Modulation of Trans-Synaptic Neurexin–Neuroligin Interaction in Pathological Pain
Abstract
1. Introduction
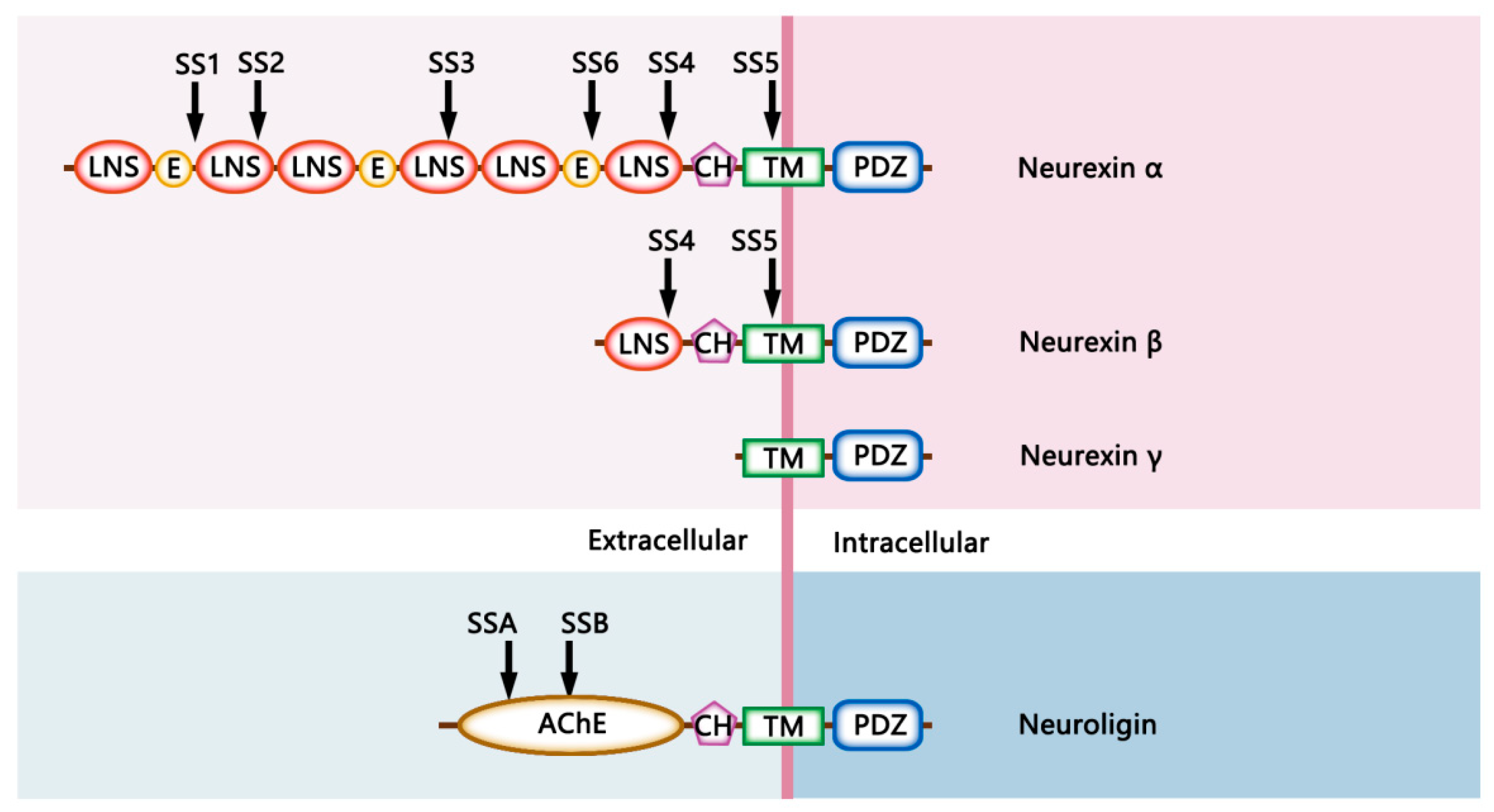
2. The Structural Characteristics of Neurexins and Neuroligins
2.1. The Structural Diversity of Neurexins
2.2. The Structural Diversity of Neuroligins
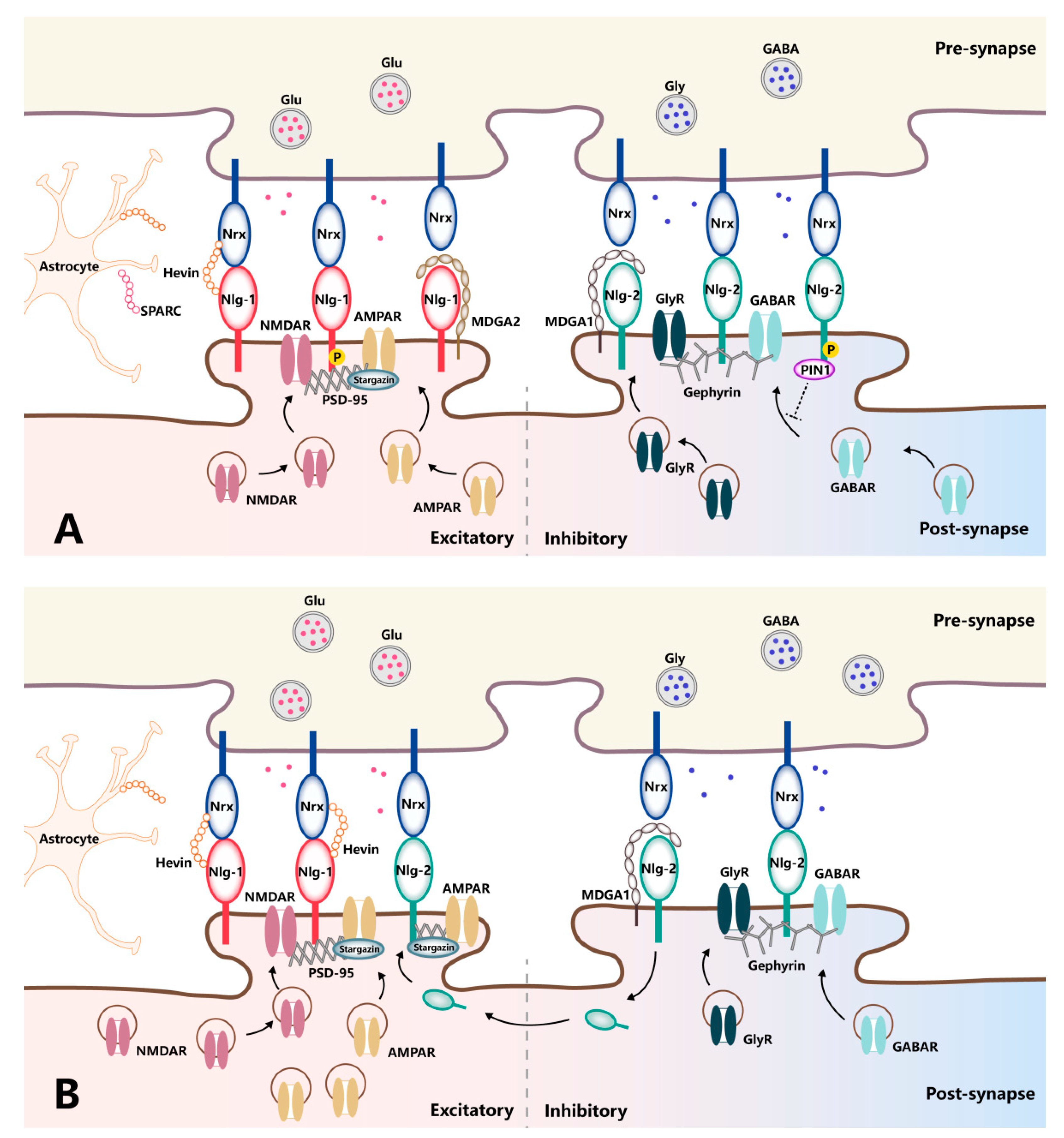
3. The Neurexin–Neuroligin Mediated Trans-Synaptic Modulation
3.1. The Dynamic Synaptic Regulation by Neurexins
3.2. The Dynamic Synaptic Regulation by Neuroligins
4. The Regulators of Neurexin–Neuroligin Interaction
4.1. Hevin
4.2. SPARC
4.3. MDGA
5. The Role of Trans-Synaptic Neurexin–Neuroligin Interaction in Pathological Pain
5.1. Disrupting Trans-Synaptic Transmission of Nociceptive Signals by Targeting Neurexins
5.2. Disrupting Trans-Synaptic Transmission of Nociceptive Signals by Targeting Neuroligins
5.3. Disrupting Trans-Synaptic Transmission of Nociceptive Signals by Targeting Regulators of the Neurexin–Neuroligin Interaction
6. Concluding Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Moura, D.M.S.; Brennan, E.J.; Brock, R.; Cocas, L.A. Neuron to oligodendrocyte precursor cell synapses: Protagonists in oligodendrocyte development and myelination, and targets for therapeutics. Front. Neurosci. 2022, 15, 779125. [Google Scholar] [CrossRef]
- Magee, J.C.; Grienberger, C. Synaptic plasticity forms and functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef]
- Tong, R.; Emptage, N.J.; Padamsey, Z. A two-compartment model of synaptic computation and plasticity. Mol. Brain 2020, 13, 79. [Google Scholar] [CrossRef]
- Jiang, X.; Sando, R.; Südhof, T.C. Multiple signaling pathways are essential for synapse formation induced by synaptic adhesion molecules. Proc. Natl. Acad. Sci. USA 2021, 118, e2000173118. [Google Scholar] [CrossRef]
- Kim, H.Y.; Um, J.W.; Ko, J. Proper synaptic adhesion signaling in the control of neural circuit architecture and brain function. Prog. Neurobiol. 2021, 200, 101983. [Google Scholar] [CrossRef]
- Gennarini, G.; Furley, A. Cell adhesion molecules in neural development and disease. Mol. Cell. Neurosci. 2017, 81, 1–3. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Guan, Y.; Wang, Y. The emerging role of kainate receptor functional dysregulation in pain. Mol. Pain 2021, 17, 1–11. [Google Scholar] [CrossRef]
- Lin, T.B.; Lai, C.Y.; Hsieh, M.C.; Jiang, J.L.; Cheng, J.K.; Chau, Y.P.; Ruan, T.; Chen, G.D.; Peng, H.Y. Neuropathic allodynia involves spinal neurexin-1β-dependent neuroligin-1/postsynaptic density-95/NR2B cascade in rats. Anesthesiology 2015, 123, 909–926. [Google Scholar] [CrossRef]
- Kim, J.V.; Megat, S.; Moy, J.K.; Asiedu, M.N.; Mejia, G.L.; Vagner, J.; Price, T.J. Neuroligin 2 regulates spinal GABAergic plasticity in hyperalgesic priming, a model of the transition from acute to chronic pain. Pain 2016, 157, 1314–1324. [Google Scholar] [CrossRef][Green Version]
- Gomez, A.M.; Traunmüller, L.; Scheiffele, P. Neurexins: Molecular codes for shaping neuronal synapses. Nat. Rev. Neurosci. 2021, 22, 137–151. [Google Scholar] [CrossRef]
- Ullrich, B.; Ushkaryov, Y.A.; Südhof, T.C. Cartography of neurexins: More than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 1995, 14, 497–507. [Google Scholar] [CrossRef]
- Liakath-Ali, K.; Südhof, T.C. The perils of navigating activity-dependent alternative splicing of neurexins. Front. Mol. Neurosci. 2021, 14, 659681. [Google Scholar] [CrossRef]
- Cuttler, K.; Hassan, M.; Carr, J.; Cloete, R.; Bardien, S. Emerging evidence implicating a role for neurexins in neurodegenerative and neuropsychiatric disorders. Open Biol. 2021, 11, 210091. [Google Scholar] [CrossRef]
- Jeong, J.; Paskus, J.D.; Roche, K.W. Posttranslational modifications of neuroligins regulate neuronal and glial signaling. Curr. Opin. Neurobiol. 2017, 45, 130–138. [Google Scholar] [CrossRef]
- Ichtchenko, K.; Nguyen, T.; Südhof, T.C. Structures, alternative splicing, and neurexin binding of multiple neuroligins. J. Biol. Chem. 1996, 271, 2676–2682. [Google Scholar] [CrossRef]
- Qin, L.; Guo, S.; Han, Y.; Wang, X.; Zhang, B. Functional mosaic organization of neuroligins in neuronal circuits. Cell. Mol. Life Sci. 2020, 77, 3117–3127. [Google Scholar] [CrossRef]
- Boucard, A.A.; Chubykin, A.A.; Comoletti, D.; Taylor, P.; Südhof, T.C. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron 2005, 48, 229–236. [Google Scholar] [CrossRef]
- Lee, H.; Dean, C.; Isacoff, E. Alternative splicing of neuroligin regulates the rate of presynaptic differentiation. J. Neurosci. 2010, 30, 11435–11446. [Google Scholar] [CrossRef]
- Oku, S.; Feng, H.; Connor, S.; Toledo, A.; Zhang, P.; Zhang, Y.; Thoumine, O.; Zhang, C.; Craig, A.M. Alternative splicing at neuroligin site A regulates glycan interaction and synaptogenic activity. Elife 2020, 9, e58668. [Google Scholar] [CrossRef]
- Graf, E.R.; Zhang, X.; Jin, S.X.; Linhoff, M.W.; Craig, A.M. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell 2004, 119, 1013–1026. [Google Scholar] [CrossRef]
- Südhof, T.C. Towards an Understanding of Synapse Formation. Neuron 2018, 100, 276–293. [Google Scholar] [CrossRef]
- Luo, F.; Sclip, A.; Merrill, S.; Südhof, T.C. Neurexins regulate presynaptic GABAB-receptors at central synapses. Nat. Commun. 2021, 12, 2380. [Google Scholar] [CrossRef]
- Roberts, B.M.; Lopes, E.F.; Cragg, S.J. Axonal modulation of striatal dopamine release by local γ-aminobutyric acid (GABA) signalling. Cells 2021, 10, 709. [Google Scholar] [CrossRef]
- Luo, F.; Sclip, A.; Jiang, M.; Südhof, T.C. Neurexins cluster Ca2+ channels within the presynaptic active zone. EMBO J. 2020, 39, e103208. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, A.C.; Arias-Aragón, F.; Romero-Barragán, M.T.; Martín-Cuevas, C.; Delgado-García, J.M.; Martinez-Mir, A.; Scholl, F.G. Selective expression of the neurexin substrate for presenilin in the adult forebrain causes deficits in associative memory and presynaptic plasticity. Exp. Neurol. 2022, 347, 113896. [Google Scholar] [CrossRef]
- Anderson, G.R.; Aoto, J.; Tabuchi, K.; Földy, C.; Covy, J.; Yee, A.X.; Wu, D.; Lee, S.J.; Chen, L.; Malenka, R.C.; et al. β-neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Cell 2015, 162, 593–606. [Google Scholar] [CrossRef]
- Aoto, J.; Martinelli, D.C.; Malenka, R.C.; Tabuchi, K.; Südhof, T.C. Presynaptic neurexin-3 alternative splicing trans-synaptically controls postsynaptic AMPA receptor trafficking. Cell 2013, 154, 75–88. [Google Scholar] [CrossRef]
- Dai, J.; Aoto, J.; Südhof, T.C. Alternative splicing of presynaptic neurexins differentially controls postsynaptic NMDA and AMPA receptor responses. Neuron 2019, 102, 993–1008.e5. [Google Scholar] [CrossRef]
- Miyazaki, T.; Morimoto-Tomita, M.; Berthoux, C.; Konno, K.; Noam, Y.; Yamasaki, T.; Verhage, M.; Castillo, P.E.; Watanabe, M.; Tomita, S. Excitatory and inhibitory receptors utilize distinct post- and trans-synaptic mechanisms in vivo. Elife 2021, 10, e59613. [Google Scholar] [CrossRef]
- Boxer, E.E.; Seng, C.; Lukacsovich, D.; Kim, J.; Schwartz, S.; Kennedy, M.J.; Földy, C.; Aoto, J. Neurexin-3 defines synapse- and sex-dependent diversity of GABAergic inhibition in ventral subiculum. Cell Rep. 2021, 37, 110098. [Google Scholar] [CrossRef]
- Sato, Y.; Iijima, Y.; Darwish, M.; Sato, T.; Iijima, T. Distinct expression of SLM2 underlies splicing-dependent trans-synaptic signaling of neurexin across GABAergic neuron subtypes. Neurochem. Res. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Trotter, J.H.; Hao, J.; Maxeiner, S.; Tsetsenis, T.; Liu, Z.; Zhuang, X.; Südhof, T.C. Synaptic neurexin-1 assembles into dynamically regulated active zone nanoclusters. J. Cell Biol. 2019, 218, 2677–2698. [Google Scholar] [CrossRef] [PubMed]
- Klatt, O.; Repetto, D.; Brockhaus, J.; Reissner, C.; El Khallouqi, A.; Rohlmann, A.; Heine, M.; Missler, M. Endogenous β-neurexins on axons and within synapses show regulated dynamic behavior. Cell Rep. 2021, 35, 109266. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.A.; Servián-Morilla, E.; Scholl, F.G. Presenilin/γ-secretase regulates neurexin processing at synapses. PLoS ONE 2011, 6, e19430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, H.; Peixoto, R.T.; Pines, M.K.; Ge, Y.; Oku, S.; Siddiqui, T.J.; Xie, Y.; Wu, W.; Archer-Hartmann, S.; et al. Heparan sulfate organizes neuronal synapses through neurexin partnerships. Cell 2018, 174, 1450–1464.e23. [Google Scholar] [CrossRef]
- Hu, X.; Luo, J.H.; Xu, J. The interplay between synaptic activity and neuroligin function in the CNS. Biomed. Res. Int. 2015, 2015, 498957. [Google Scholar] [CrossRef]
- Varoqueaux, F.; Aramuni, G.; Rawson, R.L.; Mohrmann, R.; Missler, M.; Gottmann, K.; Zhang, W.; Südhof, T.C.; Brose, N. Neuroligins determine synapse maturation and function. Neuron 2006, 51, 741–754. [Google Scholar] [CrossRef]
- Taylor, S.C.; Ferri, S.L.; Grewal, M.; Smernoff, Z.; Bucan, M.; Weiner, J.A.; Abel, T.; Brodkin, E.S. The role of synaptic cell adhesion molecules and associated scaffolding proteins in social affiliative behaviors. Biol. Psychiatry 2020, 88, 442–451. [Google Scholar] [CrossRef]
- Bemben, M.A.; Shipman, S.L.; Nicoll, R.A.; Roche, K.W. The cellular and molecular landscape of neuroligins. Trends Neurosci. 2015, 38, 496–505. [Google Scholar] [CrossRef]
- Prange, O.; Wong, T.P.; Gerrow, K.; Wang, Y.T.; El-Husseini, A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc. Natl. Acad. Sci. USA 2004, 101, 13915–13920. [Google Scholar] [CrossRef]
- Ali, H.; Marth, L.; Krueger-Burg, D. Neuroligin-2 as a central organizer of inhibitory synapses in health and disease. Sci. Signal. 2020, 13, eabd8379. [Google Scholar] [CrossRef] [PubMed]
- Chubykin, A.A.; Atasoy, D.; Etherton, M.R.; Brose, N.; Kavalali, E.T.; Gibson, J.R.; Südhof, T.C. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron 2007, 54, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Chubykin, A.A.; Atasoy, D.; Etherton, M.R.; Brose, N.; Kavalali, E.T.; Gibson, J.R.; Südhof, T.C. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proc. Natl. Acad. Sci. USA 2013, 110, 725–730. [Google Scholar]
- Jiang, M.; Polepalli, J.; Chen, L.Y.; Zhang, B.; Südhof, T.C.; Malenka, R.C. Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism. Mol. Psychiatry 2017, 22, 375–383. [Google Scholar] [CrossRef][Green Version]
- Wu, X.; Morishita, W.K.; Riley, A.M.; Hale, W.D.; Südhof, T.C.; Malenka, R.C. Neuroligin-1 signaling controls LTP and NMDA receptors by distinct molecular pathways. Neuron 2019, 102, 621–635.e3. [Google Scholar] [CrossRef] [PubMed]
- Hines, R.M.; Wu, L.; Hines, D.J.; Steenland, H.; Mansour, S.; Dahlhaus, R.; Singaraja, R.R.; Cao, X.; Sammler, E.; Hormuzdi, S.G.; et al. Synaptic imbalance, stereotypies, and impaired social interactions in mice with altered neuroligin 2 expression. J. Neurosci. 2008, 28, 6055–6067. [Google Scholar] [CrossRef]
- Troyano-Rodriguez, E.; Wirsig-Wiechmann, C.R.; Ahmad, M. Neuroligin-2 determines inhibitory synaptic transmission in the lateral septum to optimize stress-induced neuronal activation and avoidance behavior. Biol. Psychiatry 2019, 85, 1046–1055. [Google Scholar] [CrossRef]
- Uchigashima, M.; Konno, K.; Demchak, E.; Cheung, A.; Watanabe, T.; Keener, D.G.; Abe, M.; Le, T.; Sakimura, K.; Sasaoka, T.; et al. Specific Neuroligin3-αNeurexin1 signaling regulates GABAergic synaptic function in mouse hippocampus. Elife 2020, 9, e59545. [Google Scholar] [CrossRef]
- Uchigashima, M.; Leung, M.; Watanabe, T.; Cheung, A.; Le, T.; Pallat, S.; Dinis, A.L.M.; Watanabe, M.; Kawasawa, Y.I.; Futai, K. Neuroligin3 splice isoforms shape inhibitory synaptic function in the mouse hippocampus. J. Biol. Chem. 2020, 295, 8589–8595. [Google Scholar] [CrossRef]
- Bemben, M.A.; Shipman, S.L.; Hirai, T.; Herring, B.E.; Li, Y.; Badger, J.D.; Nicoll, R.A.; Diamond, J.S.; Roche, K.W. CaMKII phosphorylation of neuroligin-1 regulates excitatory synapses. Nat. Neurosci. 2014, 7, 56–64. [Google Scholar] [CrossRef]
- Jeong, J.; Pandey, S.; Li, Y.; Badger, J.D.; Lu, W.; Roche, K.W. PSD-95 binding dynamically regulates NLGN1 trafficking and function. Proc. Natl. Acad. Sci. USA 2019, 116, 12035–12044. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.; Szíber, Z.; Chamma, I.; Saphy, C.; Papasideri, I.; Tessier, B.; Sainlos, M.; Czöndör, K.; Thoumine, O. A unique intracellular tyrosine in neuroligin-1 regulates AMPA receptor recruitment during synapse differentiation and potentiation. Nat. Commun. 2018, 9, 3979. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, R.; Pizzarelli, R.; Pedroni, A.; Fritschy, J.M.; Del-Sal, G.; Cherubini, E.; Zacchi, P. Pin1-dependent signalling negatively affects GABAergic transmission by modulating neuroligin2/gephyrin interaction. Nat. Commun. 2014, 5, 5066. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.; Lagardère, M.; Tessier, B.; Janovjak, H.; Thoumine, O. Optogenetic control of excitatory post-synaptic differentiation through neuroligin-1 tyrosine phosphorylation. Elife 2020, 9, e52027. [Google Scholar] [CrossRef]
- Suzuki, K.; Hayashi, Y.; Nakahara, S.; Kumazaki, H.; Prox, J.; Horiuchi, K.; Zeng, M.; Tanimura, S.; Nishiyama, Y.; Osawa, S.; et al. Activity-dependent proteolytic cleavage of neuroligin-1. Neuron 2012, 76, 410–422. [Google Scholar] [CrossRef]
- Peixoto, R.T.; Kunz, P.A.; Kwon, H.; Mabb, A.M.; Sabatini, B.L.; Philpot, B.D.; Ehlers, M.D. Transsynaptic signaling by activity-dependent cleavage of neuroligin-1. Neuron 2012, 76, 396–409. [Google Scholar] [CrossRef]
- Gjørlund, M.D.; Carlsen, E.M.M.; Kønig, A.B.; Dmytrieva, O.; Petersen, A.V.; Jacobsen, J.; Berezin, V.; Perrier, J.F.; Owczarek, S. Soluble ectodomain of neuroligin 1 decreases synaptic activity by activating metabotropic glutamate receptor 2. Front. Mol. Neurosci. 2017, 10, 116. [Google Scholar] [CrossRef]
- Bemben, M.A.; Nguyen, T.A.; Li, Y.; Wang, T.; Nicoll, R.A.; Roche, K.W. Isoform-specific cleavage of neuroligin-3 reduces synapse strength. Mol. Psychiatry 2019, 24, 145–160. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Sage, E.H. Revisiting the matricellular concept. Matrix Biol. 2014, 37, 1–14. [Google Scholar] [CrossRef]
- Fan, S.; Gangwar, S.P.; Machius, M.; Rudenko, G. Interplay between hevin, SPARC, and MDGAs: Modulators of neurexin-neuroligin transsynaptic bridges. Structure 2021, 29, 664–678.e6. [Google Scholar] [CrossRef]
- Singh, S.K.; Stogsdill, J.A.; Pulimood, N.S.; Dingsdale, H.; Kim, Y.H.; Pilaz, L.J.; Kim, I.H.; Manhaes, A.C.; Rodrigues, W.S., Jr.; Pamukcu, A.; et al. Astrocytes assemble thalamocortical synapses by bridging NRX1α and NL1 via hevin. Cell 2016, 164, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jung, H.G.; Kim, A.; Shim, H.S.; Hyeon, S.J.; Lee, Y.S.; Han, J.; Jung, J.H.; Lee, J.; Ryu, H.; et al. Hevin-calcyon interaction promotes synaptic reorganization after brain injury. Cell Death Differ. 2021, 28, 2571–2588. [Google Scholar] [CrossRef] [PubMed]
- Lively, S.; Brown, I.R. The extracellular matrix protein SC1/hevin localizes to excitatory synapses following status epilepticus in the rat lithium-pilocarpine seizure model. J. Neurosci. Res. 2008, 86, 2895–2905. [Google Scholar] [CrossRef]
- Vincent, A.J.; Lau, P.W.; Roskams, A.J. SPARC is expressed by macroglia and microglia in the developing and mature nervous system. Dev. Dyn. 2008, 237, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Kucukdereli, H.; Allen, N.J.; Lee, A.T.; Feng, A.; Ozlu, M.I.; Conatser, L.M.; Chakraborty, C.; Workman, G.; Weaver, M.; Sage, E.H.; et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. USA 2011, 108, E440–E449. [Google Scholar] [CrossRef]
- Jones, E.V.; Bernardinelli, Y.; Tse, Y.C.; Chierzi, S.; Wong, T.P.; Murai, K.K. Astrocytes control glutamate receptor levels at developing synapses through SPARC-beta-integrin interactions. J. Neurosci. 2011, 31, 4154–4165. [Google Scholar] [CrossRef]
- Jones, E.V.; Bernardinelli, Y.; Zarruk, J.G.; Chierzi, S.; Murai, K.K. SPARC and GluA1-containing AMPA receptors promote neuronal health following CNS injury. Front. Cell Neurosci. 2018, 12, 22. [Google Scholar] [CrossRef]
- Litwack, E.D.; Babey, R.; Buser, R.; Gesemann, M.; O’Leary, D.D. Identification and characterization of two novel brain-derived immunoglobulin superfamily members with a unique structural organization. Mol. Cell. Neurosci. 2004, 25, 263–274. [Google Scholar] [CrossRef]
- Connor, S.A.; Elegheert, J.; Xie, Y.; Craig, A.M. Pumping the brakes: Suppression of synapse development by MDGA-neuroligin interactions. Curr. Opin. Neurobiol. 2019, 57, 71–80. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, D.; Won, S.Y.; Han, K.A.; Park, D.; Cho, E.; Yun, N.; An, H.J.; Um, J.W.; Kim, E.; et al. Structural insights into modulation of neurexin-neuroligin trans-synaptic adhesion by MDGA1/neuroligin-2 complex. Neuron 2017, 94, 1121–1131.e6. [Google Scholar] [CrossRef]
- Elegheert, J.; Cvetkovska, V.; Clayton, A.J.; Heroven, C.; Vennekens, K.M.; Smukowski, S.N.; Regan, M.C.; Jia, W.; Smith, A.C.; Furukawa, H.; et al. Structural mechanism for modulation of synaptic neuroligin-neurexin signaling by MDGA proteins. Neuron 2021, 109, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, Y.; Lee, S.J.; Qiang, Y.; Lee, D.; Lee, H.W.; Kim, H.; Je, H.S.; Südhof, T.C.; Ko, J. MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proc. Natl. Acad. Sci. USA 2013, 110, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.A.; Ammendrup-Johnsen, I.; Chan, A.W.; Kishimoto, Y.; Murayama, C.; Kurihara, N.; Tada, A.; Ge, Y.; Lu, H.; Yan, R.; et al. Altered cortical dynamics and cognitive function upon haploinsufficiency of the autism-linked excitatory synaptic suppressor MDGA2. Neuron 2016, 91, 1052–1068. [Google Scholar] [CrossRef]
- Xu, L.; Feng, Q.; Deng, H.; Zhang, X.; Ni, H.; Yao, M. Neurexin-2 is a potential regulator of inflammatory pain in the spinal dorsal horn of rats. J. Cell. Mol. Med. 2020, 24, 13623–13633. [Google Scholar] [CrossRef]
- Taylor, C.P.; Harris, E.W. Analgesia with gabapentin and pregabalin may involve N-Methyl-d-Aspartate receptors, neurexins, and thrombospondins. J. Pharmacol. Exp. Ther. 2020, 374, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Alves-Ferreira, M.; Quintas, M.; Sequeiros, J.; Sousa, A.; Pereira-Monteiro, J.; Alonso, I.; Neto, J.L.; Lemos, C. A genetic interaction of NRXN2 with GABRE, SYT1 and CASK in migraine patients: A case-control study. J. Headache Pain 2021, 22, 57. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Duan, X.L.; Yang, L.; Liu, J.P.; He, Y.T.; Guo, Z.; Hu, X.D.; Suo, Z.W. Activity-dependent synaptic recruitment of neuroligin 1 in spinal dorsal horn contributed to inflammatory pain. Neuroscience 2018, 388, 1–10. [Google Scholar] [CrossRef]
- Guo, R.; Li, H.; Li, X.; Xue, Z.; Sun, Y.; Ma, D.; Guan, Y.; Li, J.; Tian, M.; Wang, Y. Downregulation of neuroligin1 ameliorates postoperative pain through inhibiting neuroligin1/postsynaptic density 95-mediated synaptic targeting of α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor GluA1 subunits in rat dorsal horns. Mol. Pain 2018, 14, 1–13. [Google Scholar] [CrossRef]
- Dolique, T.; Favereaux, A.; Roca-Lapirot, O.; Roques, V.; Léger, C.; Landry, M.; Nagy, F. Unexpected association of the “inhibitory” neuroligin 2 with excitatory PSD95 in neuropathic pain. Pain 2013, 154, 2529–2546. [Google Scholar] [CrossRef]
- Guo, R.; Li, H.; Li, X.; Sun, Y.; Miao, H.; Ma, D.; Hong, F.; Zhang, Y.; Guan, Y.; Li, J.; et al. Increased neuroligin 2 levels in the postsynaptic membrane in spinal dorsal horn may contribute to postoperative pain. Neuroscience 2018, 382, 14–22. [Google Scholar] [CrossRef]
- Wang, Z.; Tao, Y.; Song, C.; Liu, P.; Wang, C.; Li, Y.; Cui, W.; Xie, K.; Zhang, L.; Wang, G. Spinal hevin mediates membrane trafficking of GluA1-containing AMPA receptors in remifentanil-induced postoperative hyperalgesia in mice. Neurosci. Lett. 2020, 722, 134855. [Google Scholar] [CrossRef] [PubMed]
- Sparcl1/Hevin drives inflammatory and neuropathic pain through astrocyte and NMDA receptor signaling. Unpublished work. 2022.
- Lee, S.; Jang, S.H.; Suzuki-Narita, M.; Gregoire, S.; Millecamps, M.; Stone, L.S. Voluntary running attenuates behavioural signs of low back pain: Dimorphic regulation of intervertebral disc inflammation in male and female SPARC-null mice. Osteoarthr. Cartil. 2022, 30, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Tajerian, M.; Alvarado, S.; Millecamps, M.; Dashwood, T.; Anderson, K.M.; Haglund, L.; Ouellet, J.; Szyf, M.; Stone, L.S. DNA methylation of SPARC and chronic low back pain. Mol. Pain 2011, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Q.; Liu, D.Q.; Chen, S.P.; Sun, J.; Zhou, X.R.; Luo, F.; Tian, Y.K.; Ye, D.W. Cellular and molecular mechanisms of calcium/calmodulin-dependent protein kinase II in chronic pain. J. Pharmacol. Exp. Ther. 2017, 363, 176–183. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Deng, X.; Jiang, C.; Pan, C.; Chen, L.; Han, Y.; Dai, W.; Hu, L.; Zhang, G.; et al. N-acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain 2016, 157, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Singh, P.; Chopra, K. Matrix metalloproteinases: Potential therapeutic target for diabetic neuropathic pain. Expert Opin. Ther. Targets. 2015, 19, 177–185. [Google Scholar] [CrossRef]
- Hashimoto, N.; Sato, T.; Yajima, T.; Fujita, M.; Sato, A.; Shimizu, Y.; Shimada, Y.; Shoji, N.; Sasano, T.; Ichikawa, H. SPARCL1-containing neurons in the human brainstem and sensory ganglion, Somatosens. Somatosens. Mot. Res. 2016, 33, 112–117. [Google Scholar] [CrossRef]
- Connor, S.A.; Ammendrup-Johnsen, I.; Kishimoto, Y.; Karimi-Tari, P.; Cvetkovska, V.; Harada, T.; Ojima, D.; Yamamoto, T.; Wang, Y.T.; Craig, A.M. Loss of synapse repressor MDGA1 enhances perisomatic inhibition, confers resistance to network excitation, and impairs cognitive function. Cell Rep. 2017, 21, 3637–3645. [Google Scholar] [CrossRef]
- Phelps, C.E.; Navratilova, E.; Porreca, F. Cognition in the chronic pain experience: Preclinical insights. Trends Cogn. Sci. 2021, 25, 365–376. [Google Scholar] [CrossRef]
- Moriarty, O.; Finn, D.P. Cognition and pain. Curr. Opin. Support. Palliat. Care. 2014, 8, 130–136. [Google Scholar] [CrossRef]
| Molecular Names | Type of Pain | Animals | Functional Receptors | Key Reference |
|---|---|---|---|---|
| Nrx-1 | SNL | Rats | NMDAR | Lin et al., 2015 [8] |
| Nrx-2 | CFA | Rats | AMPAR | Xu L et al., 2020 [74] |
| Nlg-1 | CFA | Mice/Rats | NMDAR | Zhao et al., 2018 [77] |
| Nlg-1 | Postoperative pain | Rats | AMPAR | Guo et al., 2018 [78] |
| Nlg-2 | SNL | Rats | NA | Dolique et al., 2013 [79] |
| Nlg-2 | Postoperative pain | Rats | AMPAR | Guo et al., 2018 [80] |
| Hevin | Postoperative pain | Murine | AMPAR | Wang et al., 2020 [81] |
| Hevin | Inflammation pain/CCI | Mice | NMDAR | Chen et al., 2022 [82] |
| SPARC | Chronic back pain | Mice | NA | Lee et al., 2022 [83] |
| SPARC | Chronic back pain | Human | NA | Tajerian et al., 2011 [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Guo, R.; Guan, Y.; Li, J.; Wang, Y. Modulation of Trans-Synaptic Neurexin–Neuroligin Interaction in Pathological Pain. Cells 2022, 11, 1940. https://doi.org/10.3390/cells11121940
Li H, Guo R, Guan Y, Li J, Wang Y. Modulation of Trans-Synaptic Neurexin–Neuroligin Interaction in Pathological Pain. Cells. 2022; 11(12):1940. https://doi.org/10.3390/cells11121940
Chicago/Turabian StyleLi, Huili, Ruijuan Guo, Yun Guan, Junfa Li, and Yun Wang. 2022. "Modulation of Trans-Synaptic Neurexin–Neuroligin Interaction in Pathological Pain" Cells 11, no. 12: 1940. https://doi.org/10.3390/cells11121940
APA StyleLi, H., Guo, R., Guan, Y., Li, J., & Wang, Y. (2022). Modulation of Trans-Synaptic Neurexin–Neuroligin Interaction in Pathological Pain. Cells, 11(12), 1940. https://doi.org/10.3390/cells11121940







