomal Microautophagy: Joint by a Chaperone. J. Biol. Chem. 2018, 293, 5414–5424. [Google Scholar] [CrossRef] [Green Version]
- Morozova, K.; Clement, C.C.; Kaushik, S.; Stiller, B.; Arias, E.; Ahmad, A.; Rauch, J.N.; Chatterjee, V.; Melis, C.; Scharf, B.; et al. Structural and Biological Interaction of Hsc-70 Protein with Phosphatidylserine in Endosomal Microautophagy. J. Biol. Chem. 2016, 291, 18096–18106. [Google Scholar] [CrossRef] [Green Version]
- Caballero, B.; Wang, Y.; Diaz, A.; Tasset, I.; Juste, Y.R.; Stiller, B.; Mandelkow, E.-M.; Mandelkow, E.; Cuervo, A.M. Interplay of Pathogenic Forms of Human Tau with Different Autophagic Pathways. Aging Cell 2018, 17, e12692. [Google Scholar] [CrossRef] [Green Version]
- Caballero, B.; Bourdenx, M.; Luengo, E.; Diaz, A.; Sohn, P.D.; Chen, X.; Wang, C.; Juste, Y.R.; Wegmann, S.; Patel, B.; et al. Acetylated Tau Inhibits Chaperone-Mediated Autophagy and Promotes Tau Pathology Propagation in Mice. Nat. Commun. 2021, 12, 2238. [Google Scholar] [CrossRef] [PubMed]
- Lescat, L.; Véron, V.; Mourot, B.; Péron, S.; Chenais, N.; Dias, K.; Riera-Heredia, N.; Beaumatin, F.; Pinel, K.; Priault, M.; et al. Chaperone-Mediated Autophagy in the Light of Evolution: Insight from Fish. Mol. Biol. Evol. 2020, 37, 2887–2899. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Patel, B.; Koga, H.; Cuervo, A.M.; Jenny, A. Selective Endosomal Microautophagy Is Starvation-Inducible in Drosophila. Autophagy 2016, 12, 1984–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-M.; Sun, L.-L.; Hu, W.; Ding, Y.-H.; Dong, M.-Q.; Du, L.-L. ESCRTs Cooperate with a Selective Autophagy Receptor to Mediate Vacuolar Targeting of Soluble Cargos. Mol. Cell 2015, 59, 1035–1042. [Google Scholar] [CrossRef] [Green Version]
- Lescat, L.; Herpin, A.; Mourot, B.; Véron, V.; Guiguen, Y.; Bobe, J.; Seiliez, I. CMA Restricted to Mammals and Birds: Myth or Reality? Autophagy 2018, 14, 1267–1270. [Google Scholar] [CrossRef] [PubMed]
- Dice, J.F. Peptide Sequences That Target Cytosolic Proteins for Lysosomal Proteolysis. Trends Biochem. Sci. 1990, 15, 305–309. [Google Scholar] [CrossRef]
- Lv, L.; Li, D.; Zhao, D.; Lin, R.; Chu, Y.; Zhang, H.; Zha, Z.; Liu, Y.; Li, Z.; Xu, Y.; et al. Acetylation Targets the M2 Isoform of Pyruvate Kinase for Degradation through Chaperone-Mediated Autophagy and Promotes Tumor Growth. Mol. Cell 2011, 42, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.; Cuervo, A.M. Degradation of Lipid Droplet-Associated Proteins by Chaperone-Mediated Autophagy Facilitates Lipolysis. Nat. Cell Biol. 2015, 17, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Bonhoure, A.; Vallentin, A.; Martin, M.; Senff-Ribeiro, A.; Amson, R.; Telerman, A.; Vidal, M. Acetylation of Translationally Controlled Tumor Protein Promotes Its Degradation through Chaperone-Mediated Autophagy. Eur. J. Cell Biol. 2017, 96, 83–98. [Google Scholar] [CrossRef]
- Jackson, M.P.; Hewitt, E.W. Cellular Proteostasis: Degradation of Misfolded Proteins by Lysosomes. Essays Biochem. 2016, 60, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchner, P.; Bourdenx, M.; Madrigal-Matute, J.; Tiano, S.; Diaz, A.; Bartholdy, B.A.; Will, B.; Cuervo, A.M. Proteome-Wide Analysis of Chaperone-Mediated Autophagy Targeting Motifs. PLoS Biol. 2019, 17, e3000301. [Google Scholar] [CrossRef] [PubMed]
- Stricher, F.; Macri, C.; Ruff, M.; Muller, S. HSPA8/HSC70 Chaperone Protein: Structure, Function, and Chemical Targeting. Autophagy 2013, 9, 1937–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Tang, L. The Critical Roles of HSC70 in Physiological and Pathological Processes. Curr. Pharm. Des. 2014, 20, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Ruff, M.; Muller, S. HSPA8/HSC70 in Immune Disorders: A Molecular Rheostat That Adjusts Chaperone-Mediated Autophagy Substrates. Cells 2019, 8, E849. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Singh, B. Phylogenetic Analysis of 70 KD Heat Shock Protein Sequences Suggests a Chimeric Origin for the Eukaryotic Cell Nucleus. Curr. Biol. 1994, 4, 1104–1114. [Google Scholar] [CrossRef]
- Hunt, C.; Morimoto, R.I. Conserved Features of Eukaryotic Hsp70 Genes Revealed by Comparison with the Nucleotide Sequence of Human Hsp70. Proc. Natl. Acad. Sci. USA 1985, 82, 6455–6459. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, S.; Craig, E.A. The Heat-Shock Proteins. Annu. Rev. Genet. 1988, 22, 631–677. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, Z.; Zhuo, L.; Wan, T.; Li, Y. Bioinformatic and Functional Characterization of Hsp70s in Myxococcus Xanthus. mSphere 2021, 6, e00305-21. [Google Scholar] [CrossRef]
- Werner-Washburne, M.; Craig, E.A. Expression of Members of the Saccharomyces Cerevisiae Hsp70 Multigene Family. Genome 1989, 31, 684–689. [Google Scholar] [CrossRef]
- Boorstein, W.R.; Ziegelhoffer, T.; Craig, E.A. Molecular Evolution of the HSP70 Multigene Family. J. Mol. Evol. 1994, 38, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Yoshinaga, T.; Jalufka, F.L.; Ehsan, H.; Welch, D.B.M.; Kaneko, G. The Complex Evolution of the Metazoan HSP70 Gene Family. Sci. Rep. 2021, 11, 17794. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.; Oliverio, R.; Nguyen, P.; Le, D.; Ellis, J.; Kdeiss, B.; Ord, S.; Chalkia, D.; Nikolaidis, N. Concurrent Action of Purifying Selection and Gene Conversion Results in Extreme Conservation of the Major Stress-Inducible Hsp70 Genes in Mammals. Sci. Rep. 2018, 8, 5082. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, N.; Nei, M. Concerted and Nonconcerted Evolution of the Hsp70 Gene Superfamily in Two Sibling Species of Nematodes. Mol. Biol. Evol. 2004, 21, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Kourtidis, A.; Drosopoulou, E.; Nikolaidis, N.; Hatzi, V.I.; Chintiroglou, C.C.; Scouras, Z.G. Identification of Several Cytoplasmic HSP70 Genes from the Mediterranean Mussel (Mytilus Galloprovincialis) and Their Long-Term Evolution in Mollusca and Metazoa. J. Mol. Evol. 2006, 62, 446–459. [Google Scholar] [CrossRef]
- Krenek, S.; Schlegel, M.; Berendonk, T.U. Convergent Evolution of Heat-Inducibility during Subfunctionalization of the Hsp70 Gene Family. BMC Evol. Biol. 2013, 13, 49. [Google Scholar] [CrossRef] [Green Version]
- Marzella, L.; Ahlberg, J.; Glaumann, H. Autophagy, Heterophagy, Microautophagy and Crinophagy as the Means for Intracellular Degradation. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1981, 36, 219–234. [Google Scholar] [CrossRef]
- Mortimore, G.E.; Lardeux, B.R.; Adams, C.E. Regulation of Microautophagy and Basal Protein Turnover in Rat Liver. Effects of Short-Term Starvation. J. Biol. Chem. 1988, 263, 2506–2512. [Google Scholar] [CrossRef]
- Tuttle, D.L.; Lewin, A.S.; Dunn, W.A. Selective Autophagy of Peroxisomes in Methylotrophic Yeasts. Eur. J. Cell Biol. 1993, 60, 283–290. [Google Scholar]
- Sakai, Y.; Koller, A.; Rangell, L.K.; Keller, G.A.; Subramani, S. Peroxisome Degradation by Microautophagy in Pichia Pastoris: Identification of Specific Steps and Morphological Intermediates. J. Cell Biol. 1998, 141, 625–636. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.L.; Thorsness, P.E. Escape of Mitochondrial DNA to the Nucleus in Yme1 Yeast Is Mediated by Vacuolar-Dependent Turnover of Abnormal Mitochondrial Compartments. J. Cell Sci. 1998, 111, 2455–2464. [Google Scholar] [CrossRef]
- Roberts, P.; Moshitch-Moshkovitz, S.; Kvam, E.; O’Toole, E.; Winey, M.; Goldfarb, D.S. Piecemeal Microautophagy of Nucleus in Saccharomyces Cerevisiae. Mol. Biol. Cell 2003, 14, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Zutphen, T.; Todde, V.; de Boer, R.; Kreim, M.; Hofbauer, H.F.; Wolinski, H.; Veenhuis, M.; van der Klei, I.J.; Kohlwein, S.D. Lipid Droplet Autophagy in the Yeast Saccharomyces Cerevisiae. Mol. Biol. Cell 2014, 25, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuck, S.; Gallagher, C.M.; Walter, P. ER-Phagy Mediates Selective Degradation of Endoplasmic Reticulum Independently of the Core Autophagy Machinery. J. Cell Sci. 2014, 127, 4078–4088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhang, W.; Wen, X.; Bulinski, P.J.; Chomchai, D.A.; Arines, F.M.; Liu, Y.-Y.; Sprenger, S.; Teis, D.; Klionsky, D.J.; et al. TORC1 Regulates Vacuole Membrane Composition through Ubiquitin- and ESCRT-Dependent Microautophagy. J. Cell Biol. 2020, 219, e201902127. [Google Scholar] [CrossRef]
- Schuck, S. Microautophagy—Distinct Molecular Mechanisms Handle Cargoes of Many Sizes. J. Cell Sci. 2020, 133, jcs246322. [Google Scholar] [CrossRef]
- Mesquita, A.; Glenn, J.; Jenny, A. Differential Activation of EMI by Distinct Forms of Cellular Stress. Autophagy 2021, 17, 1828–1840. [Google Scholar] [CrossRef]
- Uytterhoeven, V.; Lauwers, E.; Maes, I.; Miskiewicz, K.; Melo, M.N.; Swerts, J.; Kuenen, S.; Wittocx, R.; Corthout, N.; Marrink, S.-J.; et al. Hsc70-4 Deforms Membranes to Promote Synaptic Protein Turnover by Endosomal Microautophagy. Neuron 2015, 88, 735–748. [Google Scholar] [CrossRef] [Green Version]
- Leung, K.F.; Dacks, J.B.; Field, M.C. Evolution of the Multivesicular Body ESCRT Machinery; Retention Across the Eukaryotic Lineage. Traffic 2008, 9, 1698–1716. [Google Scholar] [CrossRef]
- Spang, A.; Saw, J.H.; Jørgensen, S.L.; Zaremba-Niedzwiedzka, K.; Martijn, J.; Lind, A.E.; van Eijk, R.; Schleper, C.; Guy, L.; Ettema, T.J.G. Complex Archaea That Bridge the Gap between Prokaryotes and Eukaryotes. Nature 2015, 521, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Hatano, T.; Palani, S.; Papatziamou, D.; Salzer, R.; Souza, D.P.; Tamarit, D.; Makwana, M.; Potter, A.; Haig, A.; Xu, W.; et al. Asgard Archaea Shed Light on the Evolutionary Origins of the Eukaryotic Ubiquitin-ESCRT Machinery. Nat. Commun. 2022, 13, 3398. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Dice, J.F. Unique Properties of Lamp2a Compared to Other Lamp2 Isoforms. J. Cell Sci. 2000, 113, 4441–4450. [Google Scholar] [CrossRef] [PubMed]
- Gough, N.R.; Hatem, C.L.; Fambrough, D.M. The Family of LAMP-2 Proteins Arises by Alternative Splicing from a Single Gene: Characterization of the Avian LAMP-2 Gene and Identification of Mammalian Homologs of LAMP-2b and LAMP-2c. DNA Cell Biol. 1995, 14, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Hatem, C.L.; Gough, N.R.; Fambrough, D.M. Multiple MRNAs Encode the Avian Lysosomal Membrane Protein LAMP-2, Resulting in Alternative Transmembrane and Cytoplasmic Domains. J. Cell Sci. 1995, 108, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Nishino, I.; Fu, J.; Tanji, K.; Yamada, T.; Shimojo, S.; Koori, T.; Mora, M.; Riggs, J.E.; Oh, S.J.; Koga, Y.; et al. Primary LAMP-2 Deficiency Causes X-Linked Vacuolar Cardiomyopathy and Myopathy (Danon Disease). Nature 2000, 406, 906–910. [Google Scholar] [CrossRef]
- Chi, C.; Leonard, A.; Knight, W.E.; Beussman, K.M.; Zhao, Y.; Cao, Y.; Londono, P.; Aune, E.; Trembley, M.A.; Small, E.M.; et al. LAMP-2B Regulates Human Cardiomyocyte Function by Mediating Autophagosome–Lysosome Fusion. Proc. Natl. Acad. Sci. USA 2019, 116, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, Y.; Furuta, A.; Kikuchi, H.; Aizawa, S.; Hatanaka, Y.; Konya, C.; Uchida, K.; Yoshimura, A.; Tamai, Y.; Wada, K.; et al. Discovery of a Novel Type of Autophagy Targeting RNA. Autophagy 2013, 9, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, Y.; Kikuchi, H.; Aizawa, S.; Furuta, A.; Hatanaka, Y.; Konya, C.; Uchida, K.; Wada, K.; Kabuta, T. Direct Uptake and Degradation of DNA by Lysosomes. Autophagy 2013, 9, 1167–1171. [Google Scholar] [CrossRef] [Green Version]
- Dehal, P.; Boore, J.L. Two Rounds of Whole Genome Duplication in the Ancestral Vertebrate. PLoS Biol. 2005, 3, e314. [Google Scholar] [CrossRef] [Green Version]
- Ravi, V.; Venkatesh, B. The Divergent Genomes of Teleosts. Annu. Rev. Anim. Biosci. 2018, 6, 47–68. [Google Scholar] [CrossRef]
- Pasquier, J.; Cabau, C.; Nguyen, T.; Jouanno, E.; Severac, D.; Braasch, I.; Journot, L.; Pontarotti, P.; Klopp, C.; Postlethwait, J.H.; et al. Gene Evolution and Gene Expression after Whole Genome Duplication in Fish: The PhyloFish Database. BMC Genom. 2016, 17, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasawa, K.; Kato, Y.; Ikami, Y.; Sakamoto, K.; Ohtake, K.; Kusano, S.; Tomabechi, Y.; Kukimoto-Niino, M.; Shirouzu, M.; Guan, J.-L.; et al. Direct Homophilic Interaction of LAMP2A with the Two-Domain Architecture Revealed by Site-Directed Photo-Crosslinks and Steric Hindrances in Mammalian Cells. Autophagy 2021, 17, 4286–4304. [Google Scholar] [CrossRef] [PubMed]
- Wilke, S.; Krausze, J.; Büssow, K. Crystal Structure of the Conserved Domain of the DC Lysosomal Associated Membrane Protein: Implications for the Lysosomal Glycocalyx. BMC Biol. 2012, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Rout, A.K.; Strub, M.-P.; Piszczek, G.; Tjandra, N. Structure of Transmembrane Domain of Lysosome-Associated Membrane Protein Type 2a (LAMP-2A) Reveals Key Features for Substrate Specificity in Chaperone-Mediated Autophagy. J. Biol. Chem. 2014, 289, 35111–35123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terasawa, K.; Tomabechi, Y.; Ikeda, M.; Ehara, H.; Kukimoto-Niino, M.; Wakiyama, M.; Podyma-Inoue, K.A.; Rajapakshe, A.R.; Watabe, T.; Shirouzu, M.; et al. Lysosome-Associated Membrane Proteins-1 and -2 (LAMP-1 and LAMP-2) Assemble via Distinct Modes. Biochem. Biophys. Res. Commun. 2016, 479, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ikami, Y.; Terasawa, K.; Sakamoto, K.; Ohtake, K.; Harada, H.; Watabe, T.; Yokoyama, S.; Hara-Yokoyama, M. The Two-Domain Architecture of LAMP2A Regulates Its Interaction with Hsc70. Exp. Cell Res. 2022, 411, 112986. [Google Scholar] [CrossRef]
- Williams, M.A.; Fukuda, M. Accumulation of Membrane Glycoproteins in Lysosomes Requires a Tyrosine Residue at a Particular Position in the Cytoplasmic Tail. J. Cell Biol. 1990, 111, 955–966. [Google Scholar] [CrossRef] [Green Version]
- Guarnieri, F.G.; Arterburn, L.M.; Penno, M.B.; Cha, Y.; August, J.T. The Motif Tyr-X-X-Hydrophobic Residue Mediates Lysosomal Membrane Targeting of Lysosome-Associated Membrane Protein 1. J. Biol. Chem. 1993, 268, 1941–1946. [Google Scholar] [CrossRef]
- Hunziker, W.; Harter, C.; Matter, K.; Mellman, I. Basolateral Sorting in MDCK Cells Requires a Distinct Cytoplasmic Domain Determinant. Cell 1991, 66, 907–920. [Google Scholar] [CrossRef]
- Harter, C.; Mellman, I. Transport of the Lysosomal Membrane Glycoprotein Lgp120 (Lgp-A) to Lysosomes Does Not Require Appearance on the Plasma Membrane. J. Cell Biol. 1992, 117, 311–325. [Google Scholar] [CrossRef] [Green Version]
- Russ, W.P.; Engelman, D.M. The GxxxG Motif: A Framework for Transmembrane Helix-Helix Association. J. Mol. Biol. 2000, 296, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Jalali, Z.; Parvaz, N. Molecular Evolution of Autophagy Rate-Limiting Factor LAMP2 in Placental Mammals. Gene 2020, 727, 144231. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnebert, S.; Goguet, M.; Vélez, E.J.; Depincé, A.; Beaumatin, F.; Herpin, A.; Seiliez, I. Diving into the Evolutionary History of HSC70-Linked Selective Autophagy Pathways: Endosomal Microautophagy and Chaperone-Mediated Autophagy. Cells 2022, 11, 1945. https://doi.org/10.3390/cells11121945
Schnebert S, Goguet M, Vélez EJ, Depincé A, Beaumatin F, Herpin A, Seiliez I. Diving into the Evolutionary History of HSC70-Linked Selective Autophagy Pathways: Endosomal Microautophagy and Chaperone-Mediated Autophagy. Cells. 2022; 11(12):1945. https://doi.org/10.3390/cells11121945
Chicago/Turabian StyleSchnebert, Simon, Maxime Goguet, Emilio J. Vélez, Alexandra Depincé, Florian Beaumatin, Amaury Herpin, and Iban Seiliez. 2022. "Diving into the Evolutionary History of HSC70-Linked Selective Autophagy Pathways: Endosomal Microautophagy and Chaperone-Mediated Autophagy" Cells 11, no. 12: 1945. https://doi.org/10.3390/cells11121945
APA StyleSchnebert, S., Goguet, M., Vélez, E. J., Depincé, A., Beaumatin, F., Herpin, A., & Seiliez, I. (2022). Diving into the Evolutionary History of HSC70-Linked Selective Autophagy Pathways: Endosomal Microautophagy and Chaperone-Mediated Autophagy. Cells, 11(12), 1945. https://doi.org/10.3390/cells11121945






