Lung Inflammation Is Associated with Preeclampsia Development in the Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Tissue Preparation
2.2. Animal Treatments
2.3. Blood Pressure
2.4. Proteinuria
2.5. RNA Isolation and Analysis
2.6. Immunofluorescence
2.7. Bronchoalveolar Lavage Fluid (BALF)
2.8. Immunoblotting
2.9. ELISA
2.10. Statistical Analysis
3. Results
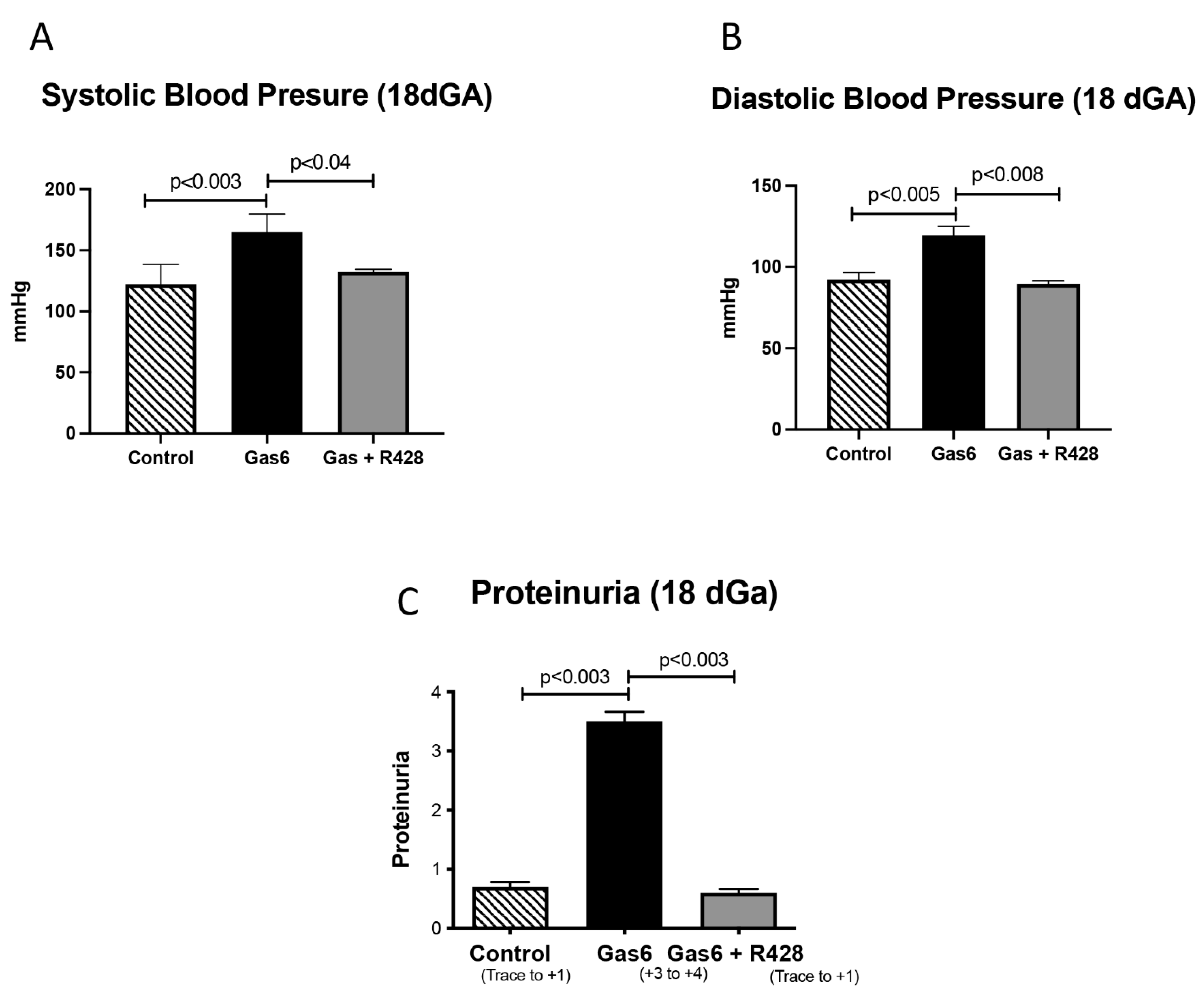
3.1. Gas6 Induces PE in Rats
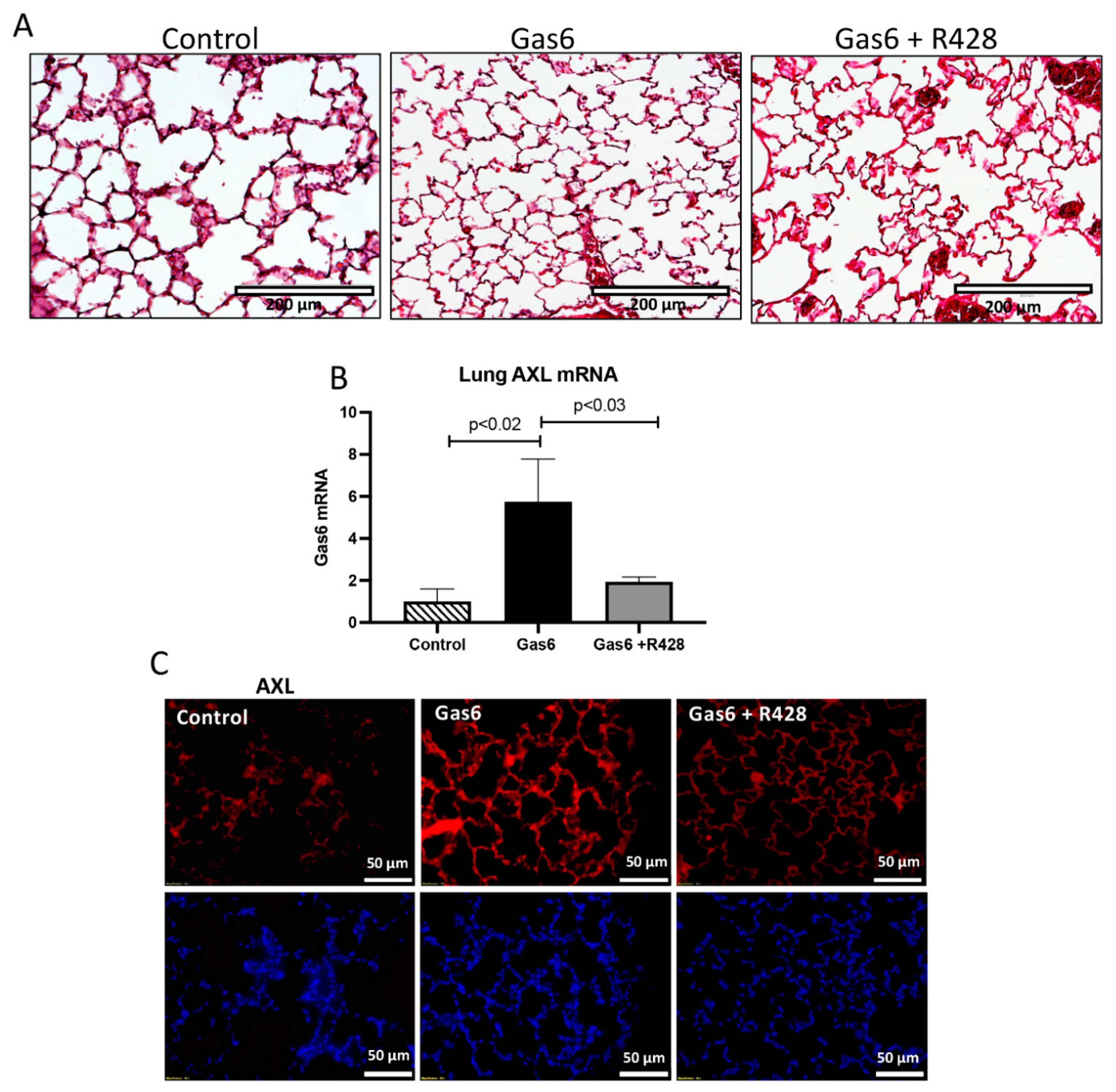
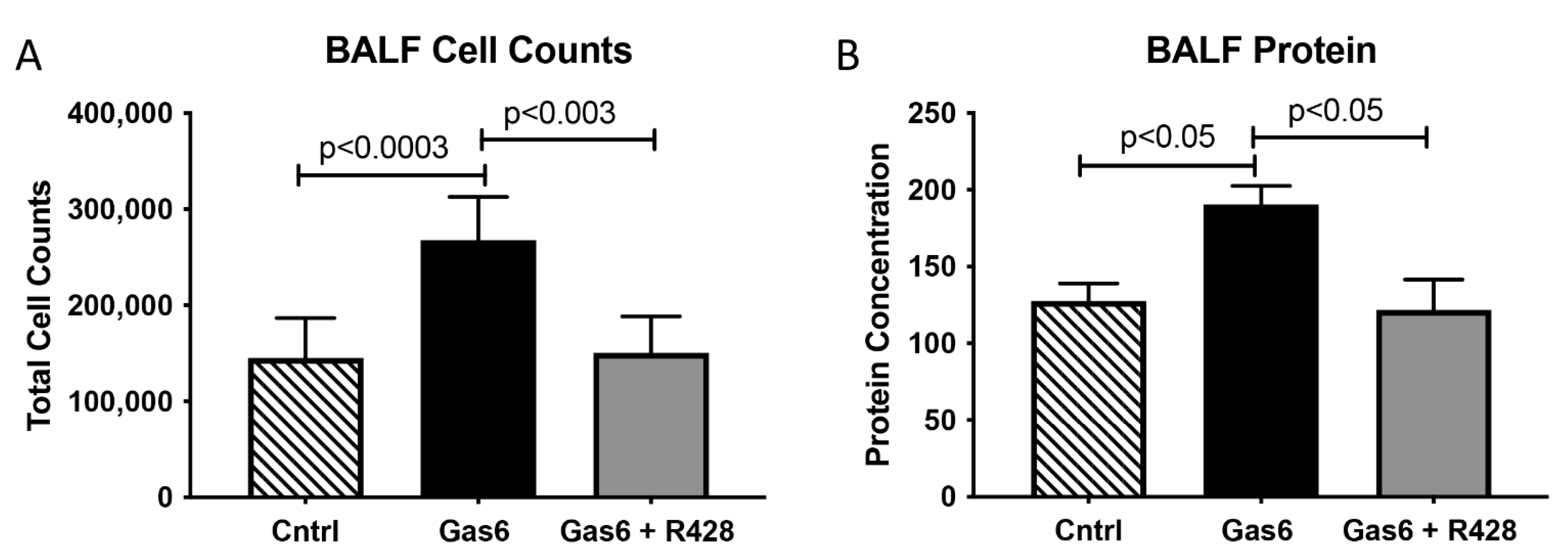
3.2. Lung and Gas6 Treatment
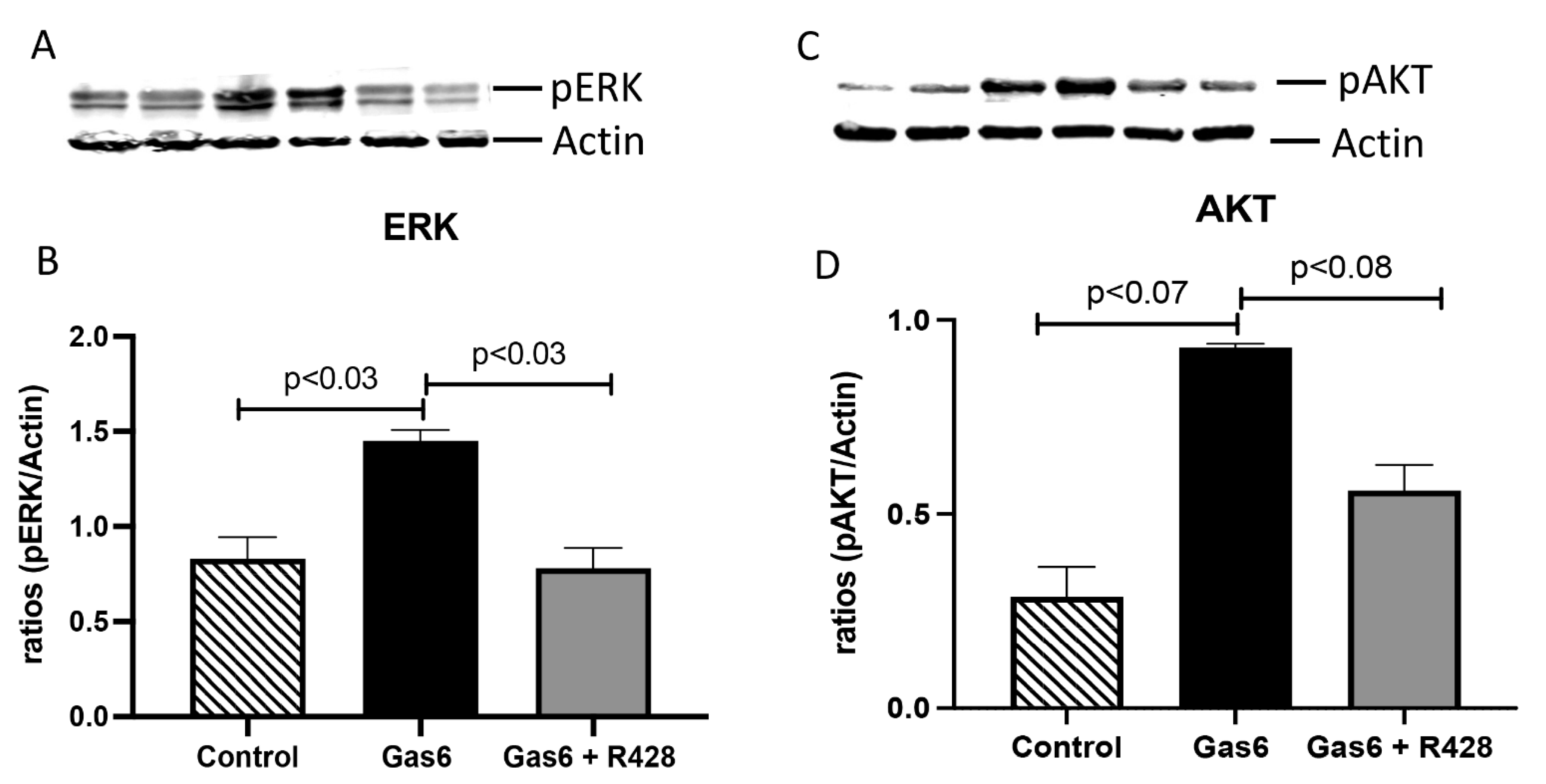
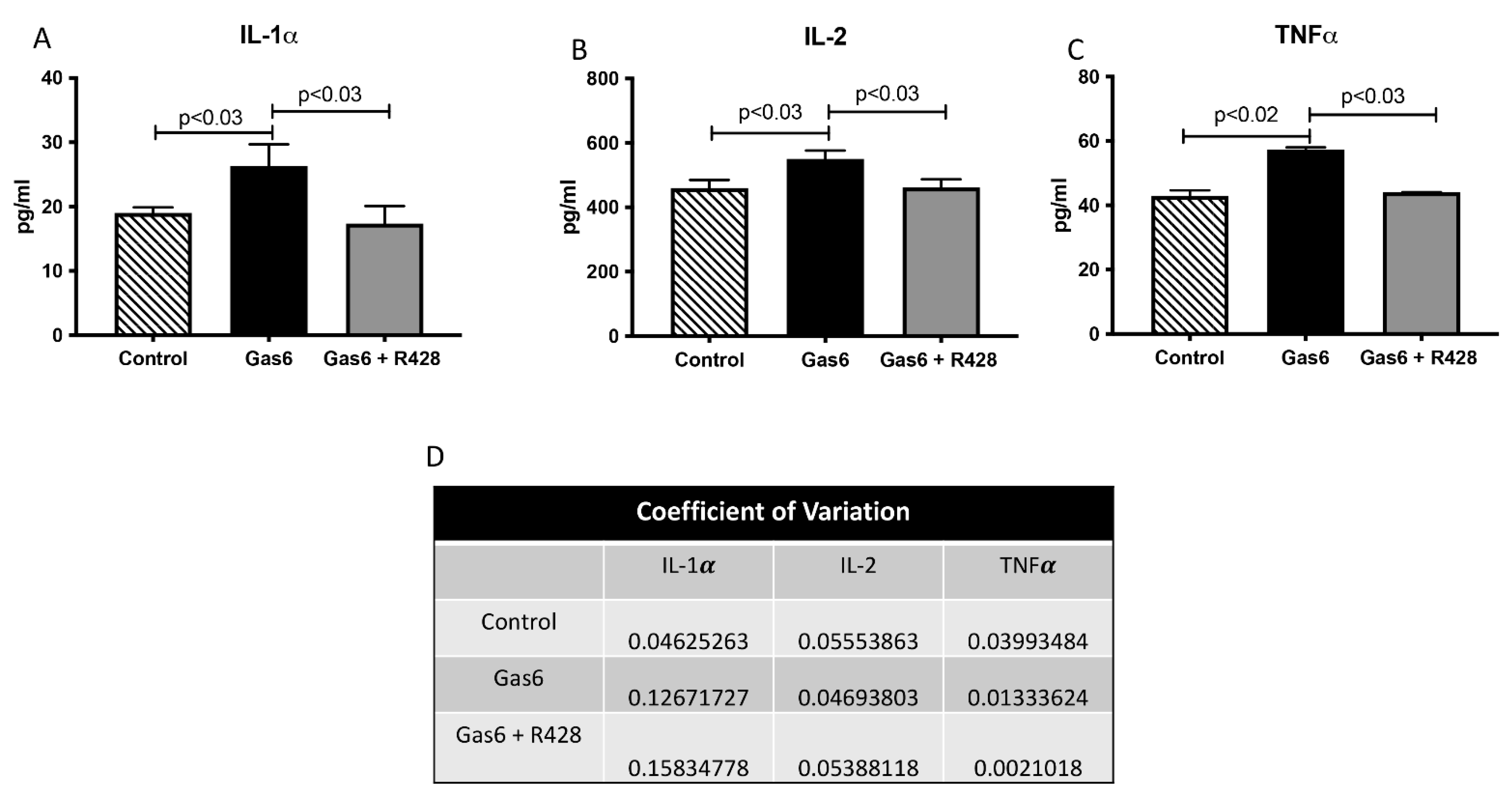
3.3. Lung-Signaling Molecules and Released Cytokines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirschi, K.M.; Tsai, K.Y.F.; Davis, T.; Clark, J.C.; Knowlton, M.N.; Bikman, B.T.; Reynolds, P.R.; Arroyo, J.A. Growth arrest-specific protein-6/AXL signaling induces preeclampsia in ratsdagger. Biol. Reprod. 2020, 102, 199–210. [Google Scholar] [PubMed]
- English, F.A.; Kenny, L.C.; McCarthy, F.P. Risk factors and effective management of preeclampsia. Integr. Blood Press. Control 2015, 8, 7–12. [Google Scholar] [PubMed] [Green Version]
- Lecarpentier, E.; Tsatsaris, V.; Goffinet, F.; Cabrol, D.; Sibai, B.; Haddad, B. Risk factors of superimposed preeclampsia in women with essential chronic hypertension treated before pregnancy. PLoS ONE 2013, 8, e62140. [Google Scholar] [CrossRef]
- Barker, D.J. The intrauterine origins of cardiovascular disease. Acta Paediatr. 1993, 82 (Suppl. 391), 93–99, discussion 100. [Google Scholar] [CrossRef] [PubMed]
- Challis, J.R.; Lye, S.J.; Gibb, W.; Whittle, W.; Patel, F.; Alfaidy, N. Understanding preterm labor. Ann. N. Y. Acad. Sci. 2001, 943, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Stepan, H.; Richter, J.; Kley, K.; Kralisch, S.; Jank, A.; Schaarschmidt, W.; Ebert, T.; Lossner, U.; Jessnitzer, B.; Kratzsch, J.; et al. Serum levels of growth arrest specific protein 6 are increased in preeclampsia. Regul. Pept. 2013, 182, 7–11. [Google Scholar] [CrossRef]
- Laurance, S.; Lemarie, C.A.; Blostein, M.D. Growth arrest-specific gene 6 (gas6) and vascular hemostasis. Adv. Nutr. 2012, 3, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Korshunov, V.A. Axl-dependent signalling: A clinical update. Clin. Sci. 2012, 122, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.Y.F.; Hirschi Budge, K.M.; Llavina, S.; Davis, T.; Long, M.; Bennett, A.; Sitton, B.; Arroyo, J.A.; Reynolds, P.R. RAGE and AXL expression following secondhand smoke (SHS) exposure in mice. Exp. Lung Res. 2019, 45, 297–309. [Google Scholar] [CrossRef]
- Buurma, A.J.; Penning, M.E.; Prins, F.; Schutte, J.M.; Bruijn, J.A.; Wilhelmus, S.; Rajakumar, A.; Bloemenkamp, K.W.; Karumanchi, S.A.; Baelde, H.J. Preeclampsia is associated with the presence of transcriptionally active placental fragments in the maternal lung. Hypertension 2013, 62, 608–613. [Google Scholar] [CrossRef] [Green Version]
- Aletta, B.; Marlies, P.; Frans, P.; Joke, S.; Jan Antonie, B.; Augustine, R.; Kitty, B.; Ananth, K.; Hans, B. OP005. Preeclampsia is associated with the presence of transcriptionally active placental fragments in the maternal lung. Pregnancy Hypertens. 2013, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Zieleskiewicz, L.; Contargyris, C.; Brun, C.; Touret, M.; Vellin, A.; Antonini, F.; Muller, L.; Bretelle, F.; Martin, C.; Leone, M. Lung ultrasound predicts interstitial syndrome and hemodynamic profile in parturients with severe preeclampsia. Anesthesiology 2014, 120, 906–914. [Google Scholar] [CrossRef]
- Qu, H.; Khalil, R.A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H661–H681. [Google Scholar] [CrossRef] [PubMed]
- Pachtman Shetty, S.L.; Koenig, S.; Tenenbaum, S.; Meirowitz, N. Point-of-care lung ultrasound patterns in late third-trimester gravidas with and without preeclampsia. Am. J. Obstet. Gynecol. MFM 2021, 3, 100310. [Google Scholar] [CrossRef]
- Diriba, K.; Awulachew, E.; Getu, E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: A systematic review and meta-analysis. Eur. J. Med. Res. 2020, 25, 39. [Google Scholar] [CrossRef] [PubMed]
- Armaly, Z.; Jadaon, J.E.; Jabbour, A.; Abassi, Z.A. Preeclampsia: Novel Mechanisms and Potential Therapeutic Approaches. Front. Physiol. 2018, 9, 973. [Google Scholar] [CrossRef] [Green Version]
- Wallace, B.; Peisl, A.; Seedorf, G.; Nowlin, T.; Kim, C.; Bosco, J.; Kenniston, J.; Keefe, D.; Abman, S.H. Anti-sFlt-1 Therapy Preserves Lung Alveolar and Vascular Growth in Antenatal Models of Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2018, 197, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.R.; Mucenski, M.L.; Le Cras, T.D.; Nichols, W.C.; Whitsett, J.A. Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J. Biol. Chem. 2004, 279, 37124–37132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, P.R.; Kasteler, S.D.; Cosio, M.G.; Sturrock, A.; Huecksteadt, T.; Hoidal, J.R. RAGE: Developmental expression and positive feedback regulation by Egr-1 during cigarette smoke exposure in pulmonary epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L1094–L1101. [Google Scholar] [CrossRef] [Green Version]
- Hirschi, K.M.; Chapman, S.; Hall, P.; Ostergar, A.; Winden, D.R.; Reynolds, P.R.; Arroyo, J.A. Gas6 protein induces invasion and reduces inflammatory cytokines in oral squamous cell carcinoma. J. Oral Pathol. Med. 2018, 47, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Rothlin, C.V.; Ghosh, S.; Zuniga, E.I.; Oldstone, M.B.; Lemke, G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 2007, 131, 1124–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, G.; Gong, K.; Ali, S.; Ali, N.; Shallwani, S.; Hatanpaa, K.J.; Pan, E.; Mickey, B.; Burma, S.; Wang, D.H.; et al. A TNF-JNK-Axl-ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma. Nat. Neurosci. 2017, 20, 1074–1084. [Google Scholar] [CrossRef] [Green Version]
- Borthwick, L.A. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin. Immunopathol. 2016, 38, 517–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.X.; Leonard, W.J. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011, 23, 598–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, S.T.; Sharma, R.; Gaskin, F.; Fu, S.M. IL-2 controls trafficking receptor gene expression and Th2 response for skin and lung inflammation. Clin. Immunol. 2012, 145, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, S.; Hoidal, J.R.; Mukherjee, T.K. Role of TNFalpha in pulmonary pathophysiology. Respir. Res. 2006, 7, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundblad, L.K.; Thompson-Figueroa, J.; Leclair, T.; Sullivan, M.J.; Poynter, M.E.; Irvin, C.G.; Bates, J.H. Tumor necrosis factor-alpha overexpression in lung disease: A single cause behind a complex phenotype. Am. J. Respir. Crit. Care Med. 2005, 171, 1363–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Ma, Z.; Hu, W.; Wang, D.; Gong, B.; Fan, C.; Jiang, S.; Li, T.; Gao, J.; Yang, Y. Molecular insights of Gas6/TAM in cancer development and therapy. Cell Death Dis. 2017, 8, e2700. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, J.H.; van der Poll, T.; van’ t Veer, C. TAM receptors, Gas6, and protein S: Roles in inflammation and hemostasis. Blood 2014, 123, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Gu, S.; Li, J.M.; Hsu, S.W.; Chen, S.J.; Chang, W.H.; Chen, C.H. Targeting the AXL Receptor in Combating Smoking-related Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2021, 64, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Boshuizen, J.; Pencheva, N.; Krijgsman, O.; Altimari, D.D.; Castro, P.G.; de Bruijn, B.; Ligtenberg, M.A.; Gresnigt-Van den Heuvel, E.; Vredevoogd, D.W.; Song, J.Y.; et al. Cooperative Targeting of Immunotherapy-Resistant Melanoma and Lung Cancer by an AXL-Targeting Antibody-Drug Conjugate and Immune Checkpoint Blockade. Cancer Res. 2021, 81, 1775–1787. [Google Scholar] [CrossRef]
- Fujino, N.; Kubo, H.; Maciewicz, R.A. Phenotypic screening identifies Axl kinase as a negative regulator of an alveolar epithelial cell phenotype. Lab. Investig. 2017, 97, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Osol, G.; Bernstein, I. Preeclampsia and maternal cardiovascular disease: Consequence or predisposition? J. Vasc. Res. 2014, 51, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Bokslag, A.; van Weissenbruch, M.; Mol, B.W.; de Groot, C.J. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum. Dev. 2016, 102, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Ozakpinar, O.B.; Sahin, S.; Verimli, N.; Simsek, G.G.; Maurer, A.M.; Eroglu, M.; Tetik, S.; Uras, F. Association between the growth arrest-specific 6 (Gas6) gene polymorphism c.834 + 7G>A and preeclampsia. J. Matern. Fetal Neonatal Med. 2016, 29, 1149–1153. [Google Scholar] [CrossRef]
- Popescu, P.; Nanea, T. Thoracic pain syndrome appearing after acute myocardial infarction. Rev. Med. Interna Neurol. Psihiatr. Neurochir. Dermatovenerol. Med. Interna 1986, 38, 323–332. [Google Scholar]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Van Eeden, S.; Leipsic, J.; Paul Man, S.F.; Sin, D.D. The relationship between lung inflammation and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2012, 186, 11–16. [Google Scholar] [CrossRef] [PubMed]
- King, P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015, 4, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimori, T.; Grabiec, A.M.; Kaur, M.; Bell, T.J.; Fujino, N.; Cook, P.C.; Svedberg, F.R.; MacDonald, A.S.; Maciewicz, R.A.; Singh, D.; et al. The Axl receptor tyrosine kinase is a discriminator of macrophage function in the inflamed lung. Mucosal Immunol. 2015, 8, 1021–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalli, G.; Colafrancesco, S.; Emmi, G.; Imazio, M.; Lopalco, G.; Maggio, M.C.; Sota, J.; Dinarello, C.A. Interleukin 1alpha: A comprehensive review on the role of IL-1alpha in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun. Rev. 2021, 20, 102763. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Function and regulation of IL-1alpha in inflammatory diseases and cancer. Immunol. Rev. 2018, 281, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Rabolli, V.; Badissi, A.A.; Devosse, R.; Uwambayinema, F.; Yakoub, Y.; Palmai-Pallag, M.; Lebrun, A.; De Gussem, V.; Couillin, I.; Ryffel, B.; et al. The alarmin IL-1alpha is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part. Fibre Toxicol. 2014, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Terlizzi, M.; Colarusso, C.; Popolo, A.; Pinto, A.; Sorrentino, R. IL-1alpha and IL-1beta-producing macrophages populate lung tumor lesions in mice. Oncotarget 2016, 7, 58181–58192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, M.; Garcia-Ruiz, I.; Maiz, N.; Rodo, C.; Garcia-Manau, P.; Serrano, B.; Lopez-Martinez, R.M.; Balcells, J.; Fernandez-Hidalgo, N.; Carreras, E.; et al. Pre-eclampsia-like syndrome induced by severe COVID-19: A prospective observational study. BJOG 2020, 127, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Rebutini, P.Z.; Zanchettin, A.C.; Stonoga, E.T.S.; Pra, D.M.M.; de Oliveira, A.L.P.; Deziderio, F.D.S.; Fonseca, A.S.; Dagostini, J.C.H.; Hlatchuk, E.C.; Furuie, I.N.; et al. Association Between COVID-19 Pregnant Women Symptoms Severity and Placental Morphologic Features. Front. Immunol. 2021, 12, 685919. [Google Scholar] [CrossRef] [PubMed]
- Rad, H.S.; Rohl, J.; Stylianou, N.; Allenby, M.C.; Bazaz, S.R.; Warkiani, M.E.; Guimaraes, F.S.F.; Clifton, V.L.; Kulasinghe, A. The Effects of COVID-19 on the Placenta During Pregnancy. Front. Immunol. 2021, 12, 743022. [Google Scholar] [CrossRef]
- Morales, A.; Rojo Rello, S.; Cristobal, H.; Fiz-Lopez, A.; Arribas, E.; Mari, M.; Tutusaus, A.; de la Cal-Sabater, P.; Nicolaes, G.A.F.; Ortiz-Perez, J.T.; et al. Growth Arrest-Specific Factor 6 (GAS6) Is Increased in COVID-19 Patients and Predicts Clinical Outcome. Biomedicines 2021, 9, 335. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtis, K.; Clarke, D.; Hanegan, M.; Stapley, B.; Wendt, R.; Beckett, N.; Litchfield, C.; Campbell, K.; Reynolds, P.; Arroyo, J. Lung Inflammation Is Associated with Preeclampsia Development in the Rat. Cells 2022, 11, 1884. https://doi.org/10.3390/cells11121884
Curtis K, Clarke D, Hanegan M, Stapley B, Wendt R, Beckett N, Litchfield C, Campbell K, Reynolds P, Arroyo J. Lung Inflammation Is Associated with Preeclampsia Development in the Rat. Cells. 2022; 11(12):1884. https://doi.org/10.3390/cells11121884
Chicago/Turabian StyleCurtis, Katrina, Derek Clarke, Makayla Hanegan, Brendan Stapley, Ryan Wendt, Nathan Beckett, Cade Litchfield, Kennedy Campbell, Paul Reynolds, and Juan Arroyo. 2022. "Lung Inflammation Is Associated with Preeclampsia Development in the Rat" Cells 11, no. 12: 1884. https://doi.org/10.3390/cells11121884
APA StyleCurtis, K., Clarke, D., Hanegan, M., Stapley, B., Wendt, R., Beckett, N., Litchfield, C., Campbell, K., Reynolds, P., & Arroyo, J. (2022). Lung Inflammation Is Associated with Preeclampsia Development in the Rat. Cells, 11(12), 1884. https://doi.org/10.3390/cells11121884








