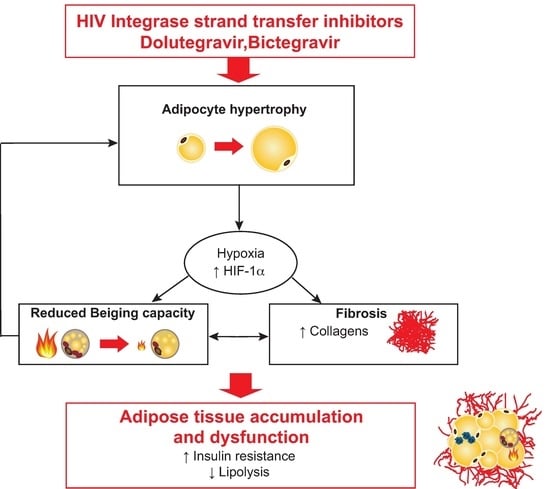
Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adipose Tissue Samples from Macaques
2.2. Isolation and Differentiation of ASCs
2.3. Protein Extraction and Western Blotting
2.4. Protein Secretion into the Cell Culture Media
2.5. RNA Isolation and Quantitative PCR
2.6. Oxidative Stress and Mitochondrial Dysfunctions
2.7. Oxygen Consumption Measurements
2.8. Lipid Metabolism
2.9. Statistical Analysis
3. Results
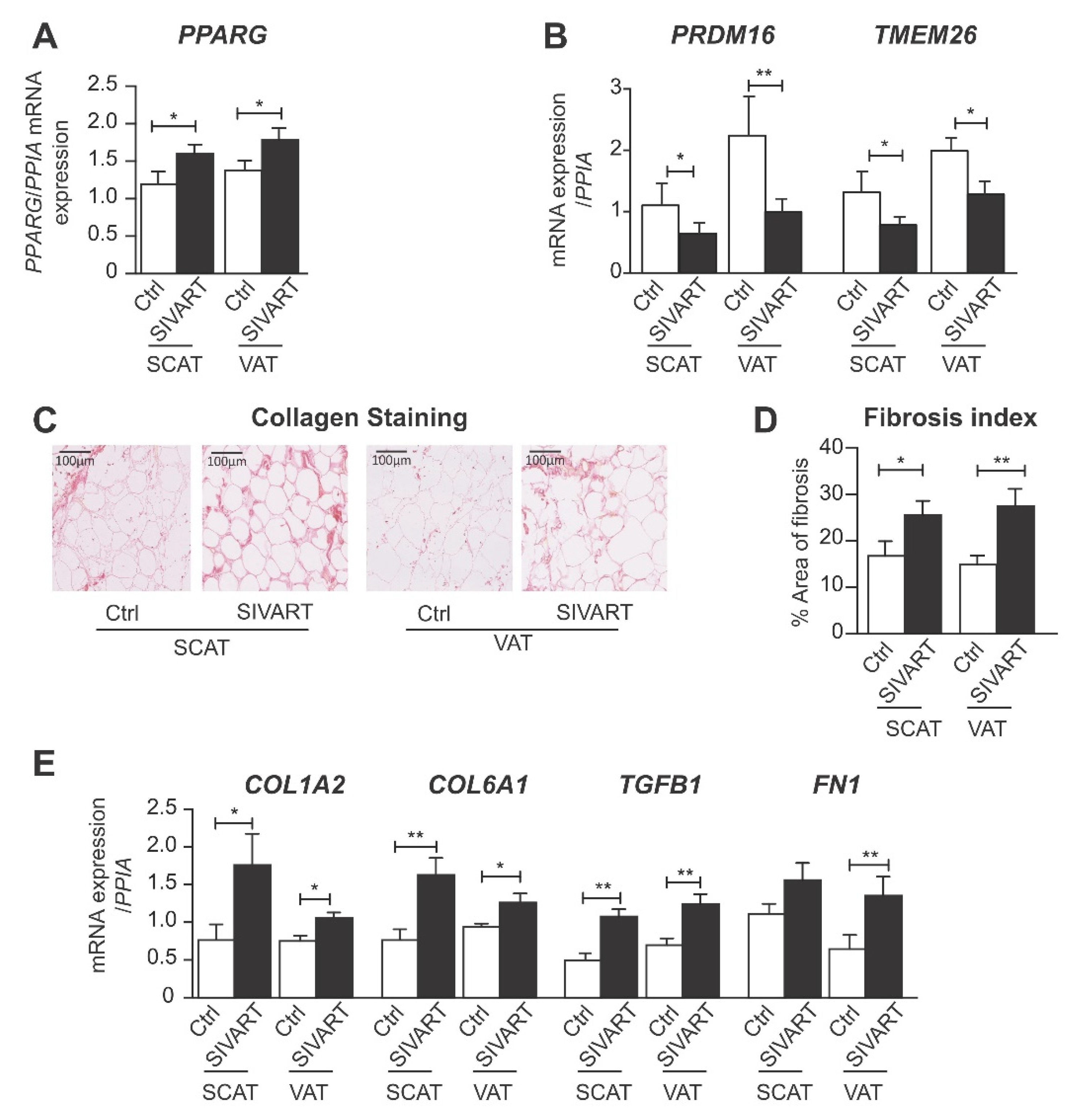
3.1. SCAT and VAT from SIV-Infected Dolutegravir-Treated Macaques Display Higher Levels of PPARG and Profibrotic Gene Expression but Lower Levels of Beige Marker Gene Expression
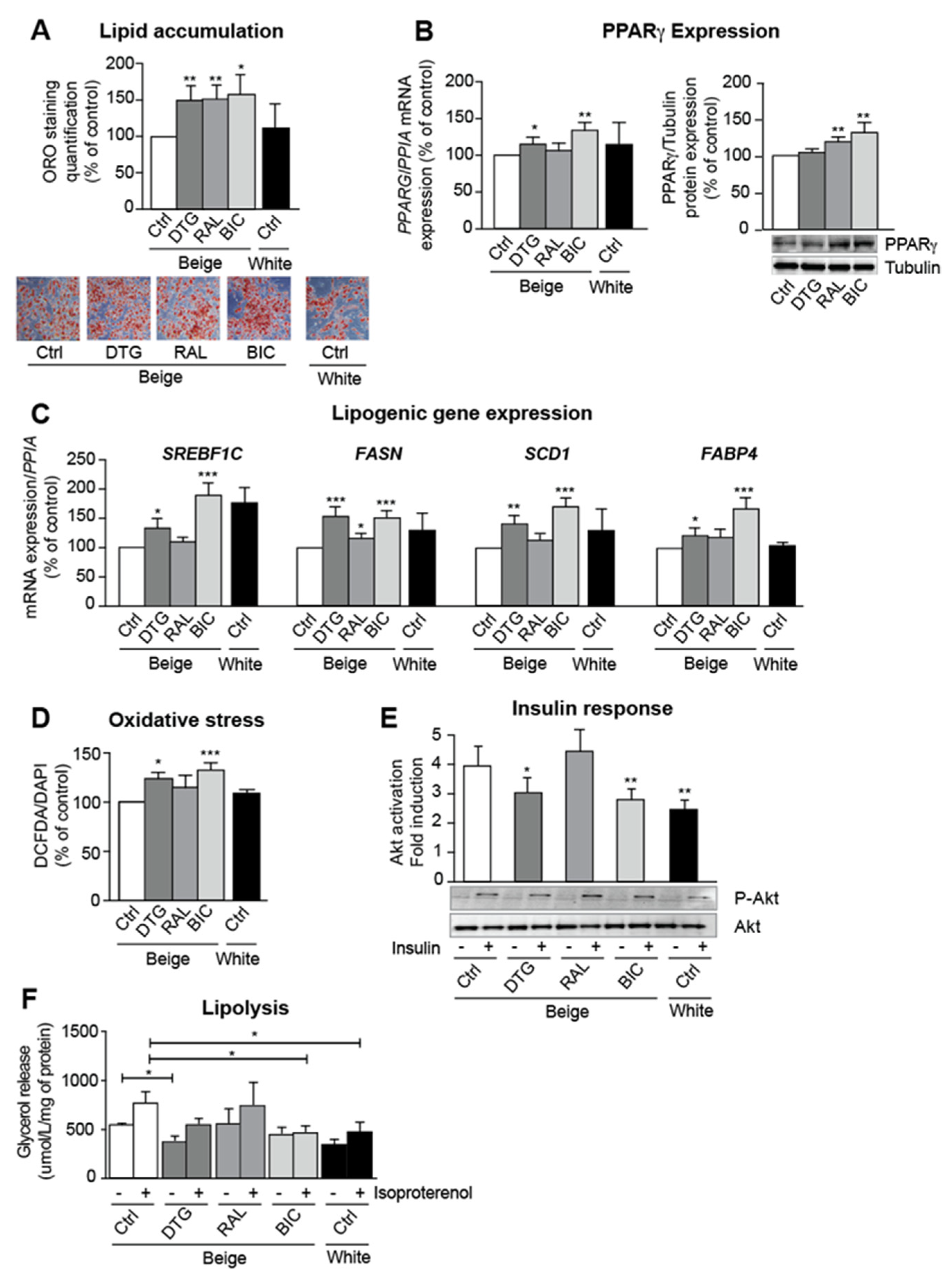
3.2. INSTIs Are Associated with Elevated Lipogenic Gene Expression and Insulin Resistance in Beige Adipocytes
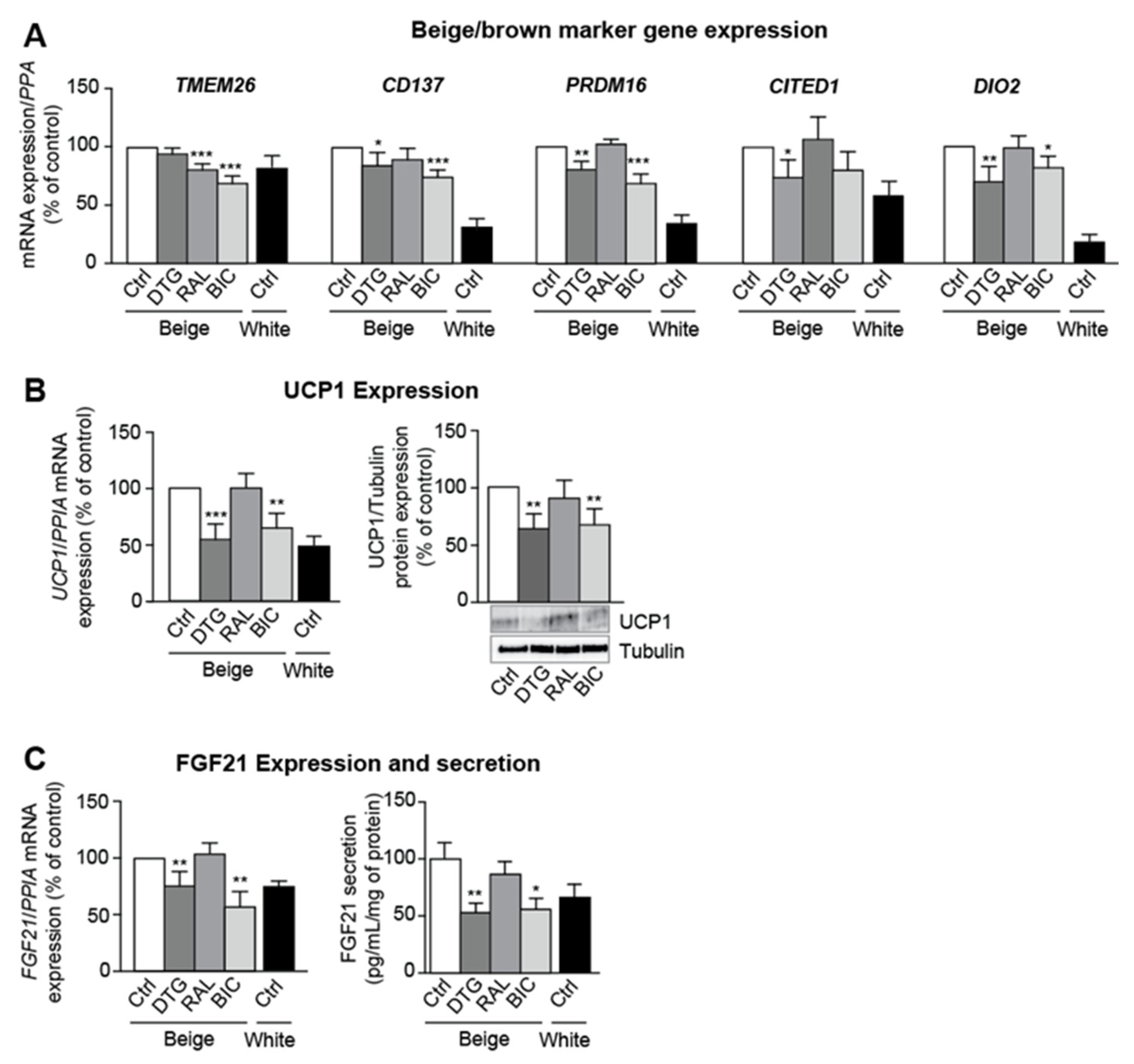
3.3. Dolutegravir and Bictegravir Inhibit Beige Differentiation
3.4. Dolutegravir and Bictegravir Are Associated with Impaired Mitochondrial Integrity in Beige Adipocytes
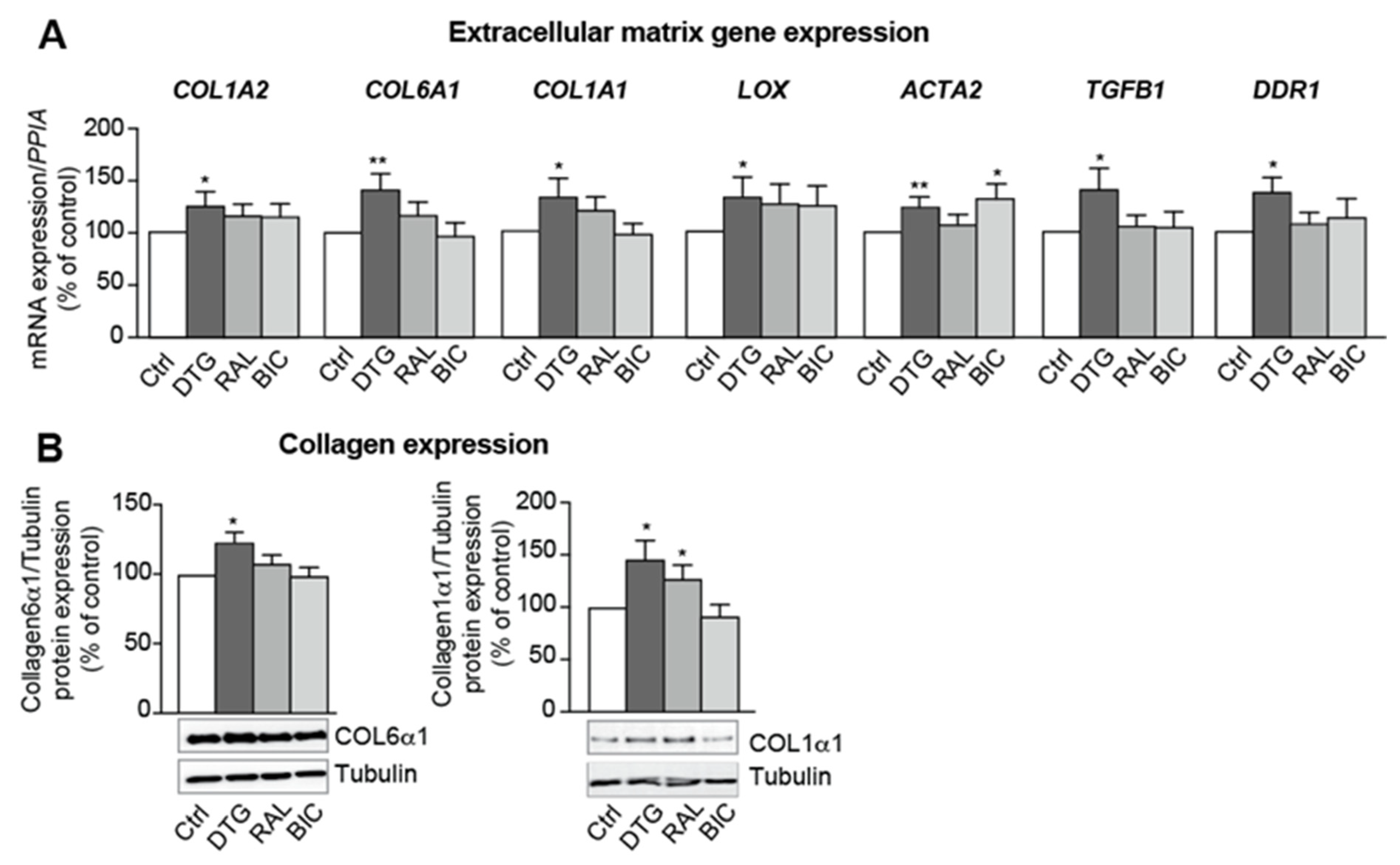
3.5. Dolutegravir Enhances the Expression of Profibrotic Genes in Beige Adipocytes
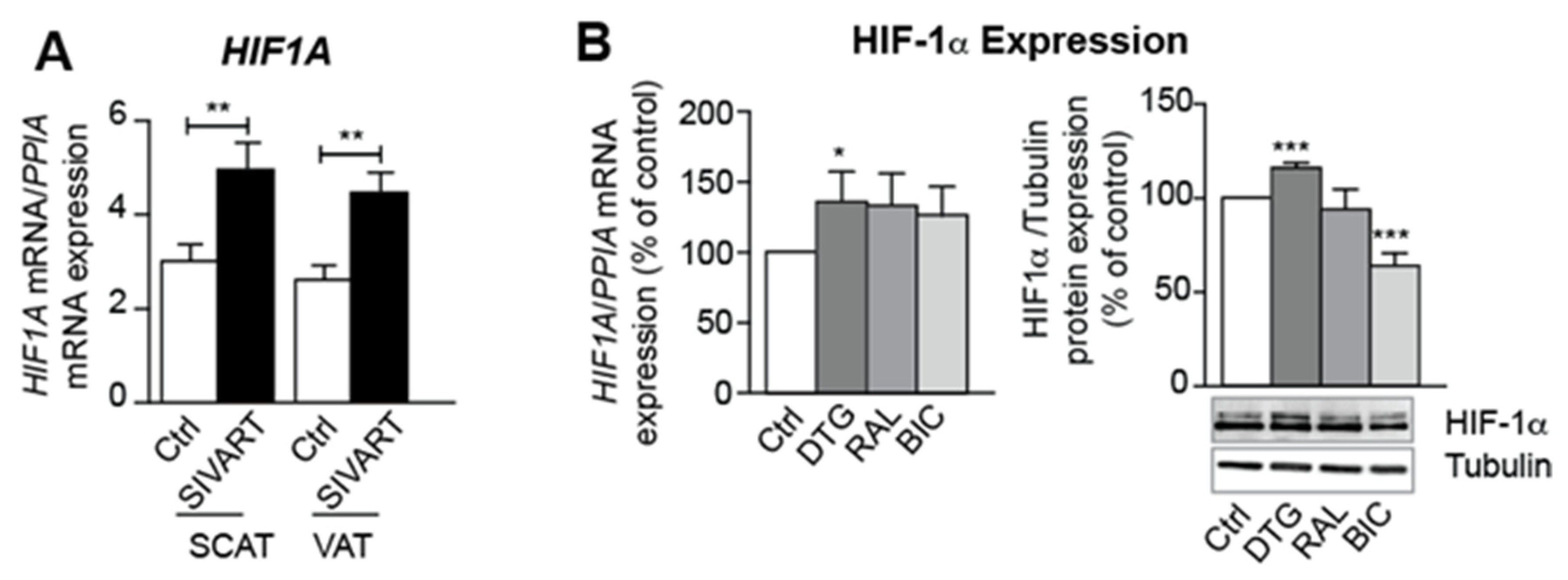
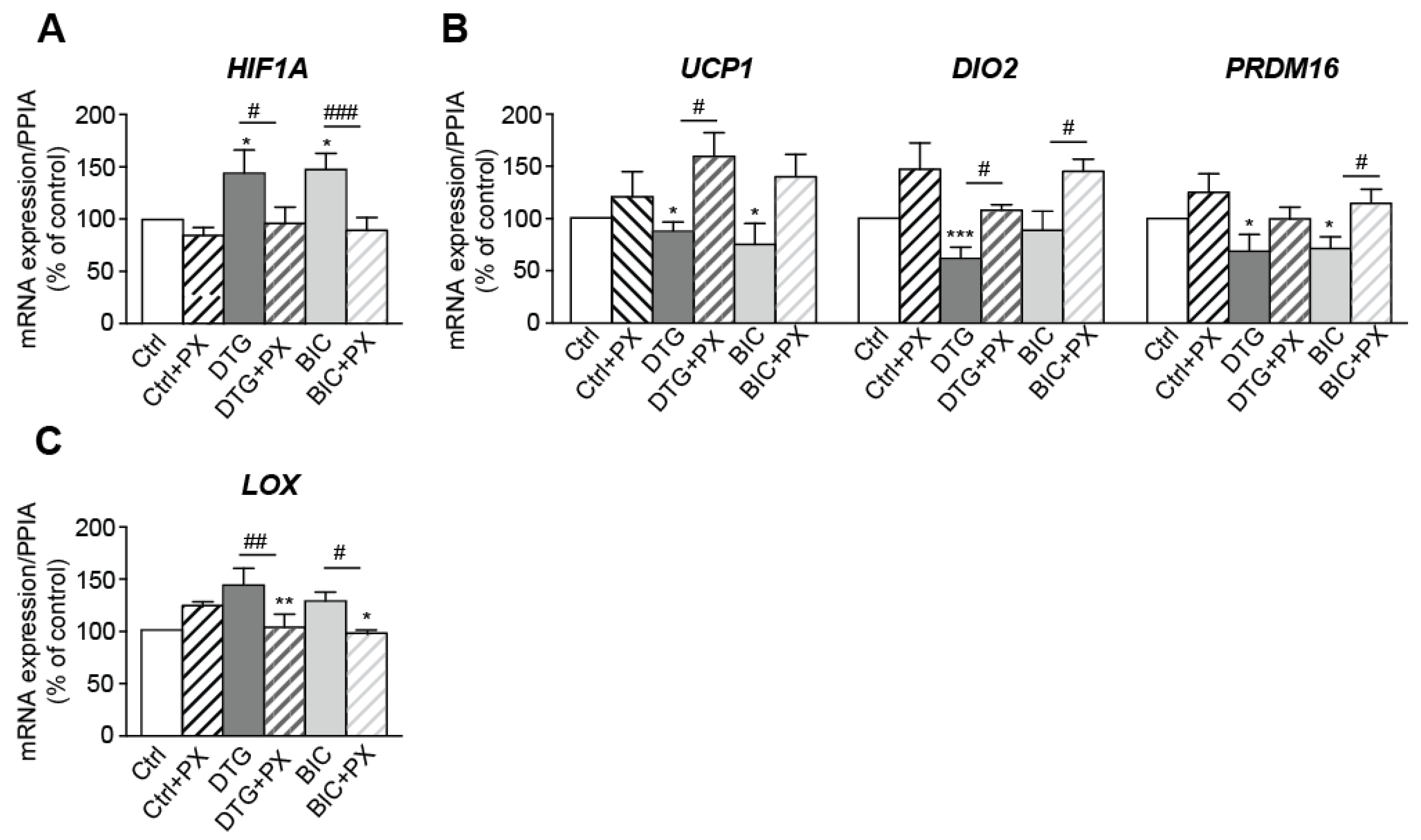
3.6. Treatment with Dolutegravir Is Associated with Elevated Expression of HIF1A, Which Is Causally Related to Beiging Inhibition and Fibrosis In Vivo and In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damouche, A.; Pourcher, G.; Pourcher, V.; Benoist, S.; Busson, E.; Lataillade, J.-J.; Le Van, M.; Lazure, T.; Adam, J.; Favier, B.; et al. High proportion of PD-1-expressing CD4(+) T cells in adipose tissue constitutes an immunomodulatory microenvironment that may support HIV persistence. Eur. J. Immunol. 2017, 47, 2113–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couturier, J.; Suliburk, J.W.; Brown, J.M.; Luke, D.J.; Agarwal, N.; Yu, X.; Nguyen, C.; Iyer, D.; Kozinetz, C.A.; Overbeek, P.A.; et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS 2015, 29, 667–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorwood, J.; Bourgeois, C.; Mantecon, M.; Atlan, M.; Pourcher, V.; Pourcher, G.; Le Grand, R.; Desjardins, D.; Fève, B.; Lambotte, O.; et al. Impact of HIV/simian immunodeficiency virus infection and viral proteins on adipose tissue fibrosis and adipogenesis. AIDS 2019, 33, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Gorwood, J.; Ejlalmanesh, T.; Bourgeois, C.; Mantecon, M.; Rose, C.; Atlan, M.; Desjardins, D.; Le Grand, R.; Fève, B.; Lambotte, O.; et al. SIV Infection and the HIV Proteins Tat and Nef Induce Senescence in Adipose Tissue and Human Adipose Stem Cells, Resulting in Adipocyte Dysfunction. Cells 2020, 9, 854. [Google Scholar] [CrossRef] [Green Version]
- Eckard, A.R.; McComsey, G.A. Weight gain and integrase inhibitors. Curr. Opin. Infect. Dis. 2020, 33, 10–19. [Google Scholar] [CrossRef]
- Wood, B.R. Do Integrase Inhibitors Cause Weight Gain? Clin. Infect. Dis. 2020, 70, 1275–1277. [Google Scholar] [CrossRef]
- Hill, A.; Waters, L.; Pozniak, A. Are new antiretroviral treatments increasing the risks of clinical obesity? J. Virus Erad. 2019, 5, 41–43. [Google Scholar] [CrossRef]
- Shah, S.; Hindley, L.; Hill, A. Are New Antiretroviral Treatments Increasing the Risk of Weight Gain? Drugs 2021, 81, 299–315. [Google Scholar] [CrossRef]
- Capeau, J.; Lagathu, C.; Bereziat, V.; Feve, B. Recent data on adipose tissue, insulin resistance, diabetes and dyslipidaemia in antiretroviral therapy controlled HIV-infected persons. Curr. Opin. HIV AIDS 2021, 16, 141–147. [Google Scholar] [CrossRef]
- Lagathu, C.; Béréziat, V.; Gorwood, J.; Fellahi, S.; Bastard, J.-P.; Vigouroux, C.; Boccara, F.; Capeau, J. Metabolic complications affecting adipose tissue, lipid and glucose metabolism associated with HIV antiretroviral treatment. Expert Opin. Drug Saf. 2019, 18, 829–840. [Google Scholar] [CrossRef]
- Lake, J.E.; Wu, K.; Bares, S.H.; Debroy, P.; Godfrey, C.; Koethe, J.R.; McComsey, G.A.; Palella, F.J.; Tassiopoulos, K.; Erlandson, K.M. Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy. Clin. Infect. Dis. 2020, 71, e471–e477. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, G.; Silveira, A.L.M.; Martins, L.B.; Ferreira, A.V.; Clement, K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J. Clin. Investig. 2019, 129, 4032–4040. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta 2014, 1842, 358–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Gorwood, J.; Bourgeois, C.; Pourcher, V.; Pourcher, G.; Charlotte, F.; Mantecon, M.; Rose, C.; Morichon, R.; Atlan, M.; Le Grand, R.; et al. The Integrase Inhibitors Dolutegravir and Raltegravir Exert Proadipogenic and Profibrotic Effects and Induce Insulin Resistance in Human/Simian Adipose Tissue and Human Adipocytes. Clin. Infect. Dis. 2020, 71, e549–e560. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Zoico, E.; Rubele, S.; De Caro, A.; Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- Thyagarajan, B.; Foster, M.T. Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20170016. [Google Scholar] [CrossRef]
- Li, S.; Gao, H.; Hasegawa, Y.; Lu, X. Fight against fibrosis in adipose tissue remodeling. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E169–E175. [Google Scholar] [CrossRef]
- Wang, W.; Ishibashi, J.; Trefely, S.; Shao, M.; Cowan, A.J.; Sakers, A.; Lim, H.W.; O’Connor, S.; Doan, M.T.; Cohen, P.; et al. A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate. Cell Metab. 2019, 30, 174–189.e5. [Google Scholar] [CrossRef]
- Yadav, H.; Quijano, C.; Kamaraju, A.K.; Gavrilova, O.; Malek, R.; Chen, W.; Zerfas, P.; Zhigang, D.; Wright, E.C.; Stuelten, C.; et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011, 14, 67–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lino, M.; Ngai, D.; Liu, A.; Mohabeer, A.; Harper, C.; Caruso, L.-L.; Schroer, S.A.; Fu, F.; McKee, T.; Giacca, A.; et al. Discoidin domain receptor 1-deletion ameliorates fibrosis and promotes adipose tissue beiging, brown fat activity, and increased metabolic rate in a mouse model of cardiometabolic disease. Mol. Metab. 2020, 39, 101006. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Ikeda, K.; Chen, Y.; Alba, D.L.; Stifler, D.; Shinoda, K.; Hosono, T.; Maretich, P.; Yang, Y.; Ishigaki, Y.; et al. Repression of Adipose Tissue Fibrosis through a PRDM16-GTF2IRD1 Complex Improves Systemic Glucose Homeostasis. Cell Metab. 2018, 27, 180–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, B.; Farrar, J.S.; Chen, S.; Lownik, J.C.; Celi, F.S. A novel role for PTK2B in cultured beige adipocyte differentiation. Biochem. Biophys. Res. Commun. 2018, 501, 851–857. [Google Scholar] [CrossRef]
- Le Pelletier, L.; Mantecon, M.; Gorwood, J.; Auclair, M.; Foresti, R.; Motterlini, R.; Laforge, M.; Atlan, M.; Fève, B.; Capeau, J.; et al. Metformin alleviates stress-induced cellular senescence of aging human adipose stromal cells and the ensuing adipocyte dysfunction. Elife 2021, 10, e62635. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, S.; Torriani, M.; Fitch, K.V.; Maehler, P.; Iyengar, S.; Feldpausch, M.; Cypess, A.M.; Grinspoon, S.K. Brief Report: Adipogenic Expression of Brown Fat Genes in HIV and HIV-Related Parameters. J. Acquir. Immune. Defic. Syndr. 2019, 82, 491–495. [Google Scholar] [CrossRef]
- Jung, I.; Tu-Sekine, B.; Jin, S.; Anokye-Danso, F.; Ahima, R.S.; Brown, T.T.; Kim, S.F. Dolutegravir Suppresses Thermogenesis via Disrupting UCP1 Expression and Mitochondrial Function in Brown/Beige Adipocytes in preclinical models. J. Infect. Dis. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Drareni, K.; Ballaire, R.; Barilla, S.; Mathew, M.J.; Toubal, A.; Fan, R.; Liang, N.; Chollet, C.; Huang, Z.; Kondili, M.; et al. GPS2 Deficiency Triggers Maladaptive White Adipose Tissue Expansion in Obesity via HIF1A Activation. Cell Rep. 2018, 24, 2957–2971.e6. [Google Scholar] [CrossRef] [Green Version]
- Vaitkus, A.; Celi, F.S. The role of adipose tissue in cancer-associated cachexia. Exp. Biol. Med. 2017, 242, 473–481. [Google Scholar] [CrossRef]
- Álvarez-Artime, A.; García-Soler, B.; Sainz, R.M.; Mayo, J.C. Emerging Roles for Browning of White Adipose Tissue in Prostate Cancer Malignant Behaviour. Int. J. Mol. Sci. 2021, 22, 5560. [Google Scholar] [CrossRef]
- Bedimo, R.; Chen, R.Y.; Accortt, N.A.; Raper, J.L.; Linn, C.; Allison, J.J.; Dubay, J.; Saag, M.S.; Hoesley, C.J. Trends in AIDS-defining and non-AIDS-defining malignancies among HIV-infected patients: 1989-2002. Clin. Infect. Dis. 2004, 39, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, L.; Ryom, L.; Neesgaard, B.; Miró, J.M.; Dahlerup Rasmussen, L.; Zangerle, R.; Grabmeier-Pfistershammer, K.; Günthard, H.F.; Kusejko, K.; Smith, C.; et al. Integrase Strand Transfer Inhibitor Use and Cancer Incidence in a Large Cohort Setting. Open Forum. Infect. Dis. 2022, 9, ofac029. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.; Seybold, U.; Roider, J.; Harter, G.; Bogner, J.R. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)-to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015-2017. Infection 2019, 47, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, W.D.; Moorhouse, M.; Sokhela, S.; Fairlie, L.; Mashabane, N.; Masenya, M.; Serenata, C.; Akpomiemie, G.; Qavi, A.; Chandiwana, N.; et al. Dolutegravir plus Two Different Prodrugs of Tenofovir to Treat HIV. N. Engl. J. Med. 2019, 381, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Torriani, M.; Srinivasa, S.; Fitch, K.V.; Thomou, T.; Wong, K.; Petrow, E.; Kahn, C.R.; Cypess, A.M.; Grinspoon, S.K. Dysfunctional Subcutaneous Fat with Reduced Dicer and Brown Adipose Tissue Gene Expression in HIV-Infected Patients. J. Clin. Endocrinol. Metab. 2016, 101, 1225–1234. [Google Scholar] [CrossRef] [Green Version]

| HIF1A | |||||
|---|---|---|---|---|---|
| SCAT | VAT | ||||
| r | p | r | p | ||
| Beiging | PRDM16 | −0.665 | 0.018 | −0.188 | 0.251 |
| Fibrosis | COL6A1 | 0.468 | 0.054 | 0.655 | 0.003 |
| COL1A2 | 0.021 | 0.475 | 0.527 | 0.027 | |
| TGFB1 | 0.617 | 0.012 | 0.744 | <0.001 | |
| Adipogenesis | PPARG | 0.622 | 0.015 | 0.073 | 0.395 |
| HIF1A | |||
|---|---|---|---|
| r | p | ||
| Beiging | PRDM16 | −0.410 | 0.009 |
| UCP1 | −0.435 | 0.008 | |
| DIO2 | −0.663 | <0.001 | |
| Fibrosis | COL6A1 | −0.040 | 0.412 |
| COL1A1 | 0.286 | 0.059 | |
| COL1A2 | 0.559 | <0.001 | |
| LOX | 0.606 | <0.001 | |
| ACTA2 | 0.561 | <0.001 | |
| TGFB1 | 0.123 | 0.250 | |
| DDR1 | 0.480 | 0.002 | |
| Lipid metabolism | PPARG | 0.05 | 0.397 |
| FABP4 | 0.438 | 0.006 | |
| Oil-Red-O | 0.357 | 0.043 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngono Ayissi, K.; Gorwood, J.; Le Pelletier, L.; Bourgeois, C.; Beaupère, C.; Auclair, M.; Foresti, R.; Motterlini, R.; Atlan, M.; Barrail-Tran, A.; et al. Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes. Cells 2022, 11, 1841. https://doi.org/10.3390/cells11111841
Ngono Ayissi K, Gorwood J, Le Pelletier L, Bourgeois C, Beaupère C, Auclair M, Foresti R, Motterlini R, Atlan M, Barrail-Tran A, et al. Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes. Cells. 2022; 11(11):1841. https://doi.org/10.3390/cells11111841
Chicago/Turabian StyleNgono Ayissi, Kenza, Jennifer Gorwood, Laura Le Pelletier, Christine Bourgeois, Carine Beaupère, Martine Auclair, Roberta Foresti, Roberto Motterlini, Michael Atlan, Aurélie Barrail-Tran, and et al. 2022. "Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes" Cells 11, no. 11: 1841. https://doi.org/10.3390/cells11111841
APA StyleNgono Ayissi, K., Gorwood, J., Le Pelletier, L., Bourgeois, C., Beaupère, C., Auclair, M., Foresti, R., Motterlini, R., Atlan, M., Barrail-Tran, A., Le Grand, R., Desjardins, D., Fève, B., Lambotte, O., Capeau, J., Béréziat, V., & Lagathu, C. (2022). Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes. Cells, 11(11), 1841. https://doi.org/10.3390/cells11111841