Maternal Vitamin D Deficiency in Mice Increases White Adipose Tissue Inflammation in Offspring
Abstract
:1. Introduction
2. Material and Methods
2.1. Animal Experiments
2.2. RNA Extraction Real Time PCR and RNA Sequencing
2.3. Gene Ontology (GO) and Ingenuity Pathway Analysis (IPA)
2.4. Evaluation of miRNA Expression
2.5. miRNA Target Prediction and Pathway Analysis
2.6. NFκB Activation and p38/MAPK Activation
2.7. Statistical Analysis
3. Results
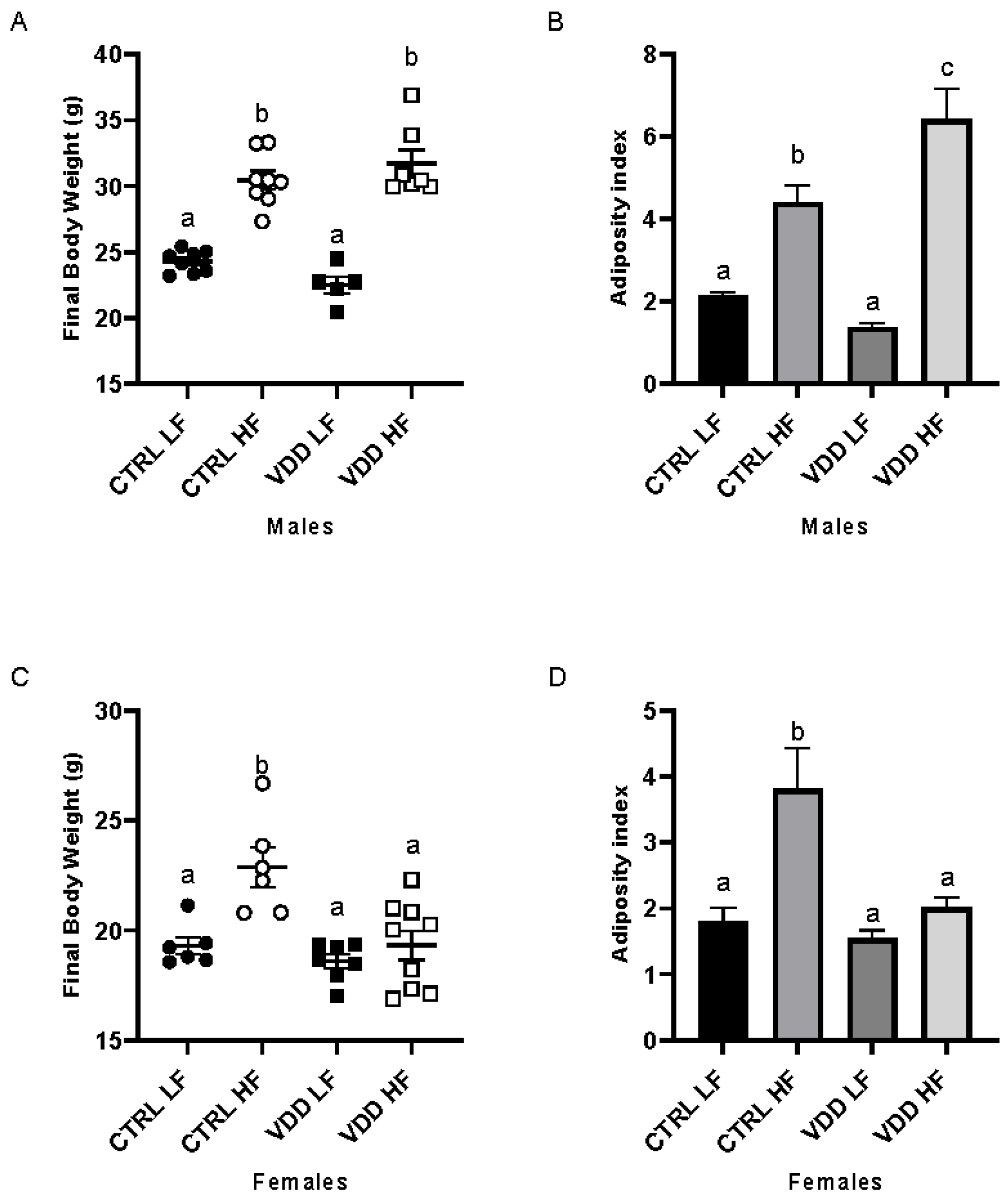
3.1. HF Diet and Maternal VDD Impact Body Weight and Adiposity Index
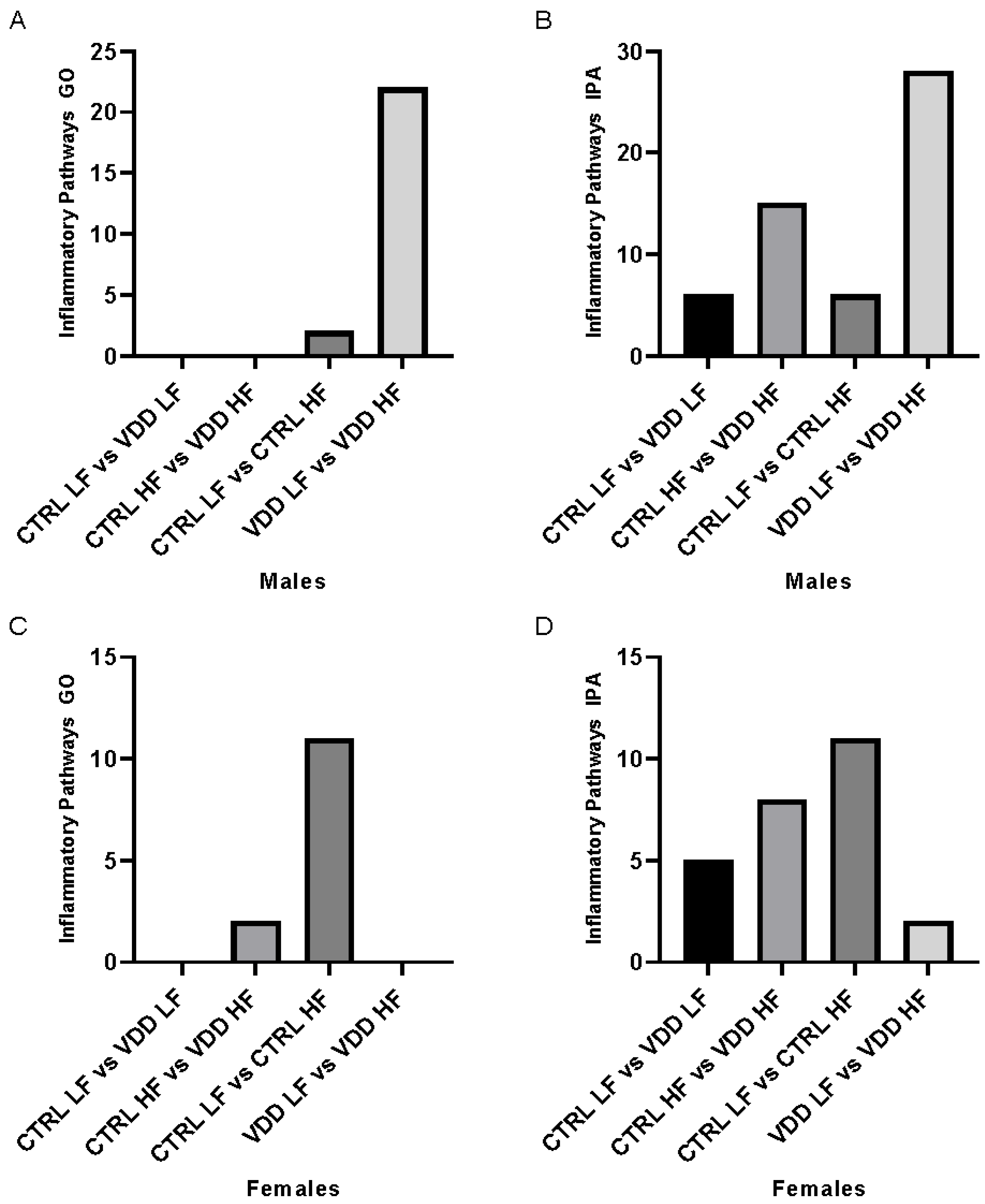
3.2. HF Diet and Maternal VDD Regulate Inflammation Pathways
3.3. HF Diet and Maternal VDD Regulate miRNA Expression and Related Inflammatory Pathways
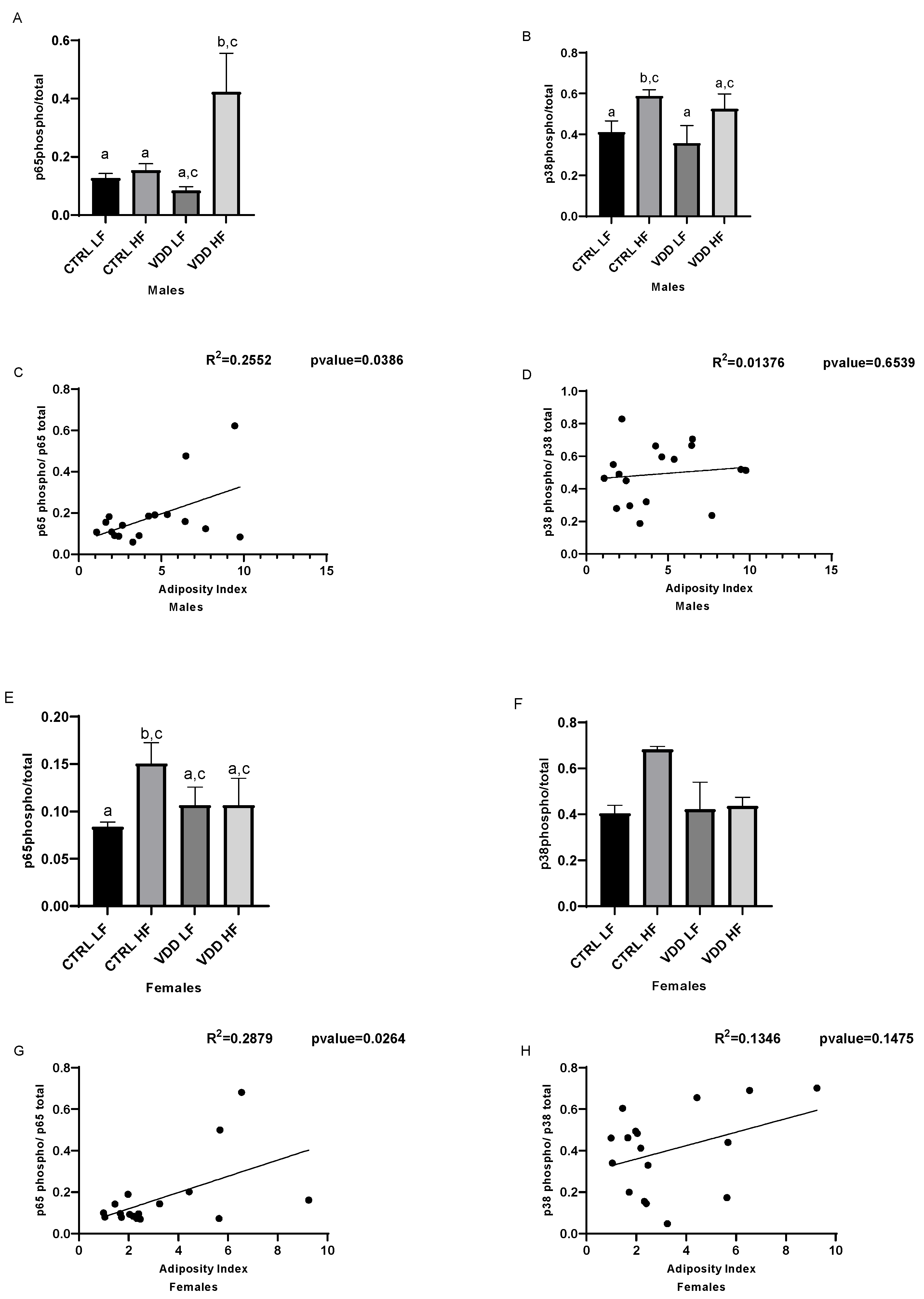
3.4. HF Diet and Maternal VDD Activate NF-kB and p38
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O.; Chen, X.; Stein, T.P. Vitamin D secondary hyperparathyroidism, and preeclampsia. Am. J. Clin. Nutr. 2013, 98, 787–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gernand, A.D.; Simhan, H.N.; Klebanoff, M.A.; Bodnar, L.M. Maternal Serum 25-Hydroxyvitamin D and Measures of Newborn and Placental Weight in a US Multicenter Cohort Study. J. Clin. Endocrinol. Metab. 2013, 98, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; Steegers, E.A.; Gaillard, R.; et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef]
- Horan, M.K.; McGowan, C.A.; Gibney, E.R.; Donnelly, J.M.; McAuliffe, F.M. The association between maternal dietary micronutrient intake and neonatal anthropometry—Secondary analysis from the ROLO study. Nutr. J. 2015, 14, 105. [Google Scholar] [CrossRef]
- Crozier, S.R.; Harvey, N.C.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Robinson, S.M.; SWS Study Group. Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: Findings from the Southampton Women’s Survey. Am. J. Clin. Nutr. 2012, 96, 57–63. [Google Scholar]
- Boyle, V.T.; Thorstensen, E.B.; Thompson, J.M.; McCowan, L.M.; Mitchell, E.A.; Godfrey, K.M.; Poston, L.; Wall, C.R.; Murphy, R.; Cutfield, W.; et al. The relationship between maternal 25-hydroxyvitamin D status in pregnancy and childhood adiposity and allergy: An observational study. Int. J. Obes. 2017, 41, 1755–1760. [Google Scholar] [CrossRef] [Green Version]
- Daraki, V.; Roumeliotaki, T.; Chalkiadaki, G.; Katrinaki, M.; Karachaliou, M.; Leventakou, V.; Vafeiadi, M.; Sarri, K.; Vassilaki, M.; Papavasiliou, S.; et al. Low maternal vitamin D status in pregnancy increases the risk of childhood obesity. Pediatr. Obes. 2018, 13, 467–475. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Veena, S.R.; Winder, N.R.; Hill, J.C.; Noonan, K.; Boucher, B.J.; Karat, S.C.; Fall, C.H. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: The Mysore Parthenon Study. Am. J. Clin. Nutr. 2011, 93, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Seipelt, E.M.; Tourniaire, F.; Couturier, C.; Astier, J.; Loriod, B.; Vachon, H.; Puceat, M.; Mounien, L.; Landrier, J.F. Prenatal maternal vitamin D deficiency sex-dependently programs adipose tissue metabolism and energy homeostasis in offspring. FASEB J. 2020, 34, 14905–14919. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hong, Q.; Wang, X.; Zhu, L.; Wu, T.; Xu, P.; Fu, Z.; You, L.; Wang, X.; Ji, C.; et al. The effect of maternal vitamin D deficiency during pregnancy on body fat and adipogenesis in rat offspring. Sci. Rep. 2018, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Reichetzeder, C.; Chen, H.; Föller, M.; Slowinski, T.; Li, J.; Chen, Y.P.; Lang, F.; Hocher, B. Maternal Vitamin D Deficiency and Fetal Programming—Lessons Learned from Humans and Mice. Kidney Blood Press. Res. 2014, 39, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.A.; Ceciliano, T.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Transgenerational effects on the liver and pancreas resulting from maternal vitamin D restriction in mice. J. Nutr. Sci. Vitaminol. 2013, 59, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Schoenrock, S.A.; Valdar, W.; Tarantino, L.M.; Ideraabdullah, F.Y. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin. Epigenet. 2016, 8, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.F. Recent insights into vitamin D, adipocyte, and adipose tissue biology. Obes. Rev. 2022, e13453. [Google Scholar] [CrossRef]
- Landrier, J.F.; Karkeni, E.; Marcotorchino, J.; Bonnet, L.; Tourniaire, F. Vitamin D modulates adipose tissue biology: Possible consequences for obesity? Proc. Nutr. Soc. 2016, 75, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Landrier, J.F.; Derghal, A.; Mounien, L. MicroRNAs in Obesity and Related Metabolic Disorders. Cells 2019, 8, 859. [Google Scholar] [CrossRef] [Green Version]
- Tourniaire, F.; Romier-Crouzet, B.; Lee, J.H.; Marcotorchino, J.; Gouranton, E.; Salles, J.; Malezet, C.; Astier, J.; Darmon, P.; Blouin, E.; et al. Chemokine Expression in Inflamed Adipose Tissue Is Mainly Mediated by NF-kappaB. PLoS ONE 2013, 8, e66515. [Google Scholar]
- Marcotorchino, J.; Gouranton, E.; Romier, B.; Tourniaire, F.; Astier, J.; Malezet, C.; Amiot, M.-J.; Landrier, J.-F. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol. Nutr. Food Res. 2012, 56, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Marcotorchino, J.; Tourniaire, F.; Astier, J.; Karkeni, E.; Canault, M.; Amiot, M.-J.; Bendahan, D.; Bernard, M.; Martin, J.-C.; Giannesini, B.; et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J. Nutr. Biochem. 2014, 25, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Marziou, A.; Philouze, C.; Couturier, C.; Astier, J.; Obert, P.; Landrier, J.-F.; Riva, C. Vitamin D Supplementation Improves Adipose Tissue Inflammation and Reduces Hepatic Steatosis in Obese C57BL/6J Mice. Nutrients 2020, 12, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marziou, A.; Aubert, B.; Couturier, C.; Astier, J.; Philouze, C.; Obert, P.; Landrier, J.-F.; Riva, C. Combined Beneficial Effect of Voluntary Physical Exercise and Vitamin D Supplementation in Diet-induced Obese C57BL/6J Mice. Med. Sci. Sports Exerc. 2021, 53, 1883–1894. [Google Scholar] [CrossRef]
- Karkeni, E.; Bonnet, L.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Ye, J.; Landrier, J.-F. Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: A new mechanism for the regulation of inflammation by vitamin D. Epigenetics 2018, 13, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Karkeni, E.; Marcotorchino, J.; Tourniaire, F.; Astier, J.; Peiretti, F.; Darmon, P.; Landrier, J.-F. Vitamin D limits chemokine expression in adipocytes and macrophage migration in vitro and in male mice. Endocrinology 2015, 156, 1782–1793. [Google Scholar] [CrossRef]
- Landrier, J.-F.; Malezet-Desmoulins, C.; Reboul, E.; Lorec, A.M.; Amiot, M.J.; Borel, P. Comparison of different vehicles to study the effect of tocopherols on gene expression in intestinal cells. Free Radic. Res. 2008, 42, 523–530. [Google Scholar] [CrossRef]
- Gouranton, E.; El Yazidi, C.; Cardinault, N.; Amiot, M.J.; Borel, P.; Landrier, J.-F. Purified low-density lipoprotein and bovine serum albumin efficiency to internalise lycopene into adipocytes. Food Chem. Toxicol. 2008, 46, 3832–3836. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Karkeni, E.; Bonnet, L.; Astier, J.; Couturier, C.; Dalifard, J.; Tourniaire, F.; Landrier, J.F. All-trans-retinoic acid represses chemokine expression in adipocytes and adipose tissue by inhibiting NF-kappaB signaling. J. Nutr. Biochem. 2017, 42, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Saraf, R.; Morton, S.M.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, L.; Bouvattier, C.; Christin-Maitre, S. Impact of intra-uterine life on future health. Ann. Endocrinol. 2022, 83, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Arner, P.; Kulyte, A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat. Rev. Endocrinol. 2015, 11, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Brichard, S.; Yi, X.; Li, Q. microRNAs as a new mechanism regulating adipose tissue inflammation in obesity and as a novel therapeutic strategy in the metabolic syndrome. J. Immunol. Res. 2014, 2014, 987285. [Google Scholar] [CrossRef] [Green Version]
- Karkeni, E.; Astier, J.; Tourniaire, F.; El Abed, M.; Romier, B.; Gouranton, E.; Wan, L.; Borel, P.; Salles, J.; Walrand, S.; et al. Obesity-associated Inflammation Induces microRNA-155 Expression in Adipocytes and Adipose Tissue: Outcome on Adipocyte Function. J. Clin. Endocrinol. Metab. 2016, 101, 1615–1626. [Google Scholar] [CrossRef]
- Roos, J.; Enlund, E.; Funcke, J.-B.; Tews, D.; Holzmann, K.; Debatin, K.-M.; Wabitsch, M.; Fischer-Posovszky, P. miR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci. Rep. 2016, 6, 38339. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Song, F.; Lu, X.; Chen, W.; Huang, C.; Li, L.; Liang, D.; Cao, S.; Dai, H. MicroRNA-322 inhibits inflammatory cytokine expression and promotes cell proliferation in LPS-stimulated murine macrophages by targeting NF-κB1 (p50). Biosci. Rep. 2017, 37, BSR20160239. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, R.; Zhou, C. Recent Advances in Understanding the Role of IKKbeta in Cardiometabolic Diseases. Front. Cardiovasc. Med. 2021, 8, 752337. [Google Scholar] [CrossRef]
- Leiva, M.; Matesanz, N.; Pulgarín-Alfaro, M.; Nikolic, I.; Sabio, G. Uncovering the Role of p38 Family Members in Adipose Tissue Physiology. Front. Endocrinol. 2020, 11, 572089. [Google Scholar] [CrossRef]
- Mutt, S.J.; Karhu, T.; Lehtonen, S.; Lehenkari, P.; Carlberg, C.; Saarnio, J.; Sebert, S.; Hyppönen, E.; Järvelin, M.; Herzig, K. Inhibition of cytokine secretion from adipocytes by 1,25-dihydroxyvitamin D(3) via the NF-kappaB pathway. FASEB J. 2012, 26, 4400–4407. [Google Scholar] [CrossRef]
- Gao, D.; Trayhurn, P.; Bing, C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int. J. Obes. 2012, 37, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorente-Cebrián, S.; Eriksson, A.; Dunlop, T.; Mejhert, N.; Dahlman, I.; Åström, G.; Sjölin, E.; Wåhlén, K.; Carlberg, C.; Laurencikiene, J.; et al. Differential effects of 1alpha,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur. J. Nutr. 2012, 51, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Raffi, F.A.M. Dual-Specificity Phosphatases in Immunity and Infection: An Update. Int. J. Mol. Sci. 2019, 20, 2710. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Total mRNA Differentially Expressed | Up-Regulated | Down-Regulated | |
|---|---|---|---|
| Males | |||
| CTRL LF vs. VDD LF | 348 | 146 | 20 |
| CTL HF vs. VDD HF | 907 | 650 | 257 |
| CTRL LF vs. CTRL HF | 1065 | 581 | 484 |
| VDD LF vs. VDD HF | 2406 | 1315 | 1091 |
| Females | |||
| CTRL LF vs. VDD LF | 1323 | 820 | 503 |
| CTL HF vs. VDD HF | 1451 | 719 | 732 |
| CTRL LF vs. CTRL HF | 2234 | 1250 | 984 |
| VDD LF vs. VDD HF | 363 | 185 | 178 |
| Total miRNA Differentially Expressed | Up-Regulated | Down-Regulated | |
|---|---|---|---|
| Males | |||
| CTRL LF vs. VDD LF | 1 | 1 | 0 |
| CTL HF vs. VDD HF | 0 | 0 | 0 |
| CTRL LF vs. CTRL HF | 0 | 0 | 0 |
| VDD LF vs. VDD HF | 5 | 0 | 5 |
| Females | |||
| CTRL LF vs. VDD LF | 4 | 4 | 0 |
| CTL HF vs. VDD HF | 1 | 0 | 1 |
| CTRL LF vs. CTRL HF | 1 | 1 | 0 |
| VDD LF vs. VDD HF | 3 | 0 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haroun, N.; Bennour, I.; Seipelt, E.; Astier, J.; Couturier, C.; Mounien, L.; Landrier, J.-F. Maternal Vitamin D Deficiency in Mice Increases White Adipose Tissue Inflammation in Offspring. Cells 2022, 11, 2024. https://doi.org/10.3390/cells11132024
Haroun N, Bennour I, Seipelt E, Astier J, Couturier C, Mounien L, Landrier J-F. Maternal Vitamin D Deficiency in Mice Increases White Adipose Tissue Inflammation in Offspring. Cells. 2022; 11(13):2024. https://doi.org/10.3390/cells11132024
Chicago/Turabian StyleHaroun, Nicole, Imene Bennour, Eva Seipelt, Julien Astier, Charlene Couturier, Lourdes Mounien, and Jean-François Landrier. 2022. "Maternal Vitamin D Deficiency in Mice Increases White Adipose Tissue Inflammation in Offspring" Cells 11, no. 13: 2024. https://doi.org/10.3390/cells11132024
APA StyleHaroun, N., Bennour, I., Seipelt, E., Astier, J., Couturier, C., Mounien, L., & Landrier, J.-F. (2022). Maternal Vitamin D Deficiency in Mice Increases White Adipose Tissue Inflammation in Offspring. Cells, 11(13), 2024. https://doi.org/10.3390/cells11132024







