Perturbation of Cortical Excitability in a Conditional Model of PCDH19 Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Viral Injections
2.3. In Vivo Local Field Potential Recordings
2.4. EEG Recordings in Behaving Mice
2.5. In Vivo 2-Photon Calcium Imaging
2.6. Data Analyses
3. Results
3.1. Behavioural Phenotype of PCDH19 Mice
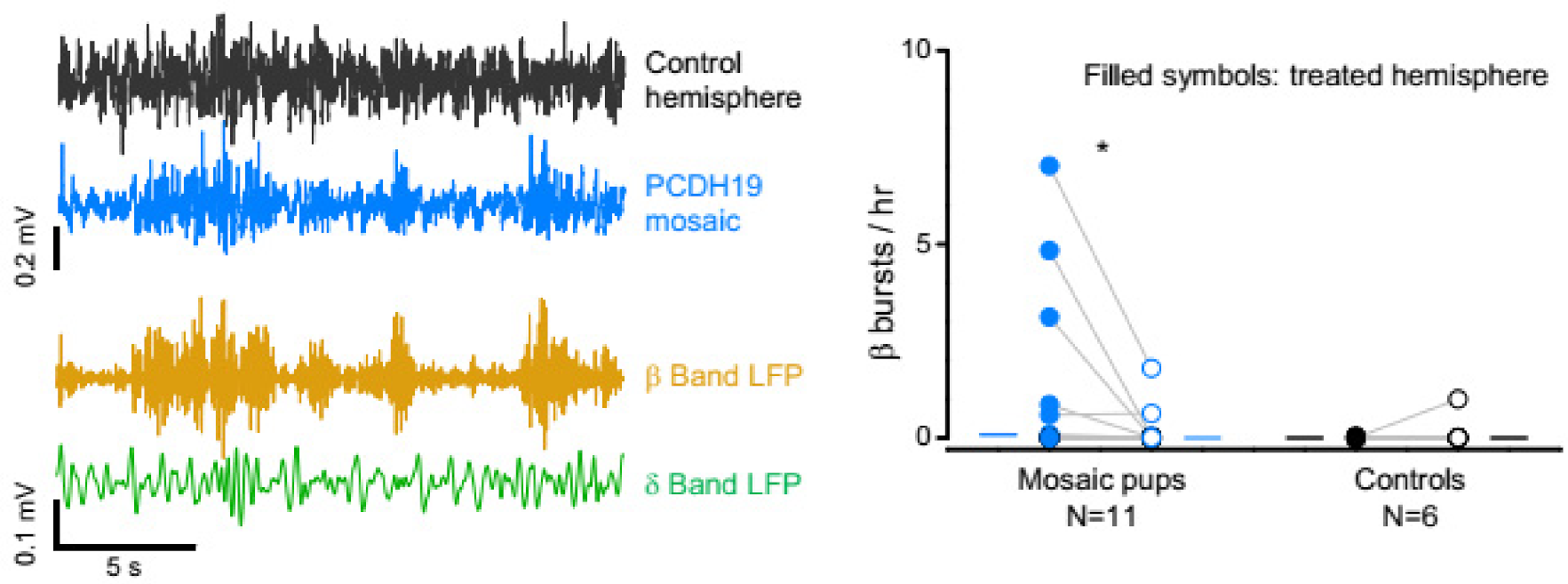
3.2. Hyperexcitability of PCDH19 Mosaic Mice
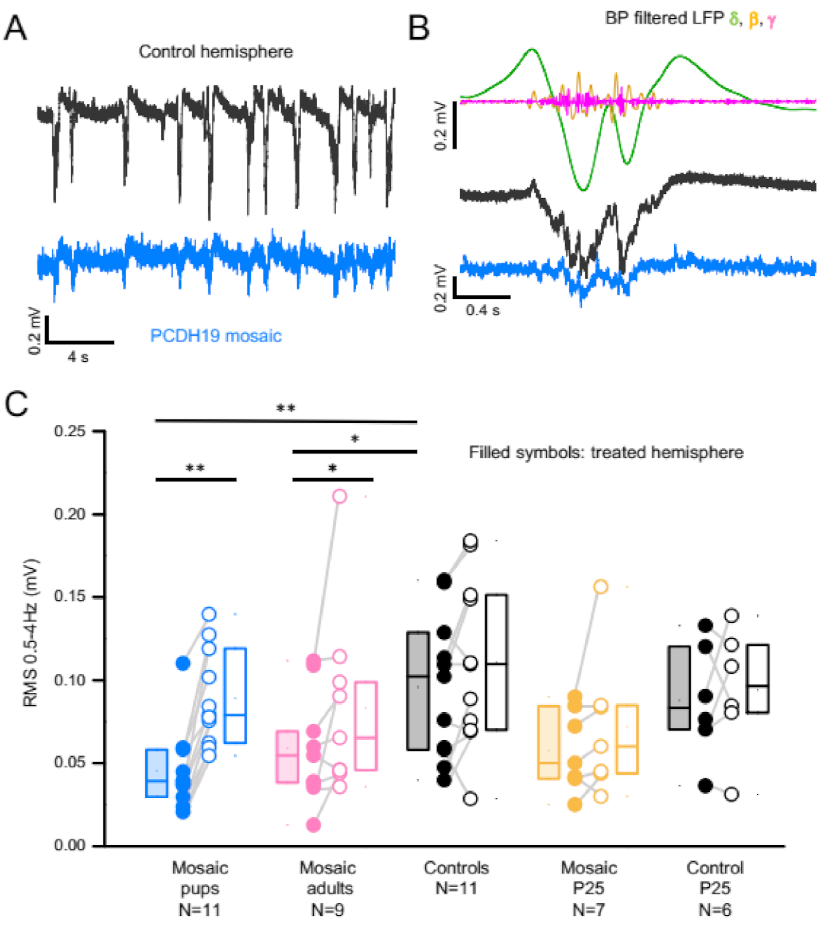
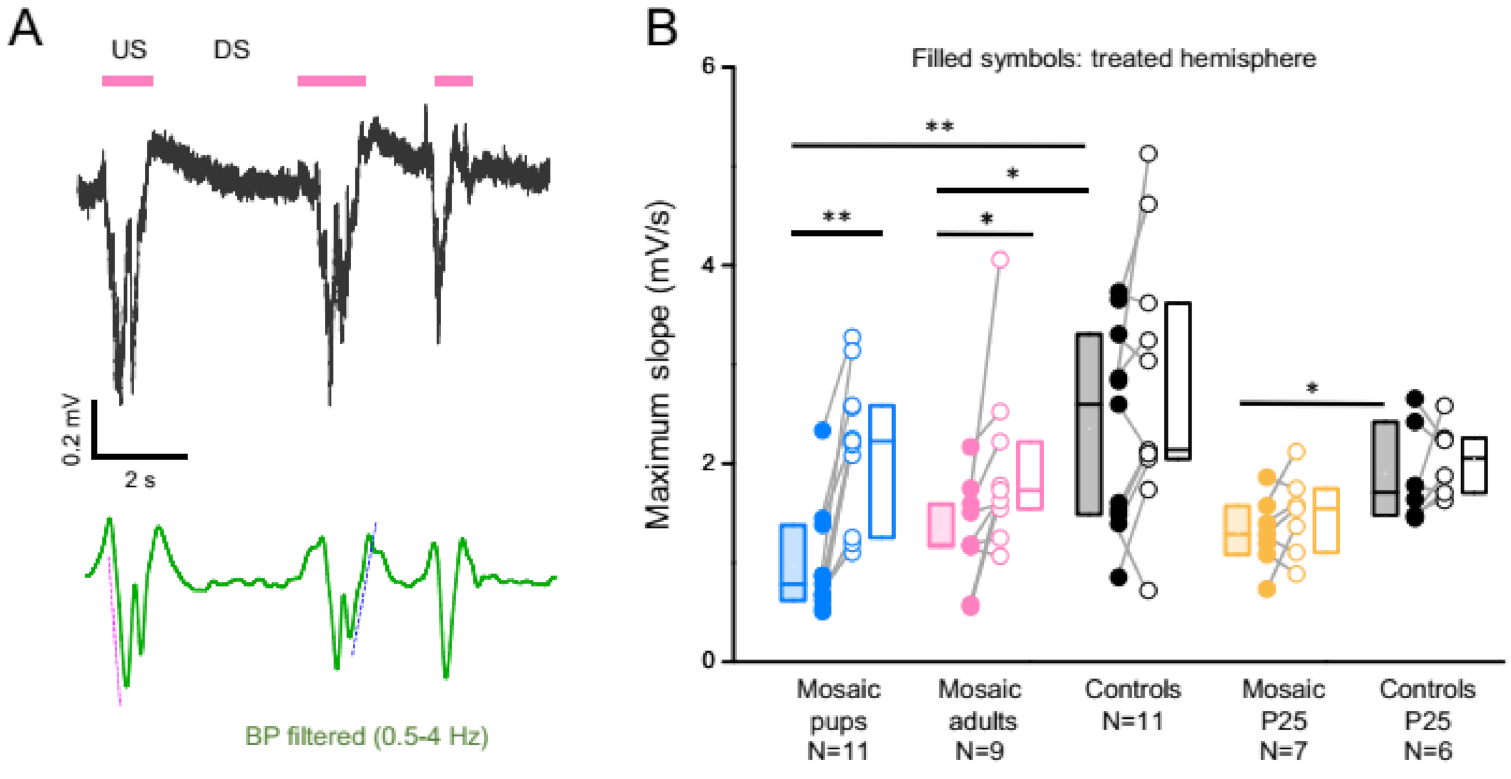
3.3. PCDH19 Mosaicism Causes Disruption of SWA and Network Synchronization
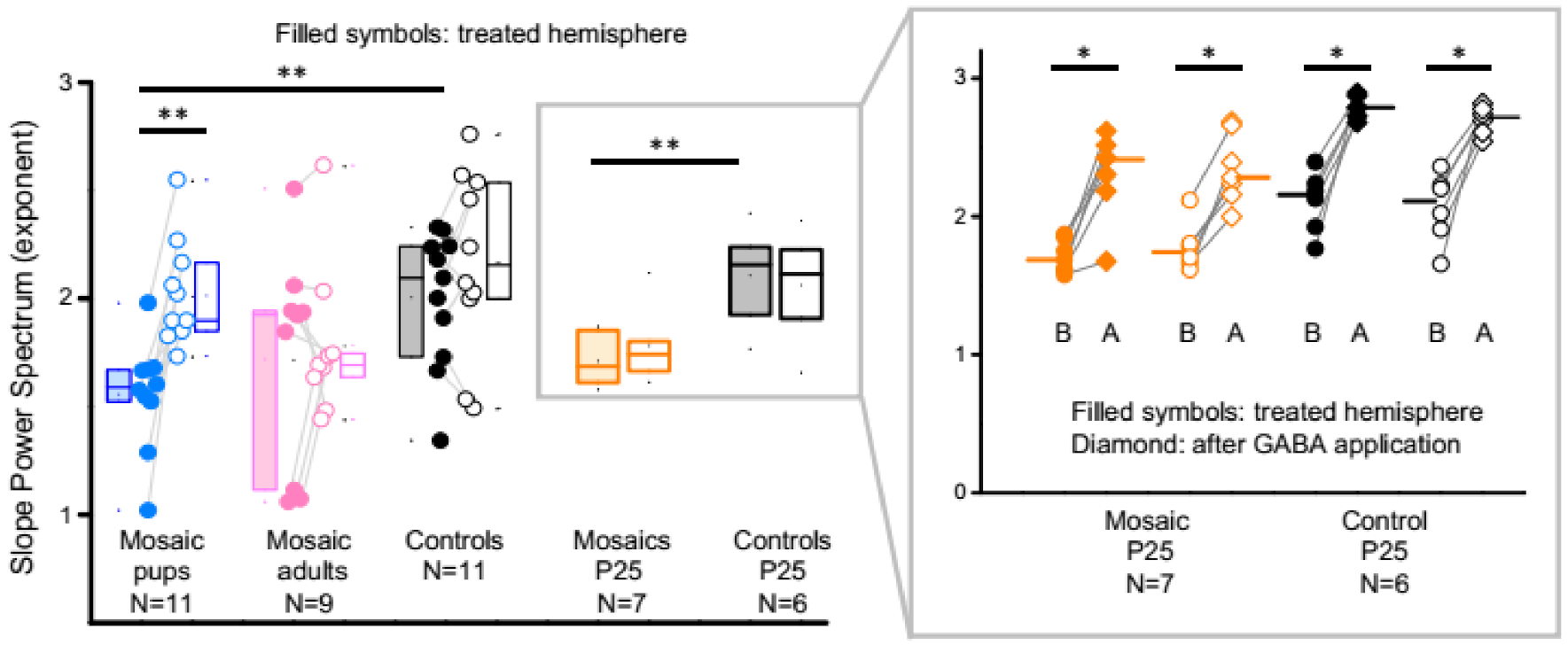
3.4. PCDH19 Mosaic Animals Have an Increased Excitation to Inhibition Ratio
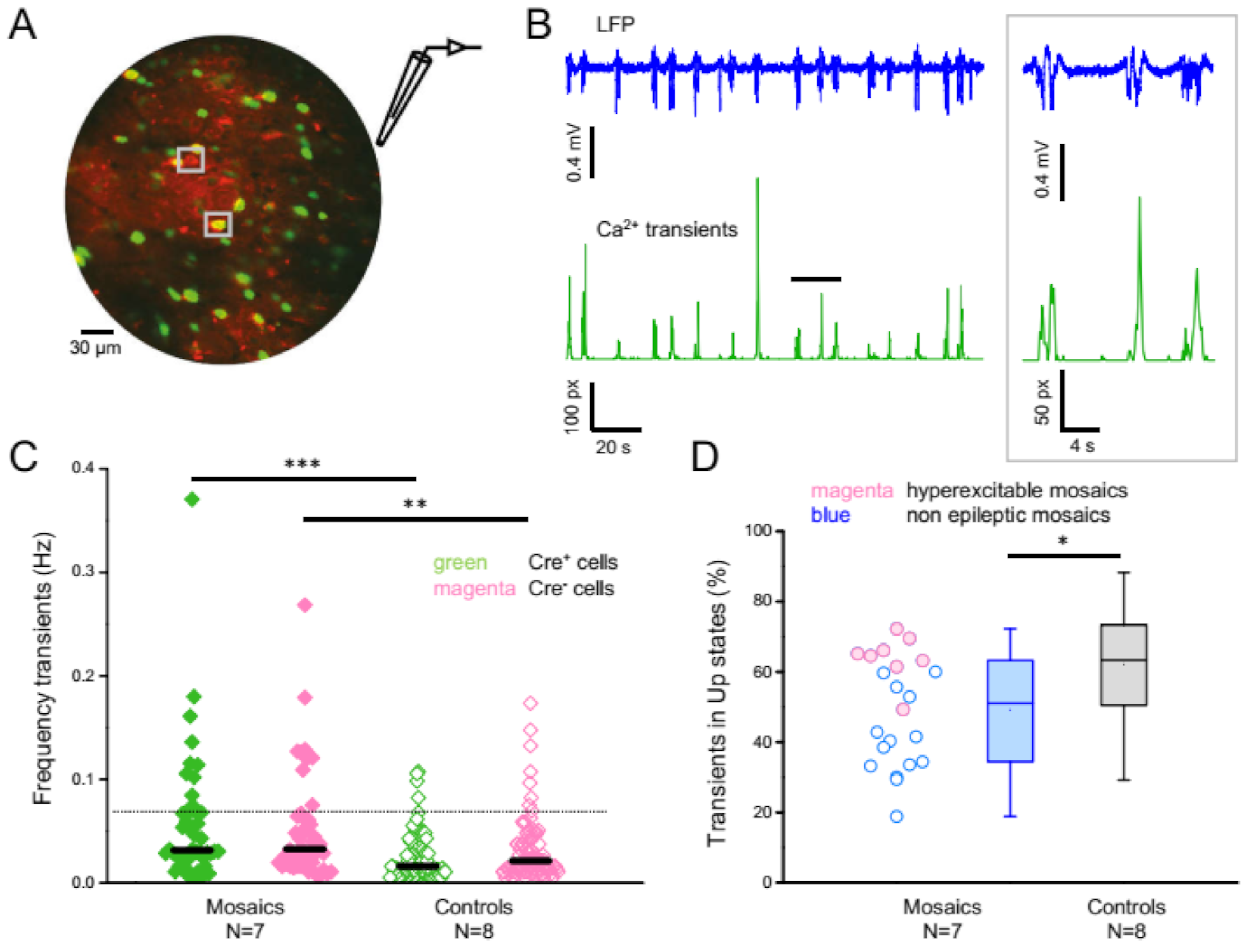
3.5. In Vivo 2-Photon Calcium Imaging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duszyc, K.; Terczynska, I.; Hoffman-Zacharska, D. Epilepsy and mental retardation restricted to females: X-linked epileptic infantile encephalopathy of unusual inheritance. J. Appl. Genet. 2015, 56, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Turner, S.J.; Dibbens, L.M.; Bayly, M.A.; Friend, K.; Hodgson, B.; Burrows, L.; Shaw, M.; Wei, C.; Ullmann, R.; et al. Epilepsy and mental retardation limited to females: An under-recognized disorder. Brain 2008, 131, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Depienne, C.; Bouteiller, D.; Keren, B.; Cheuret, E.; Poirier, K.; Trouillard, O.; Benyahia, B.; Quelin, C.; Carpentier, W.; Julia, S.; et al. Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles dravet syndrome but mainly affects females. PLoS Genet. 2009, 5, e1000381. [Google Scholar] [CrossRef]
- Kolc, K.L.; Sadleir, L.G.; Scheffer, I.E.; Ivancevic, A.; Roberts, R.; Pham, D.H.; Gecz, J. A systematic review and meta-analysis of 271 PCDH19-variant individuals identifies psychiatric comorbidities, and association of seizure onset and disease severity. Mol. Psychiatry 2018, 24, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Vlaskamp, D.R.M.; Bassett, A.S.; Sullivan, J.E.; Robblee, J.; Sadleir, L.G.; Scheffer, I.E.; Andrade, D.M. Schizophrenia is a later-onset feature of PCDH19 girls clustering epilepsy. Epilepsia 2019, 60, 429–440. [Google Scholar] [CrossRef]
- Depienne, C.; Trouillard, O.; Bouteiller, D.; Gourfinkel-An, I.; Poirier, K.; Rivier, F.; Berquin, P.; Nabbout, R.; Chaigne, D.; Steschenko, D.; et al. Mutations and deletions in PCDH19 account for various familial or isolated epilepsies in females. Hum. Mutat. 2011, 32, 1959–1975. [Google Scholar] [CrossRef] [Green Version]
- Specchio, N.; Marini, C.; Terracciano, A.; Mei, D.; Trivisano, M.; Sicca, F.; Fusco, L.; Cusmai, R.; Darra, F.; Bernardina, B.D.; et al. Spectrum of phenotypes in female patients with epilepsy due to protocadherin 19 mutations. Epilepsia 2011, 52, 1251–1257. [Google Scholar] [CrossRef]
- Dibbens, L.M.; Tarpey, P.S.; Hynes, K.; Bayly, M.; Scheffer, I.E.; Smith, R.; Bomar, J.; Sutton, E.; Vandeleur, L.; Shoubridge, C.; et al. X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat. Genet. 2008, 40, 776–781. [Google Scholar]
- Thiffault, I.; Farrow, E.; Smith, L.; Lowry, J.; Zellmer, L.; Black, B.; Abdelmoity, A.; Miller, N.; Soden, S.; Saunders, C. PCDH19-related epileptic encephalopathy in a male mosaic for a truncating variant. Am. J. Med. Genet. Part A 2016, 170, 1585–1589. [Google Scholar] [CrossRef]
- de Lange, I.M.; Rump, P.; Neuteboom, R.F.; Augustijn, P.B.; Hodges, K.; Kistemaker, A.I.; Brouwer, O.F.; Mancini, G.M.S.; Newman, H.A.; Vos, Y.J.; et al. Male patients affected by mosaic PCDH19 mutations: Five new cases. Neurogenetics 2017, 18, 147–153. [Google Scholar] [CrossRef]
- Pederick, D.T.; Richards, K.L.; Piltz, S.G.; Kumar, R.; Mincheva-Tasheva, S.; Mandelstam, S.A.; Dale, R.C.; Scheffer, I.E.; Gecz, J.; Petrou, S.; et al. Abnormal cell sorting underlies the unique X-linked inheritance of PCDH19 epilepsy. Neuron 2018, 97, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, S.; Inoue, Y.; Hattori, S.; Kaneko, M.; Shioi, G.; Miyakawa, T.; Takeichi, M. Loss of X-linked protocadherin-19 differentially affects the behavior of heterozygous female and hemizygous male mice. Sci. Rep. 2017, 7, 5801. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, N.; Johnson-Venkatesh, E.M.; Hoshina, M.; Umemori, H. Female-specific synaptic dysfunction and cognitive impairment in a mouse model of PCDH19 disorder. Science 2021, 372, eaaz3893. [Google Scholar] [CrossRef] [PubMed]
- Bassani, S.; Cwetsch, A.W.; Gerosa, L.; Serratto, G.M.; Folci, A.; Hall, I.F.; Mazzanti, M.; Cancedda, L.; Passafaro, M. The female epilepsy protein PCDH19 is a new GABAAR-binding partner that regulates GABAergic transmission as well as migration and morphological maturation of hippocampal neurons. Hum. Mol. Genet. 2018, 27, 1027–1038. [Google Scholar] [CrossRef]
- Serratto, G.M.; Pizzi, E.; Murru, L.; Mazzoleni, S.; Pelucchi, S.; Marcello, E.; Mazzanti, M.; Passafaro, M.; Bassani, S. The epilepsy-related protein PCDH19 regulates tonic inhibition, GABAAR kinetics, and the intrinsic excitability of hippocampal neurons. Mol. Neurobiol. 2020, 57, 5336–5351. [Google Scholar] [CrossRef]
- Brust, V.; Schindler, P.M.; Lewejohann, L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front. Zool. 2015, 12, S17. [Google Scholar] [CrossRef] [Green Version]
- Steriade, M.; Nuñez, A.; Amzica, F. A novel slow oscillation of neocortical neurons in vivo: Depolarizing and hyperpolarizing components. J. Neurosci. 1993, 13, 3252–3265. [Google Scholar] [CrossRef] [Green Version]
- Neske, G.T. The slow oscillation in cortical and thalamic networks: Mechanisms and functions. Front. Neural Circuits 2016, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Trovato, F.; Parra, R.; Pracucci, E.; Landi, S.; Cozzolino, O.; Nardi, G.; Cruciani, F.; Pillai, V.; Mosti, L.; Cwetsch, A.; et al. Modelling genetic mosaicism of neurodevelopmental disorders in vivo by a Cre-amplifying fluorescent reporter. Nat. Commun. 2020, 11, 6194. [Google Scholar] [CrossRef]
- De Curtis, M.; Avanzini, G. Interictal spikes in focal epileptogenesis. Prog. Neurobiol. 2001, 63, 541–567. [Google Scholar] [CrossRef]
- Fisher, R.S.; Van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J. Epileptic seizures and epilepsy: Definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Petrucco, L.; Pracucci, E.; Brondi, M.; Ratto, G.M.; Landi, S. Epileptiform activity in the mouse visual cortex interferes with cortical processing in connected areas. Sci. Rep. 2017, 7, 40054. [Google Scholar] [CrossRef] [PubMed]
- Esser, S.K.; Hill, S.L.; Tononi, G. Sleep Homeostasis and cortical synchronization: I. modeling the effects of synaptic strength on sleep slow waves. Sleep 2007, 30, 1617–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyazovskiy, V.V.; Riedner, B.A.; Cirelli, C.; Tononi, G. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat. Sleep 2007, 30, 1631–1642. [Google Scholar] [CrossRef]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Antoine, M.W.; Langberg, T.; Schnepel, P.; Feldman, D.E. Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron 2019, 101, 648–661. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Peterson, E.J.; Voytek, B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage 2017, 158, 70–78. [Google Scholar] [CrossRef]
- Dana, H.; Mohar, B.; Sun, Y.; Narayan, S.; Gordus, A.; Hasseman, J.P.; Tsegaye, G.; Holt, G.T.; Hu, A.; Walpita, D.; et al. Sensitive red protein calcium indicators for imaging neural activity. Elife 2016, 5, e12727. [Google Scholar] [CrossRef]
- Neske, G.T.; Patrick, S.L.; Connors, B.W. Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex. J. Neurosci. 2015, 35, 1089–1105. [Google Scholar] [CrossRef] [Green Version]
- Busche, M.A.; Eichhoff, G.; Adelsberger, H.; Abramowski, D.; Wiederhold, K.; Haass, C.; Staufenbiel, M.; Konnerth, A.; Garaschuk, O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of alzheimer’s disease. Science 2008, 321, 1686–1690. [Google Scholar] [CrossRef] [Green Version]
- Cwetsch, A.W.; Ziogas, I.; Narducci, R.; Savardi, A.; Bolla, M.; Pinto, B.; Perlini, L.E.; Bassani, S.; Passafaro, M.; Cancedda, L. A rat model of a focal mosaic expression of PCDH19 replicates human brain developmental abnormalities and behaviours. Brain Commun. 2022, 4, fcac091. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.G.; Chance, P.F.; Zou, C.; Spinner, N.B.; Golden, J.A.; Smietana, S. Epilepsy and mental retardation limited to females: An X-linked dominant disorder with male sparing. Nat. Genet. 1997, 17, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.; Korff, C.M.; Ranza, E.; Bernasconi, A.; Lübbig, A.; Nangia, S.; Ramelli, G.P.; Wohlrab, G.; Nordli, D.R.; Bast, T. Focal cortical malformations in children with early infantile epilepsy and PCDH19 mutations: Case report. Dev. Med. Child Neurol. 2018, 6, 100–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenge, M.; Marini, C.; Canale, E.; Napolitano, A.; De Masi, S.; Trivisano, M.; Mei, D.; Longo, D.; Camilla, M.; Espagnet, M.C.R.; et al. Quantitative MRI-based analysis identifies developmental limbic abnormalities in PCDH19 encephalopathy. Cereb. Cortex 2020, 30, 6039–6050. [Google Scholar] [CrossRef]
- Kolc, K.L.; Møller, R.S.; Sadleir, L.G.; Scheffer, I.E.; Kumar, R.; Gecz, J. PCDH19 pathogenic variants in males: Expanding the phenotypic spectrum. Adv. Exp. Med. Biol. 2020, 1298, 177–187. [Google Scholar]
- Parisi, P.; Moavero, R.; Verrotti, A.; Curatolo, P. Attention deficit hyperactivity disorder in children with epilepsy. Brain Dev. 2010, 32, 10–16. [Google Scholar] [CrossRef]
- Terzano, M.G.; Parrino, L.; Garofalo, P.G.; Durisotti, C.; Filati-roso, C. Activation of partial seizures with motor signs during cyclic alternating pattern in human sleep. Epilepsy Res. 1991, 10, 166–173. [Google Scholar] [CrossRef]
- Sammaritano, M.; Gigli, G.L.; Gotman, J. Interictal spiking during wakefulness and sleep and the localization of foci in temporal lobe epilepsy. Neurology 1991, 41, 290–297. [Google Scholar] [CrossRef]
- De Guzman, P.H.; Nazer, F.; Dickson, C.T. Short-duration epileptic discharges show a distinct phase preference during ongoing hippocampal slow oscillations. J. Neurophysiol. 2010, 104, 2194–2202. [Google Scholar] [CrossRef]
- Frauscher, B.; Von Ellenrieder, N.; Ferrari-Marinho, T.; Avoli, M.; Dubeau, F.; Gotman, J. Facilitation of epileptic activity during sleep is mediated by high amplitude slow waves. Brain 2015, 138, 1629–1641. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, H.; Imai, K.; Ikeda, H.; Shigematsu, H.; Takahashi, Y.; Inoue, Y.; Higurashi, N.; Hirose, S. Characteristic phasic evolution of convulsive seizure in PCDH19-related epilepsy. Epileptic Disord. 2016, 18, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Liu, L.; Meng, Q.; Du, C.; Li, K.; Dong, S.; Zhang, Y.; Li, H.; Zhang, H. Decreased expression of the clock gene Bmal1 is involved in the pathogenesis of temporal lobe epilepsy. Mol. Brain 2021, 14, 113. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.; Born, J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn. Sci. 2007, 11, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Rasch, B.; Born, J. About sleep’s role in memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef]
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic to cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef] [Green Version]
- Vyazovskiy, V.V.; Cirelli, C.; Pfister-genskow, M.; Faraguna, U.; Tononi, G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 2008, 11, 200–208. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Olcese, U.; Lazimy, Y.M.; Faraguna, U.; Esser, S.K.; Williams, J.C.; Cirelli, C.; Tononi, G. Cortical firing and sleep homeostasis. Neuron 2009, 63, 865–878. [Google Scholar] [CrossRef]
- de Vivo, L.; Bellesi, M.; Marshall, W.; Bushong, E.A.; Ellisman, M.H.; Tononi, G.; Cirelli, C. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 2017, 355, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Watson, B.O.; Levenstein, D.; Greene, J.P.; Gelinas, J.N.; Buzsaki, G. Network homeostasis and state dynamics of neocortical sleep. Neuron 2016, 90, 839–852. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.; Singhal, N.; El Achkar, C.M.; Truglio, G.; Sheidley, B.R.; Sullivan, J.; Poduri, A. PCDH19-related epilepsy is associated with a broad neurodevelopmental spectrum. Epilepsia 2018, 59, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Rupin-Mas, M.; Gourfinkel-An, I.; Arnulf, I. N3 sleep with rapid eye movements in a PCDH19 mutation: A new dissociate state between N3 and REM sleep. Sleep Med. 2020, 74, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Bozzi, Y.; Provenzano, G.; Casarosa, S. Neurobiological bases of autism–epilepsy comorbidity: A focus on excitation/inhibition imbalance. Eur. J. Neurosci. 2018, 47, 534–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; Ogshea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yu, Y. Synaptic E-I balance underlies efficient neural coding. Front. Neurosci. 2018, 12, 46. [Google Scholar] [CrossRef] [Green Version]
- Dani, V.S.; Chang, Q.; Maffei, A.; Turrigiano, G.G.; Jaenisch, R.; Nelson, S.B. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett Syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 12560–12565. [Google Scholar] [CrossRef] [Green Version]
- Gnatkovsky, V.; Librizzi, L.; Trombin, F.; De Curtis, M. Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann. Neurol. 2008, 64, 674–686. [Google Scholar] [CrossRef]
- Avoli, M.; de Curtis, M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog. Neurobiol. 2011, 95, 104–132. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.; Portera-Cailliau, C. Autism in the balance: Elevated E-I ratio as a homeostatic stabilization of synaptic drive. Neuron 2019, 101, 543–545. [Google Scholar] [CrossRef] [Green Version]
- Kastanenka, K.V.; Hou, S.S.; Shakerdge, N.; Logan, R.; Feng, D.; Wegmann, S.; Chopra, V.; Hawkes, J.M.; Chen, X.; Bacskai, B.J. Optogenetic restoration of disrupted slow oscillations halts amyloid deposition and restores calcium homeostasis in an animal model of Alzheimer’s disease. PLoS ONE 2017, 12, e0170275. [Google Scholar] [CrossRef]
- Trovato, F.; Parra, R.; Pracucci, E.; Landi, S.; Cozzolino, O.; Nardi, G.; Cruciani, F.; Mosti, L.; Cwetsch, A.; Cancedda, L.; et al. A Cre-amplifier to generate and detect genetic mosaics in vivo. bioRxiv 2019, 715490. [Google Scholar] [CrossRef]
- Mazziotti, R.; Cacciante, F.; Sagona, G.; Lupori, L.; Gennaro, M.; Putignano, E.; Alessandrì, M.G.; Ferrari, A.; Battini, R.; Cioni, G.; et al. Novel translational phenotypes and biomarkers for creatine transporter deficiency. Brain Commun. 2020, 2. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; De Rosa, A.; Russo, R.; Di Maio, A.; Garofalo, M.; Federici, M.; Migliarini, S.; LeDonne, A.; Rizzo, F.R.; Avallone, L.; et al. The striatal-enriched protein Rhes is a critical modulator of cocaine-induced molecular and behavioral responses. Sci. Rep. 2019, 9, 15294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiroga, R.Q.; Nadasdy, Z.; Ben-Shaul, Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural. Comput. 2004, 16, 1661–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donoghue, T.; Haller, M.; Peterson, E.J.; Varma, P.; Sebastian, P.; Gao, R.; Noto, T.; Lara, A.H.; Wallis, J.D.; Knight, R.T.; et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat. Neurosci. 2020, 23, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Bokil, H.; Andrews, P.; Kulkarni, J.E.; Mehta, S.; Mitra, P. Chronux: A Platform for Analyzing Neural Signals. J. Neurosci. Methods 2010, 192, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bastianini, S.; Berteotti, C.; Gabrielli, A.; Del Vecchio, F.; Amici, R.; Alexandre, C.; Scammell, T.E.; Gazea, M.; Kimura, M.; Martire, V.L.; et al. SCOPRISM: A new algorithm for automatic sleep scoring in mice. J. Neurosci. Methods 2014, 235, 277–284. [Google Scholar] [CrossRef]
- Cozzolino, O.; Sicca, F.; Paoli, E.; Trovato, F.; Santorelli, F.M.; Ratto, G.M.; Marchese, M. Evolution of Epileptiform Activity in Zebrafish by Statistical-Based Integration of Electrophysiology and 2-Photon Ca2+ Imaging. Cells 2020, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Pederick, D.; Homan, C.; Jaehne, E.; Piltz, S.G.; Haines, B.P.; Baune, B.T.; Jolly, L.; Hughes, J.N.; Gecz, J.; Thomas, P.Q. Pcdh19 Loss-of-Function Increases Neuronal Migration In Vitro but is Dispensable for Brain Development in Mice. Sci. Rep. 2016, 6, 26765. [Google Scholar] [CrossRef]
- Krishna-K, K.; Hertel, N.; Redies, C. Cadherin expression in the somatosensory cortex: Evidence for a combinatorial molecular code at the single-cell level. Neuroscience 2011, 175, 37–48. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamers, D.; Landi, S.; Mezzena, R.; Baroncelli, L.; Pillai, V.; Cruciani, F.; Migliarini, S.; Mazzoleni, S.; Pasqualetti, M.; Passafaro, M.; et al. Perturbation of Cortical Excitability in a Conditional Model of PCDH19 Disorder. Cells 2022, 11, 1939. https://doi.org/10.3390/cells11121939
Lamers D, Landi S, Mezzena R, Baroncelli L, Pillai V, Cruciani F, Migliarini S, Mazzoleni S, Pasqualetti M, Passafaro M, et al. Perturbation of Cortical Excitability in a Conditional Model of PCDH19 Disorder. Cells. 2022; 11(12):1939. https://doi.org/10.3390/cells11121939
Chicago/Turabian StyleLamers, Didi, Silvia Landi, Roberta Mezzena, Laura Baroncelli, Vinoshene Pillai, Federica Cruciani, Sara Migliarini, Sara Mazzoleni, Massimo Pasqualetti, Maria Passafaro, and et al. 2022. "Perturbation of Cortical Excitability in a Conditional Model of PCDH19 Disorder" Cells 11, no. 12: 1939. https://doi.org/10.3390/cells11121939
APA StyleLamers, D., Landi, S., Mezzena, R., Baroncelli, L., Pillai, V., Cruciani, F., Migliarini, S., Mazzoleni, S., Pasqualetti, M., Passafaro, M., Bassani, S., & Ratto, G. M. (2022). Perturbation of Cortical Excitability in a Conditional Model of PCDH19 Disorder. Cells, 11(12), 1939. https://doi.org/10.3390/cells11121939






