Enoxaparin Increases D6 Receptor Expression and Restores Cytoskeleton Organization in Trophoblast Cells from Preeclampsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Cell Cultures
2.3. Western Blotting
2.4. Confocal Microscopy Analysis of D6 Expression in Trophoblast Cells
2.5. F-Actin Immunofluorescent Staining
2.6. Measure of Cytoskeletal Fiber Alignment
2.7. Statistical Analysis
3. Results
3.1. Study Population
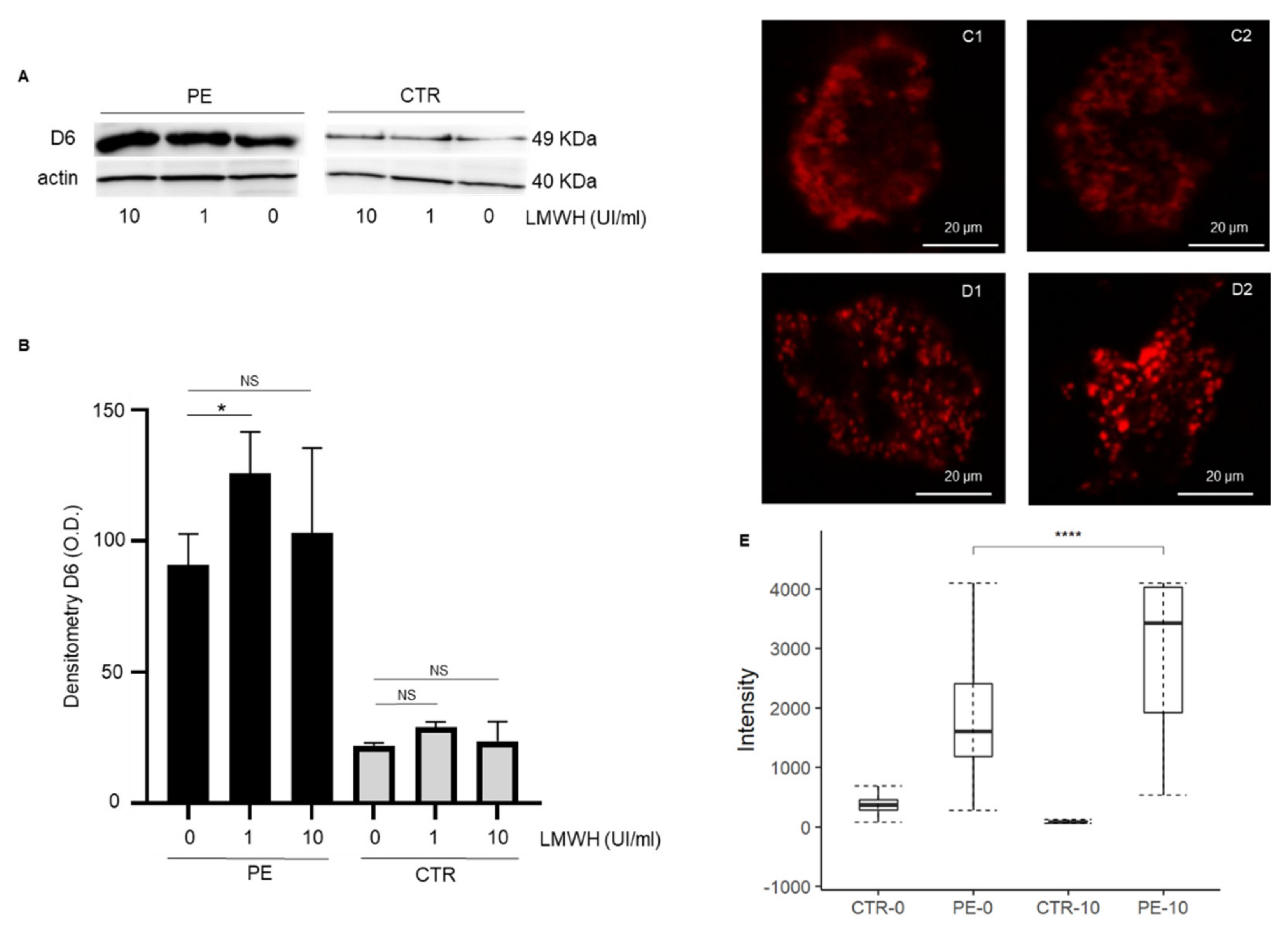
3.2. LMWH Increases D6 Expression of Trophoblast Cells from PE
3.3. LMWH Restores Cytoskeleton Organization of Trophoblast Cells from PE
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villar, J.; Say, L.; Gulmezoglu, A.M.; Meraldi, M.; Lindheimer, M.D.; Betran, A.P.P.G. Eclampsia and Preeclampsia: A Health Problem for 2000 Years. In Pre-Eclampsia; RCOG Press: London, UK, 2003; pp. 189–207. [Google Scholar]
- Ronsmans, C.; Graham, W.J. Maternal mortality: Who, when, where, and why. Lancet 2006, 368, 1189–1200. [Google Scholar] [CrossRef]
- Madigan, J.; Freeman, D.J.; Menzies, F.; Forrow, S.; Nelson, S.M.; Young, A.; Sharkey, A.; Moffett, A.; Graham, G.J.; Greer, I.A.; et al. Chemokine scavenger D6 is expressed by trophoblasts and aids the survival of mouse embryos transferred into allogeneic recipients. J. Immunol. 2010, 184, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- Yarim, G.F.; Karahan, S.; Nisbet, C. Elevated plasma levels of interleukin 1 β, tumour necrosis factor α and monocyte chemotactic protein 1 are associated with pregnancy toxaemia in ewes. Vet. Res. Commun. 2007, 31, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kauma, S.; Takacs, P.; Scordalakes, C.; Walsh, S.; Green, K.; Peng, T. Increased endothelial monocyte chemoattractant protein-1 and interleukin-8 in preeclampsia. Obstet. Gynecol. 2002, 100, 706–714. [Google Scholar]
- Mellembakken, J.R.; Solum, N.O.; Ueland, T.; Videm, V.; Aukrust, P. Increased concentrations of soluble CD40 ligand, RANTES and GRO-α in preeclampsia-possible role of platelet activation. Thromb. Haemost. 2001, 86, 1272–1276. [Google Scholar]
- Tersigni, C.; Di Nicuolo, F.; Maulucci, G.; Rolfo, A.; Giuffrida, D.; Veglia, M.; De Spirito, M.; Scambia, G.; Todros, T.; Di Simone, N. Placental Chemokine Receptor D6 Is Functionally Impaired in Pre-Eclampsia. PLoS ONE 2016, 11, e0164747. [Google Scholar] [CrossRef]
- Murphy, P.M. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 1994, 12, 593–633. [Google Scholar] [CrossRef]
- Murphy, P.M.; Baggiolini, M.; Charo, I.F.; He’bert, C.A.; Horuk, R.; Matsushima, K.; Miller, L.H.; Oppenheim, J.J.; Power, C.A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000, 52, 145–176. [Google Scholar]
- Locati, M.; Torre, Y.M.; Galliera, E.; Bonecchi, R.; Bodduluri, H.; Vago, G.; Vecchi, A.; Mantovani, A. Silent chemoattractant receptors: D6 as a decoy and scavenger receptor for inflammatory CC chemokines. Cytokine Growth Factor Rev. 2005, 16, 679–686. [Google Scholar] [CrossRef]
- Mantovani, A.; Bonecchi, R.; Locati, M. Tuning inflammation and immunity by chemokine sequestration: Decoys and more. Nat. Rev. Immunol. 2006, 6, 907–918. [Google Scholar] [CrossRef]
- Weber, M.; Blair, E.; Simpson, C.V.; O’Hara, M.; Blackburn, P.E.; Rot, A.; Graham, G.J.; Nibbs, R.J.B. The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol. Biol. Cell 2004, 15, 2492–2508. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R.; Borroni, E.M.; Anselmo, A.; Doni, A.; Savino, B.; Mirolo, M.; Fabbri, M.; Jala, V.R.; Haribabu, B.; Mantovani, A.; et al. Regulation of D6 chemokine scavenging activity by ligand- and Rab11-dependent surface up-regulation. Blood 2008, 112, 493–503. [Google Scholar] [CrossRef][Green Version]
- Fra, A.M.; Locati, M.; Otero, K.; Sironi, M.; Signorelli, P.; Massardi, M.L.; Gobbi, M.; Vecchi, A.; Sozzani, S.; Mantovani, A. Cutting edge: Scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J. Immunol. 2003, 170, 2279–2282. [Google Scholar] [CrossRef] [PubMed]
- Borroni, E.M.; Cancellieri, C.; Vacchini, A.; Benureau, Y.; Lagane, B.; Bachelerie, F.; Arenzana-Seisdedos, F.; Mizuno, K.; Mantovani, A.; Bonecchi, R.; et al. β-arrestin-dependent activation of the cofilin pathway is required for the scavenging activity of the atypical chemokine receptor D6. Sci. Signal. 2013, 6, ra30. [Google Scholar] [CrossRef]
- Nibbs, R.J.; Wylie, S.M.; Yang, J.; Landau, N.R.; Graham, G.J. Cloning and characterization of a novel promiscuous human β-chemokine receptor D6. J. Biol. Chem. 1997, 272, 32078–32083. [Google Scholar] [CrossRef] [PubMed]
- Martinez de la Torre, Y.; Buracchi, C.; Borroni, E.M.; Dupor, J.; Bonecchi, R.; Nebuloni, M.; Pasqualini, F.; Doni, A.; Lauri, E.; Agostinis, C.; et al. Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc. Natl. Acad. Sci. USA 2007, 104, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Gris, J.C.; Chauleur, C.; Molinari, N.; Marès, P.; Fabbro-Peray, P.; Quéré, I.; Lefrant, J.Y.; Haddad, B.; Dauzat, M. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre-eclampsia. The pilot randomised controlled NOH-PE trial. Thromb. Haemost. 2011, 106, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Rodger, M.A.; Langlois, N.J.; de Vries, J.I.; Rey, E.; Gris, J.C.; Martinelli, I.; Schleussner, E.; Ramsay, T.; Mallick, R.; Skidmore, B.; et al. Low-molecular-weight heparin for prevention of placenta-mediated pregnancy complications: Protocol for a systematic review and individual patient data meta-analysis (AFFIRM). Syst. Rev. 2014, 26, 69. [Google Scholar] [CrossRef][Green Version]
- Groom, K.M.; McCowan, L.M.; Mackay, L.K.; Lee, A.C.; Said, J.M.; Kane, S.C.; Walker, S.P.; van Mens, T.E.; Hannan, N.J.; Tong, S.; et al. Enoxaparin for the prevention of preeclampsia and intrauterine growth restriction in women with a history: A randomized trial. Am. J. Obstet. Gynecol. 2017, 216, 296.e1–296.e14. [Google Scholar] [CrossRef]
- Rodger, M.A.; Gris, J.C.; de Vries, J.I.P.; Martinelli, I.; Rey, É.; Schleussner, E.; Middeldorp, S.; Kaaja, R.; Langlois, N.J.; Ramsay, T.; et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: A meta-analysis of individual patient data from randomised controlled trials. Lancet 2016, 388, 2629–2641. [Google Scholar] [CrossRef]
- De Vries, J.I.; van Pampus, M.G.; Hague, W.M.; Bezemer, P.D.; Joosten, J.H.; FRUIT Investigators. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: The FRUIT-RCT. J. Thromb. Haemost. 2012, 10, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, I.; Ruggenenti, P.; Cetin, I.; Pardi, G.; Perna, A.; Vergani, P.; Acaia, B.; Facchinetti, F.; La Sala, G.B.; Bozzo, M.; et al. Heparin in pregnant women with previous placenta-mediated pregnancy complications: A prospective, randomized, multicenter, controlled clinical trial. Blood 2012, 119, 3269–3275. [Google Scholar] [CrossRef] [PubMed]
- Voudouri, K.; Nikitovic, D.; Berdiaki, A.; Papachristou, D.J.; Tsiaoussis, J.; Spandidos, D.A.; Tsatsakis, A.M.; Tzanakakis, G.N. Heparin regulates B6FS cell motility through a FAK/actin cytoskeleton axis. Oncol. Rep. 2016, 36, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Di Simone, N.; Caliandro, D.; Castellani, R.; Ferrazzani, S.; Caruso, A. Interleukin-3 and human trophoblast: In vitro explanations for the effect of interleukin in patients with antiphospholipid antibody syndrome. Fertil. Steril. 2000, 73, 1194–1200. [Google Scholar] [CrossRef]
- Bianchetti, G.; Di Giacinto, F.; De Spirito, M.; Maulucci, G. Machine-learning assisted confocal imaging of intracellular sites of triglycerides and cholesteryl esters formation and storage. Anal. Chim. Acta 2020, 1121, 57–66. [Google Scholar] [CrossRef]
- Marcotti, S.; de Freitas, D.B.; Troughton, L.D.; Kenny, F.N.; Shaw, T.J.; Stramer, B.M.; Oakes, P.W. A workflow for rapid unbiased quantification of fibrillar feature alignment in biological images. Front. Comput. Sci. 2021, 3, 745831. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L. Placental stress and pre-eclampsia: A revised view. Placenta 2009, 30, S38–S42. [Google Scholar] [CrossRef]
- Redman, C.W.; Sargent, I.L.; Staff, A.C. IFPA Senior Award Lecture: Making sense of PE- two placental causes of PE? Placenta 2014, 35, S20–S25. [Google Scholar] [CrossRef]
- Ganguly, S.; Saxena, R.; Chattopadhyay, A. Reorganization of the actin cytoskeleton upon G-protein coupled receptor signaling. Biochim. Biophys. Acta 2011, 1808, 1921–1929. [Google Scholar] [CrossRef]
- Hu, R.; Jin, H.; Zhou, S.; Yang, P.; Li, X. Proteomic analysis of hypoxia-induced responses in the syncytiali zation of human placental cell line BeWo. Placenta 2007, 28, 399–407. [Google Scholar] [CrossRef] [PubMed]
- D’Ippolito, S.; Di Nicuolo, F.; Marana, R.; Castellani, R.; Stinson, J.; Tersigni, C.; Scambia, G.; Di Simone, N. Emerging nonanticoagulant role of low molecular weight heparins on extravillous trophoblast functions and on heparin binding-epidermal growth factor and cystein-rich angiogenic inducer 61 expression. Fertil. Steril. 2012, 98, 1028–1036.e2. [Google Scholar] [CrossRef] [PubMed]
- Di Simone, N.; Caliandro, D.; Castellani, R.; Ferrazzani, S.; De Carolis, S.; Caruso, A. Low-molecular weight heparin restores in-vitro trophoblast invasiveness and differentiation in presence of immunoglobulin G fractions obtained from patients with antiphospholipid syndrome. Hum. Reprod. 1999, 14, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G.; Redecha, P.; Salmon, J.E. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat. Med. 2004, 10, 1222–1226. [Google Scholar] [CrossRef]
- Fritzsche, J.; Simonis, D.; Bendas, G. Melanoma cell adhesion can be blocked by heparin in vitro: Suggestion of VLA-4 as a novel target for antimetastatic approaches. Thromb. Haemost. 2008, 100, 1166–1175. [Google Scholar]
- Moyano, J.V.; Maqueda, A.; Albar, J.P.; Garcia-Pardo, A. A synthetic peptide from the heparin-binding domain III (repeats III4-5) of fibronectin promotes stress-fibre and focal-adhesion formation in melanoma cells. Biochem. J. 2003, 371, 565–571. [Google Scholar] [CrossRef]
- Chalkiadaki, G.; Nikitovic, D.; Berdiaki, A.; Katonis, P.; Karamanos, N.K.; Tzanakakis, G.N. Heparin plays a key regulatory role via a p53/FAK-dependent signaling in melanoma cell adhesion and migration. IUBMB Life 2011, 63, 109–119. [Google Scholar] [CrossRef]
- Chalkiadaki, G.; Nikitovic, D.; Katonis, P.; Berdiaki, A.; Tsatsakis, A.; Kotsikogianni, I.; Karamanos, N.K.; Tzanakakis, G.N. Low molecular weight heparin inhibits melanoma cell adhesion and migration through a PKCa/JNK signaling pathway inducing actin cytoskeleton changes. Cancer Lett. 2011, 312, 235–244. [Google Scholar] [CrossRef]

| Characteristics | Preeclampsia (n = 9) | Controls (n = 9) | p Value |
|---|---|---|---|
| Age (years) | 33 (21–42) ** | 33 (21–44) ** | 0.63 |
| Smokers | 1 (10%) | 0 (0.0%) | 0.84 |
| Nulliparous | 6 (60.0%) | 5 (50.0%) | 0.09 |
| BMI at booking (Kg/m2) | 26.3 (6.8) * | 21.6 (3.2) * | <0.001 |
| Gestational age at PE onset (weeks) | 31 (19–40) ** | N/A | |
| PE onset | N/A | ||
| Early (<32 weeks) | 7 (70.0%) | ||
| Late (>32 weeks) | 3 (30.0%) | ||
| Gestational age at delivery (weeks) | 32 (22–41) ** | 40 (37–41) ** | <0.001 |
| Birth weight (g) | 1597 (844) * | 3401 (365) * | <0.001 |
| Birth weight percentile | 24 (24) * | 58 (21) * | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tersigni, C.; Maulucci, G.; Castellani, R.; Bianchetti, G.; Onori, M.; Franco, R.; Barbaro, G.; De Spirito, M.; Lanzone, A.; Scambia, G.; et al. Enoxaparin Increases D6 Receptor Expression and Restores Cytoskeleton Organization in Trophoblast Cells from Preeclampsia. Cells 2022, 11, 2036. https://doi.org/10.3390/cells11132036
Tersigni C, Maulucci G, Castellani R, Bianchetti G, Onori M, Franco R, Barbaro G, De Spirito M, Lanzone A, Scambia G, et al. Enoxaparin Increases D6 Receptor Expression and Restores Cytoskeleton Organization in Trophoblast Cells from Preeclampsia. Cells. 2022; 11(13):2036. https://doi.org/10.3390/cells11132036
Chicago/Turabian StyleTersigni, Chiara, Giuseppe Maulucci, Roberta Castellani, Giada Bianchetti, Marianna Onori, Rita Franco, Greta Barbaro, Marco De Spirito, Antonio Lanzone, Giovanni Scambia, and et al. 2022. "Enoxaparin Increases D6 Receptor Expression and Restores Cytoskeleton Organization in Trophoblast Cells from Preeclampsia" Cells 11, no. 13: 2036. https://doi.org/10.3390/cells11132036
APA StyleTersigni, C., Maulucci, G., Castellani, R., Bianchetti, G., Onori, M., Franco, R., Barbaro, G., De Spirito, M., Lanzone, A., Scambia, G., & Di Simone, N. (2022). Enoxaparin Increases D6 Receptor Expression and Restores Cytoskeleton Organization in Trophoblast Cells from Preeclampsia. Cells, 11(13), 2036. https://doi.org/10.3390/cells11132036










