Yeast Chronological Lifespan: Longevity Regulatory Genes and Mechanisms
Abstract
1. Introduction
2. The Methods
3. Mutation Rate Detection during CLS
4. Genetic Determinants of Longevity Discovered by Chronological Lifespan
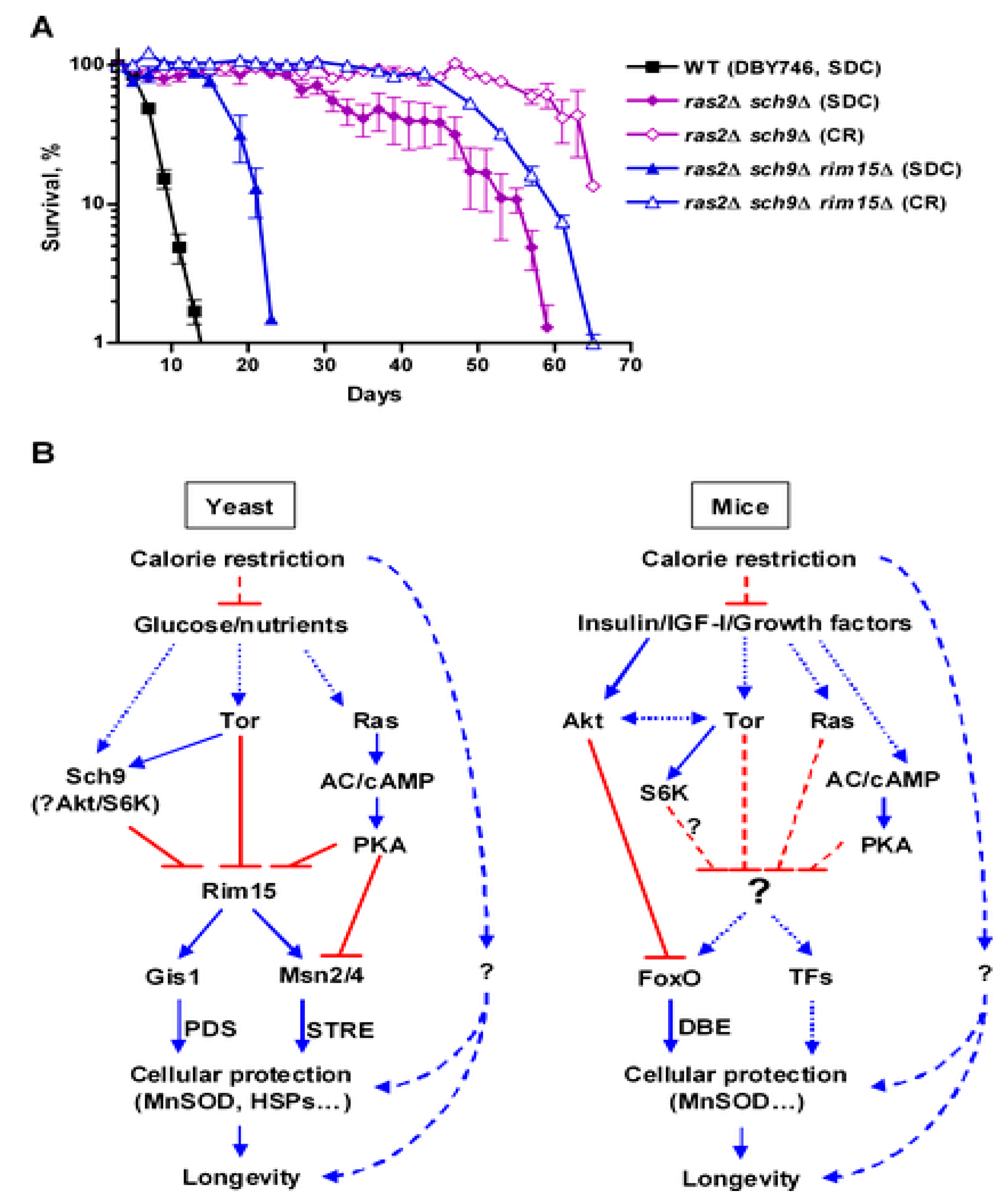
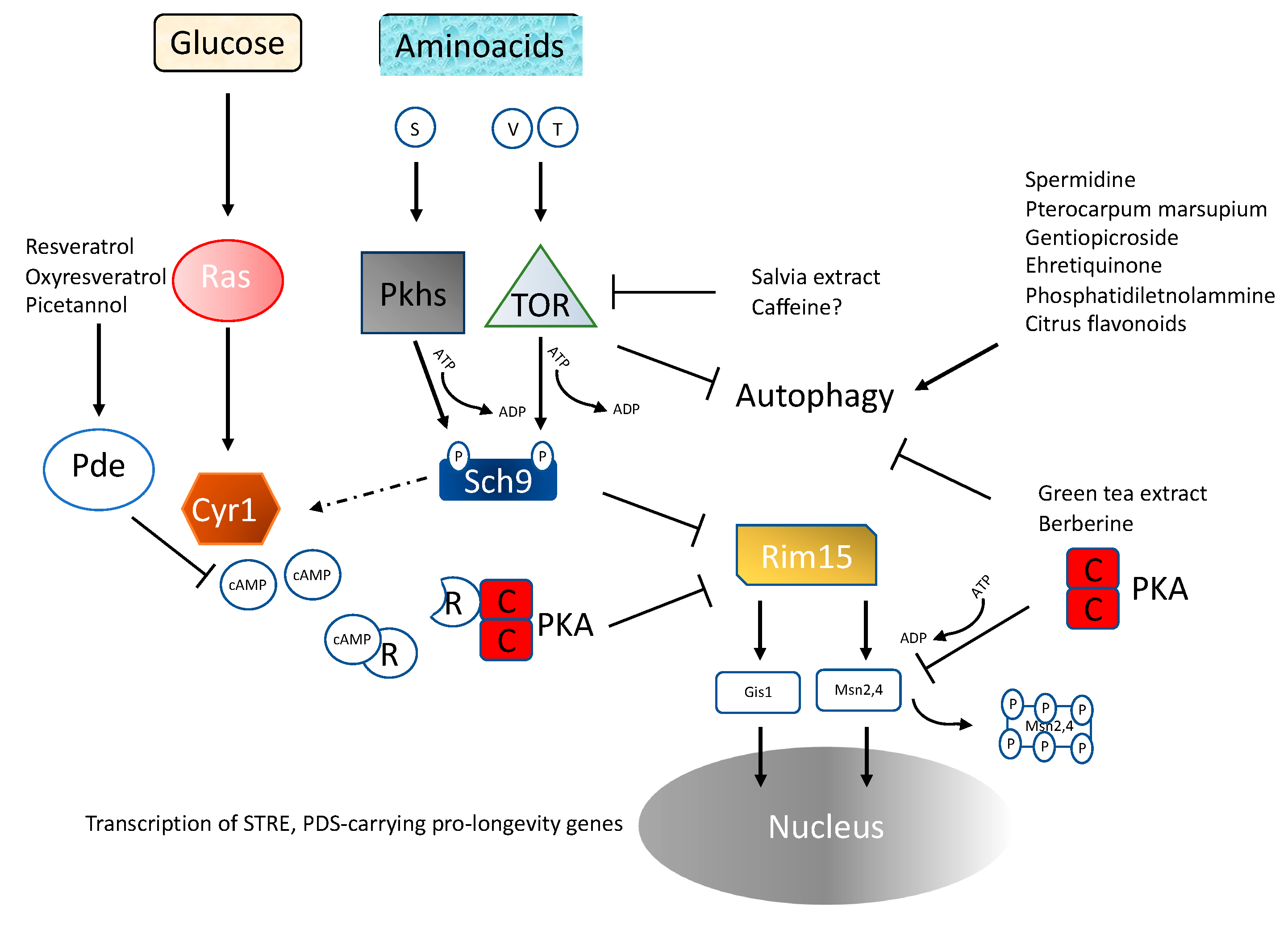
4.1. Ras/PKA Pathway
4.2. TOR/Sch9
4.3. Genomic Instability
4.4. Amino Acid Availability
4.5. Inhibition of Ras-PKA Signaling
4.6. Inhibition of TOR-Sch9 Signaling
4.7. Substances Capable of Affecting Autophagy
4.8. Oxidants Regulation
4.9. Lipid Metabolism
| Affected Pathway | Substance | Effect on CLS | Note | Reference |
|---|---|---|---|---|
| Ras-PKA | Resveratrol | ↑ | [80] | |
| Oxyresveratrol | ↑ | [80] | ||
| Picetannol | ↑ | [80] | ||
| TOR/Sch9 | Cryptotanshinone | ↑ | [82] | |
| Caffeine | ↑ | Only fission yeast | [83] | |
| Autophagy | Spermidine | ↑ | [88] | |
| Green tea | ↓ | [89] | ||
| Berberine | ↓ | [89] | ||
| Pterocarpum marsupium | ↑ | [89] | ||
| Gentiopicroside | ↑ | [90] | ||
| Ehretiquinone | ↑ | [92,103] | ||
| Phosphatidiletanolammine | ↑ | [95] | ||
| Citrus flavonoids | ↑ | [96] | ||
| Oxidant regulation | Astaxantin | ↑↓ | Dose-dependent | [108,109,110] |
| Magnolol | ↑↓ | Dose-dependent | [108,109,110] | |
| Glyceofillin | ↑↓ | Dose-dependent | [108,109,110] | |
| Pyrogallol | ↑ | [112] | ||
| Myricetin | ↑ | sod2 delta background | [112] | |
| Cocoa polyphenol | ↑ | [113] | ||
| Cucurbitacin B | ↑ | [114] | ||
| Allicin | ↑ | [105] | ||
| Lipid metabolism | PUFA | ↓ | [120] | |
| Litocholic acid | ↑ | [121,122] | ||
| Salix alba extract | ↑ | [123] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kataoka, T.; Powers, S.; Cameron, S.; Fasano, O.; Goldfarb, M.; Broach, J.; Wigler, M. Functional homology of mammalian and yeast RAS genes. Cell 1985, 40, 19–26. [Google Scholar] [CrossRef]
- Foury, F. Human genetic diseases: A cross-talk between man and yeast. Gene 1997, 195, 1–10. [Google Scholar] [CrossRef]
- Karathia, H.; Vilaprinyo, E.; Sorribas, A.; Alves, R. Saccharomyces cerevisiae as a model organism: A comparative study. PLoS ONE 2011, 6, e16015. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, R.K.; Johnston, J.R. Life span of individual yeast cells. Nature 1959, 183, 1751–1752. [Google Scholar] [CrossRef]
- Veitia, R.A. DNA Content, Cell Size and Cell Senescence. Trends Biochem. Sci. 2019, 44, 645–647. [Google Scholar] [CrossRef]
- Neurohr, G.E.; Terry, R.L.; Lengefeld, J.; Bonney, M.; Brittingham, G.P.; Moretto, F.; Miettinen, T.P.; Vaites, L.P.; Soares, L.M.; Paulo, J.A.; et al. Excessive Cell Growth Causes Cytoplasm Dilution and Contributes to Senescence. Cell 2019, 176, 1083–1097.e18. [Google Scholar] [CrossRef] [PubMed]
- Steffen, K.K.; Kennedy, B.K.; Kaeberlein, M. Measuring replicative life span in the budding yeast. J. Vis. Exp. 2009, 25, 1209. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhu, Z.; Zhang, Z.; Xu, F.; Marchisio, M.A.; Wang, Z.; Pan, D.; Zhao, X.; Huang, Q.A. Design and 3D modeling investigation of a microfluidic electrode array for electrical impedance measurement of single yeast cells. Electrophoresis 2021, 42, 1996–2009. [Google Scholar] [CrossRef]
- Longo, V.D.; Gralla, E.B.; Valentine, J.S. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J. Biol. Chem. 1996, 271, 12275–12280. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Ellerby, L.M.; Bredesen, D.E.; Valentine, J.S.; Gralla, E.B. Human Bcl-2 reverses survival defects in yeast lacking superoxide dismutase and delays death of wild-type yeast. J. Cell Biol. 1997, 137, 1581–1588. [Google Scholar] [CrossRef]
- Mirisola, M.G.; Longo, V.D. Acetic acid and acidification accelerate chronological and replicative aging in yeast. Cell Cycle 2012, 11, 3532–3533. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.V.; Petsko, G.A.; Johnston, G.C.; Ringe, D.; Singer, R.A.; Werner-Washburne, M. “Sleeping beauty”: Quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2004, 68, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, P.; Gattazzo, C.; Battistella, L.; Wei, M.; Cheng, C.; McGrew, K.; Longo, V.D. Sir2 blocks extreme life-span extension. Cell 2005, 123, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, P.; Battistella, L.; Vardavas, R.; Gattazzo, C.; Liou, L.L.; Diaspro, A.; Dossen, J.W.; Gralla, E.B.; Longo, V.D. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J. Cell Biol. 2004, 166, 1055–1067. [Google Scholar] [CrossRef]
- Herker, E.; Jungwirth, H.; Lehmann, K.A.; Maldener, C.; Fröhlich, K.U.; Wissing, S.; Büttner, S.; Fehr, M.; Sigrist, S.; Madeo, F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004, 164, 501–507. [Google Scholar] [CrossRef]
- Gulli, J.; Cook, E.; Kroll, E.; Rosebrock, A.; Caudy, A.; Rosenzweig, F. Diverse conditions support near-zero growth in yeast: Implications for the study of cell lifespan. Microb. Cell 2019, 6, 397–413. [Google Scholar] [CrossRef]
- Fabrizio, P.; Liou, L.L.; Moy, V.N.; Diaspro, A.; Valentine, J.S.; Gralla, E.B.; Longo, V.D. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics 2003, 163, 35–46. [Google Scholar] [CrossRef]
- Burtner, C.R.; Murakami, C.J.; Kennedy, B.K.; Kaeberlein, M. A molecular mechanism of chronological aging in yeast. Cell Cycle 2009, 8, 1256–1270. [Google Scholar] [CrossRef]
- Orlandi, I.; Casatta, N.; Vai, M. Lack of Ach1 CoA-Transferase Triggers Apoptosis and Decreases Chronological Lifespan in Yeast. Front. Oncol. 2012, 2, 67. [Google Scholar] [CrossRef]
- Orlandi, I.; Ronzulli, R.; Casatta, N.; Vai, M. Ethanol and acetate acting as carbon/energy sources negatively affect yeast chronological aging. Oxid Med. Cell Longev. 2013, 2013, 802870. [Google Scholar] [CrossRef]
- Kitanovic, A.; Bonowski, F.; Heigwer, F.; Ruoff, P.; Kitanovic, I.; Ungewiss, C.; Wölfl, S. Acetic acid treatment in S. cerevisiae creates significant energy deficiency and nutrient starvation that is dependent on the activity of the mitochondrial transcriptional complex Hap2-3-4-5. Front. Oncol. 2012, 2, 118. [Google Scholar] [CrossRef] [PubMed]
- Aung-Htut, M.T.; Lam, Y.T.; Lim, Y.L.; Rinnerthaler, M.; Gelling, C.L.; Yang, H.; Breitenbach, M.; Dawes, I.W. Maintenance of mitochondrial morphology by autophagy and its role in high glucose effects on chronological lifespan of Saccharomyces cerevisiae. Oxid Med. Cell Longev. 2013, 2013, 636287. [Google Scholar] [CrossRef] [PubMed]
- Wasko, B.M.; Carr, D.T.; Tung, H.; Doan, H.; Schurman, N.; Neault, J.R.; Feng, J.; Lee, J.; Zipkin, B.; Mouser, J.; et al. Buffering the pH of the culture medium does not extend yeast replicative lifespan. F1000Research 2013, 2, 216. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Umezu, K.; Kolodner, R.D. Chromosomal rearrangements occur in S. cerevisiae rfa1 mutator mutants due to mutagenic lesions processed by double-strand-break repair. Mol. Cell 1998, 2, 9–22. [Google Scholar] [CrossRef]
- Guthrie, C.; Fink, G. Guide to Yeast Genetics and Molecular Biology; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Capizzi, R.L.; Jameson, J.W. A table for the estimation of the spontaneous mutation rate of cells in culture. Mutat. Res. 1973, 17, 147–148. [Google Scholar] [CrossRef]
- Chen, C.; Kolodner, R.D. Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat. Genet. 1999, 23, 81–85. [Google Scholar] [CrossRef]
- Longo, V.D. Mutations in signal transduction proteins increase stress resistance and longevity in yeast, nematodes, fruit flies and mammalian neuronal cells. Neurobiol. Aging 1999, 20, 479–486. [Google Scholar] [CrossRef]
- Fabrizio, P.; Pozza, F.; Pletcher, S.D.; Gendron, C.M.; Longo, V.D. Regulation of longevity and stress resistance by Sch9 in yeast. Science 2001, 292, 288–290. [Google Scholar] [CrossRef]
- de Magalhães, J.P. Paternal genome effects on aging: Evidence for a role of Rasgrf1 in longevity determination? Mech. Ageing Dev. 2011, 132, 72–73. [Google Scholar] [CrossRef]
- Mirisola, M.G.; Longo, V.D. Conserved role of Ras-GEFs in promoting aging: From yeast to mice. Aging 2011, 3, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Mirisola, M.G.; Seidita, G.; Verrotti, A.C.; Di Blasi, F.; Fasano, O. Mutagenic alteration of the distal switch II region of RAS blocks CDC25-dependent signaling functions. J. Biol. Chem. 1994, 269, 15740–15748. [Google Scholar] [CrossRef]
- Wei, M.; Fabrizio, P.; Hu, J.; Ge, H.; Cheng, C.; Li, L.; Longo, V.D. Life span extension by calorie restriction depends on Rim15 and transcription factors downstream of Ras/PKA, Tor and Sch9. PLoS Genet. 2008, 4, e13. [Google Scholar] [CrossRef] [PubMed]
- Mirisola, M.G.; Taormina, G.; Fabrizio, P.; Wei, M.; Hu, J.; Longo, V.D. Serine- and threonine/valine-dependent activation of PDK and Tor orthologs converge on Sch9 to promote aging. PLoS Genet. 2014, 10, e1004113. [Google Scholar] [CrossRef] [PubMed]
- Görner, W.; Durchschlag, E.; Martinez-Pastor, M.T.; Estruch, F.; Ammerer, G.; Hamilton, B.; Ruis, H.; Schüller, C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998, 12, 586–597. [Google Scholar] [CrossRef]
- Zaman, S.; Lippman, S.I.; Schneper, L.; Slonim, N.; Broach, J.R. Glucose regulates transcription in yeast through a network of signaling pathways. Mol. Syst. Biol. 2009, 5, 245, Erratum in Mol. Syst. Biol. 2009, 5, 257. [Google Scholar] [CrossRef]
- Taormina, G.; Mirisola, M.G. Longevity: Epigenetic and biomolecular aspects. Biomol. Concepts 2015, 6, 105–117. [Google Scholar] [CrossRef]
- Yu, R.; Cao, X.; Sun, L.; Zhu, J.Y.; Wasko, B.M.; Liu, W.; Crutcher, E.; Liu, H.; Jo, M.C.; Qin, L.; et al. Inactivating histone deacetylase HDA promotes longevity by mobilizing trehalose metabolism. Nat. Commun. 2021, 12, 1981. [Google Scholar] [CrossRef]
- Hanasaki, M.; Yaku, K.; Yamauchi, M.; Nakagawa, T.; Masumoto, H. Deletion of the GAPDH gene contributes to genome stability in Saccharomyces cerevisiae. Sci. Rep. 2020, 10, 21146. [Google Scholar] [CrossRef]
- Urban, J.; Soulard, A.; Huber, A.; Lippman, S.; Mukhopadhyay, D.; Deloche, O.; Wanke, V.; Anrather, D.; Ammerer, G.; Riezman, H.; et al. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol. Cell 2007, 26, 663–674. [Google Scholar] [CrossRef]
- Kapahi, P.; Zid, B.M.; Harper, T.; Koslover, D.; Sapin, V.; Benzer, S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004, 14, 885–890. [Google Scholar] [CrossRef]
- Polak, P.; Hall, M.N. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 2009, 21, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, M.; Powers, R.W., 3rd; Steffen, K.K.; Westman, E.A.; Hu, D.; Dang, N.; Kerr, E.O.; Kirkland, K.T.; Fields, S.; Kennedy, B.K. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 2005, 310, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Powers, R.W., 3rd; Kaeberlein, M.; Caldwell, S.D.; Kennedy, B.K.; Fields, S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006, 20, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Bonawitz, N.D.; Chatenay-Lapointe, M.; Pan, Y.; Shadel, G.S. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab. 2007, 5, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Kupiec, M.; Weisman, R. TOR links starvation responses to telomere length maintenance. Cell Cycle 2012, 11, 2268–2271. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Cabral, W.A.; Tavarez, U.L.; Beeram, I.; Yeritsyan, D.; Boku, Y.D.; Eckhaus, M.A.; Nazarian, A.; Erdos, M.R.; Collins, F.S. Genetic reduction of mTOR extends lifespan in a mouse model of Hutchinson-Gilford Progeria syndrome. Aging Cell 2021, 20, e13457. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Fabrizio, P. Chronological aging in Saccharomyces cerevisiae. Aging Res. Yeast 2012, 57, 101–121. [Google Scholar] [CrossRef]
- Noguchi, C.; Wang, L.; Shetty, M.; Mell, J.C.; Sell, C.; Noguchi, E. Maf1 limits RNA polymerase III-directed transcription to preserve genomic integrity and extend lifespan. Cell Cycle 2021, 20, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Carolina de Souza-Guerreiro, T.; Meng, X.; Dacheux, E.; Firczuk, H.; McCarthy, J. Translational control of gene expression noise and its relationship to ageing in yeast. FEBS J. 2021, 288, 2278–2293. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Ahn, H.; Duan, R.; Liu, Y.; Ryu, H.Y.; Ahn, S.H. The Spt7 subunit of the SAGA complex is required for the regulation of lifespan in both dividing and nondividing yeast cells. Mech. Ageing Dev. 2021, 196, 111480. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Lorente, M.A.; Cano-Martin, A.C.; Blasco, M.A. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 2019, 10, 4723. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, K.; Vera, E.; Martínez-Nevado, E.; Sanpera, C.; Blasco, M.A. Telomere shortening rate predicts species life span. Proc. Natl. Acad. Sci. USA 2019, 116, 15122–15127. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ong, E.B.B. Genomic Instability and Cellular Senescence: Lessons from the Budding Yeast. Front. Cell Dev. Biol. 2021, 8, 619126. [Google Scholar] [CrossRef]
- Harkness, T.A.; Shea, K.A.; Legrand, C.; Brahmania, M.; Davies, G.F. A functional analysis reveals dependence on the anaphase-promoting complex for prolonged life span in yeast. Genetics 2004, 168, 759–774. [Google Scholar] [CrossRef]
- Vasileva, B.; Staneva, D.; Krasteva, N.; Miloshev, G.; Georgieva, M. Changes in Chromatin Organization Eradicate Cellular Stress Resilience to UVA/B Light and Induce Premature Aging. Cells 2021, 10, 1755. [Google Scholar] [CrossRef]
- Maxwell, P.H. Growth conditions that increase or decrease lifespan in Saccharomyces cerevisiae lead to corresponding decreases or increases in rates of interstitial deletions and non-reciprocal translocations. BMC Genet. 2016, 17, 140. [Google Scholar] [CrossRef]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Wang, Z.; Liu, J.P. Roles of Telomere Biology in Cell Senescence, Replicative and Chronological Ageing. Cells 2019, 8, 54. [Google Scholar] [CrossRef]
- 5Neil, A.J.; Hisey, J.A.; Quasem, I.; McGinty, R.J.; Hitczenko, M.; Khristich, A.N.; Mirkin, S.M. Replication-independent instability of Friedreich’s ataxia GAA repeats during chronological aging. Proc. Natl. Acad. Sci. USA 2021, 118, e2013080118. [Google Scholar] [CrossRef]
- Kwan, E.X.; Foss, E.; Kruglyak, L.; Bedalov, A. Natural polymorphism in BUL2 links cellular amino acid availability with chronological aging and telomere maintenance in yeast. PLoS Genet. 2011, 7, e1002250. [Google Scholar] [CrossRef] [PubMed]
- Czachor, J.; Miłek, M.; Galiniak, S.; Stępień, K.; Dżugan, M.; Mołoń, M. Coffee Extends Yeast Chronological Lifespan through Antioxidant Properties. Int. J. Mol. Sci. 2020, 21, 9510. [Google Scholar] [CrossRef] [PubMed]
- Sudharshan, S.J.; Dyavaiah, M. Astaxanthin protects oxidative stress mediated DNA damage and enhances longevity in Saccharomyces cerevisiae. Biogerontology 2021, 22, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Richie, J.P., Jr.; Leutzinger, Y.; Parthasarathy, S.; Malloy, V.; Orentreich, N.; Zimmerman, J.A. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994, 8, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; McMahon, A.C.; Ballard, J.W.; Ruohonen, K.; Wu, L.E.; Cogger, V.C.; Warren, A.; Huang, X.; Pichaud, N.; Melvin, R.G.; et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging and longevity in ad libitum-fed mice. Cell Metab. 2014, 19, 418–430. [Google Scholar] [CrossRef]
- Piper, M.D.; Partridge, L.; Raubenheimer, D.; Simpson, S.J. Dietary restriction and aging: A unifying perspective. Cell Metab. 2011, 14, 154–160. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.Q.; Huang, D. Dietary restriction depends on nutrient composition to extend chronological lifespan in budding yeast Saccharomyces cerevisiae. PLoS ONE 2013, 8, e64448. [Google Scholar] [CrossRef]
- Cangemi, A.; Fanale, D.; Rinaldi, G.; Bazan, V.; Galvano, A.; Perez, A.; Barraco, N.; Massihnia, D.; Castiglia, M.; Vieni, S.; et al. Dietary restriction: Could it be considered as speed bump on tumor progression road? Tumour Biol. 2016, 37, 7109–7118. [Google Scholar] [CrossRef]
- Diaz-Ruiz, A.; Rhinesmith, T.; Pomatto-Watson, L.C.D.; Price, N.L.; Eshaghi, F.; Ehrlich, M.R.; Moats, J.M.; Carpenter, M.; Rudderow, A.; Brandhorst, S.; et al. Diet composition influences the metabolic benefits of short cycles of very low caloric intake. Nat. Commun. 2021, 12, 6463. [Google Scholar] [CrossRef]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Wu, Z.; Song, L.; Liu, S.Q.; Huang, D. Independent and additive effects of glutamic acid and methionine on yeast longevity. PLoS ONE 2013, 8, e79319. [Google Scholar] [CrossRef]
- Nishimura, A.; Yoshikawa, Y.; Ichikawa, K.; Takemoto, T.; Tanahashi, R.; Takagi, H. Longevity Regulation by Proline Oxidation in Yeast. Microorganisms 2021, 9, 1650. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Hesles, E.; Smith, D.L., Jr.; Maqani, N.; Wierman, M.B.; Sutcliffe, M.D.; Fine, R.D.; Kalita, A.; Santos, S.M.; Muehlbauer, M.J.; Bain, J.R.; et al. A cell-nonautonomous mechanism of yeast chronological aging regulated by caloric restriction and one-carbon metabolism. J. Biol. Chem. 2021, 296, 100125. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, H.; Kato, T.; Sato, T.; Shimasaki, T.; Kojima, T.; Aiba, H. Leucine depletion extends the lifespans of leucine-auxotrophic fission yeast by inducing Ecl1 family genes via the transcription factor Fil1. Mol. Genet. Genom. 2019, 294, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Peeters, K.; Van Leemputte, F.; Fischer, B.; Bonini, B.M.; Quezada, H.; Tsytlonok, M.; Haesen, D.; Vanthienen, W.; Bernardes, N.; Gonzalez-Blas, C.B.; et al. Fructose-1,6-bisphosphate couples glycolytic flux to activation of Ras. Nat. Commun. 2017, 8, 922. [Google Scholar] [CrossRef]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.; Giannopoulou, E.G.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef]
- Flaig, T.W.; Gustafson, D.L.; Su, L.J.; Zirrolli, J.A.; Crighton, F.; Harrison, G.S.; Pierson, A.S.; Agarwal, R.; Glodé, L.M. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investig. New Drugs 2007, 25, 139–146. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shi, R.; Witt, S.N. The small molecule triclabendazole decreases the intracellular level of cyclic AMP and increases resistance to stress in Saccharomyces cerevisiae. PLoS ONE 2013, 8, e64337. [Google Scholar] [CrossRef]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Characterization of Resveratrol, Oxyresveratrol, Piceatannol and Roflumilast as Modulators of Phosphodiesterase Activity. Study of Yeast Lifespan. Pharmaceuticals 2020, 13, 225. [Google Scholar] [CrossRef]
- Aiello, A.; Accardi, G.; Candore, G.; Gambino, C.M.; Mirisola, M.; Taormina, G.; Virruso, C.; Caruso, C. Nutrient sensing pathways as therapeutic targets for healthy ageing. Expert Opin. Ther. Targets 2017, 21, 371–380. [Google Scholar] [CrossRef]
- Wu, Z.; Song, L.; Liu, S.Q.; Huang, D. Tanshinones extend chronological lifespan in budding yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2014, 98, 8617–8628. [Google Scholar] [CrossRef] [PubMed]
- Rallis, C.; Codlin, S.; Bähler, J. TORC1 signaling inhibition by rapamycin and caffeine affect lifespan, global gene expression and cell proliferation of fission yeast. Aging Cell 2013, 12, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, P.; Hoon, S.; Shamalnasab, M.; Galbani, A.; Wei, M.; Giaever, G.; Nislow, C.; Longo, V.D. Genome-wide screen in Saccharomyces cerevisiae identifies vacuolar protein sorting, autophagy, biosynthetic and tRNA methylation genes involved in life span regulation. PLoS Genet. 2010, 6, e1001024. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Clark, W.; Luo, G.; Wang, X.; Fu, Y.; Wei, J.; Wang, X.; Hao, Z.; Dai, Q.; Zheng, G.; et al. ALKBH1-Mediated tRNA Demethylation Regulates Translation. Cell 2016, 167, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Mohler, K.; Mann, R.; Kyle, A.; Reynolds, N.; Ibba, M. Aminoacyl-tRNA quality control is required for efficient activation of the TOR pathway regulator Gln3p. RNA Biol. 2018, 15, 594–603. [Google Scholar] [CrossRef]
- Alugoju, P.; Janardhanshetty, S.S.; Subaramanian, S.; Periyasamy, L.; Dyavaiah, M. Quercetin Protects Yeast Saccharomyces cerevisiae pep4 Mutant from Oxidative and Apoptotic Stress and Extends Chronological Lifespan. Curr. Microbiol. 2018, 75, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Lee, M.B.; Kiflezghi, M.G.; Tsuchiya, M.; Wasko, B.; Carr, D.T.; Uppal, P.A.; Grayden, K.A.; Elala, Y.C.; Nguyen, T.A.; Wang, J.; et al. Pterocarpus marsupium extract extends replicative lifespan in budding yeast. Geroscience 2021, 43, 2595–2609. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, L.; Matsuura, A.; Xiang, L.; Qi, J. Gentiopicroside, a Secoiridoid Glycoside from Gentiana rigescens Franch, Extends the Lifespan of Yeast via Inducing Mitophagy and Antioxidative Stress. Oxid Med. Cell Longev. 2020, 2020, 9125752. [Google Scholar] [CrossRef]
- Liao, P.C.; Wolken, D.M.A.; Serrano, E.; Srivastava, P.; Pon, L.A. Mitochondria-Associated Degradation Pathway (MAD) Function beyond the Outer Membrane. Cell Rep. 2020, 32, 107902. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Liu, Y.; Disasa, D.; Akira, M.; Xiang, L.; Qi, J. A New Geniposidic Acid Derivative Exerts Antiaging Effects through Antioxidative Stress and Autophagy Induction. Antioxidants 2021, 10, 987. [Google Scholar] [CrossRef] [PubMed]
- Plummer, J.D.; Johnson, J.E. Extension of Cellular Lifespan by Methionine Restriction Involves Alterations in Central Carbon Metabolism and Is Mitophagy-Dependent. Front. Cell Dev. Biol. 2019, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Ruckenstuhl, C.; Netzberger, C.; Entfellner, I.; Carmona-Gutierrez, D.; Kickenweiz, T.; Stekovic, S.; Gleixner, C.; Schmid, C.; Klug, L.; Hajnal, I.; et al. Autophagy extends lifespan via vacuolar acidification. Microb. Cell 2014, 1, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Rockenfeller, P.; Koska, M.; Pietrocola, F.; Minois, N.; Knittelfelder, O.; Sica, V.; Franz, J.; Carmona-Gutierrez, D.; Kroemer, G.; Madeo, F. Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ. 2015, 22, 499–508. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, H.; Guan, X.; Zhou, Z. The Anti-Aging Potential of Neohesperidin and Its Synergistic Effects with Other Citrus Flavonoids in Extending Chronological Lifespan of Saccharomyces Cerevisiae BY4742. Molecules 2019, 24, 4093. [Google Scholar] [CrossRef]
- Alvers, A.L.; Fishwick, L.K.; Wood, M.S.; Hu, D.; Chung, H.S.; Dunn, W.A., Jr.; Aris, J.P. Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae. Aging Cell 2009, 8, 353–369. [Google Scholar] [CrossRef]
- Alvers, A.L.; Wood, M.S.; Hu, D.; Kaywell, A.C.; Dunn, W.A., Jr.; Aris, J.P. Autophagy is required for extension of yeast chronological life span by rapamycin. Autophagy 2009, 5, 847–849. [Google Scholar] [CrossRef]
- Takeda, K.; Yanagida, M. In quiescence of fission yeast, autophagy and the proteasome collaborate for mitochondrial maintenance and longevity. Autophagy 2010, 6, 564–565. [Google Scholar] [CrossRef]
- Longo, V.D.; Nislow, C.; Fabrizio, P. Endosomal protein sorting and autophagy genes contribute to the regulation of yeast life span. Autophagy 2010, 6, 1227–1228. [Google Scholar] [CrossRef]
- Heo, J.M.; Livnat-Levanon, N.; Taylor, E.B.; Jones, K.T.; Dephoure, N.; Ring, J.; Xie, J.; Brodsky, J.L.; Madeo, F.; Gygi, S.P.; et al. A stress-responsive system for mitochondrial protein degradation. Mol. Cell 2010, 40, 465–480. [Google Scholar] [CrossRef]
- Chen, L.B.; Ma, S.; Jiang, T.X.; Qiu, X.B. Transcriptional upregulation of proteasome activator Blm10 antagonizes cellular aging. Biochem. Biophys. Res. Commun. 2020, 532, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Fujii, R.; Farooq, U.; Cheng, L.; Matsuura, A.; Qi, J.; Xiang, L. Ehretiquinone from Onosma bracteatum Wall Exhibits Antiaging Effect on Yeasts and Mammals through Antioxidative Stress and Autophagy Induction. Oxid Med. Cell Longev. 2021, 2021, 5469849. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Fröhlich, E.; Fröhlich, K.U. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 1997, 139, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Mirisola, M.G.; Longo, V.D. A radical signal activates the epigenetic regulation of longevity. Cell Metab. 2013, 17, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Abid, M.; Elamrani, A.; Drouet, S.; Addi, M.; Hano, C. Almond Skin Extracts and Chlorogenic Acid Delay Chronological Aging and Enhanced Oxidative Stress Response in Yeast. Life 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, S.; Alugoju, P.; Sj, S.; Veerabhadrappa, B.; Dyavaiah, M. Magnolol protects Saccharomyces cerevisiae antioxidant-deficient mutants from oxidative stress and extends yeast chronological life span. FEMS Microbiol. Lett. 2019, 366, fnz065. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Z.; Feng, S.; Yang, X.; Huang, D. Hormesis of glyceollin I, an induced phytoalexin from soybean, on budding yeast chronological lifespan extension. Molecules 2014, 19, 568–580. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Burdyliuk, N.I.; Izers’ka, L.I.; Lushchak, V.I. Concentration-Dependent Effects of Rhodiola Rosea on Long-Term Survival and Stress Resistance of Yeast Saccharomyces Cerevisiae: The Involvement of YAP 1 and MSN2/4 Regulatory Proteins. Dose Response 2013, 12, 93–109. [Google Scholar] [CrossRef]
- Ross, E.M.; Maxwell, P.H. Low doses of DNA damaging agents extend Saccharomyces cerevisiae chronological lifespan by promoting entry into quiescence. Exp. Gerontol. 2018, 108, 189–200. [Google Scholar] [CrossRef]
- Mendes, V.; Vilaça, R.; de Freitas, V.; Ferreira, P.M.; Mateus, N.; Costa, V. Effect of myricetin, pyrogallol and phloroglucinol on yeast resistance to oxidative stress. Oxid Med. Cell Longev. 2015, 2015, 782504. [Google Scholar] [CrossRef] [PubMed]
- Baiges, I.; Arola, L. COCOA (Theobroma cacao) Polyphenol-Rich Extract Increases the Chronological Lifespan of Saccharomyces cerevisiae. J. Frailty Aging 2016, 5, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kotakeyama, Y.; Li, J.; Pan, Y.; Matsuura, A.; Ohya, Y.; Yoshida, M.; Xiang, L.; Qi, J. Cucurbitacin B Exerts Antiaging Effects in Yeast by Regulating Autophagy and Oxidative Stress. Oxid Med. Cell Longev. 2019, 2019, 4517091. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Schlembach, I.; Leontiev, R.; Uebachs, A.; Gollwitzer, P.U.G.; Weiss, A.; Delaunay, A.; Toledano, M.; Slusarenko, A.J. Yap1p, the central regulator of the S. cerevisiae oxidative stress response, is activated by allicin, a natural oxidant and defence substance of garlic. Free Radic. Biol. Med. 2017, 108, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Aerts, A.M.; François, I.E.; Bammens, L.; Cammue, B.P.; Smets, B.; Winderickx, J.; Accardo, S.; De Vos, D.E.; Thevissen, K. Level of M(IP)2C sphingolipid affects plant defensin sensitivity, oxidative stress resistance and chronological life-span in yeast. FEBS Lett. 2006, 580, 1903–1907. [Google Scholar] [CrossRef]
- Vilaça, R.; Silva, E.; Nadais, A.; Teixeira, V.; Matmati, N.; Gaifem, J.; Hannun, Y.A.; Sá Miranda, M.C.; Costa, V. Sphingolipid signalling mediates mitochondrial dysfunctions and reduced chronological lifespan in the yeast model of Niemann-Pick type C1. Mol. Microbiol. 2014, 91, 438–451. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Pereira, C.; Osório, H.; Moradas-Ferreira, P.; Costa, V. The ceramide-activated protein phosphatase Sit4p controls lifespan, mitochondrial function and cell cycle progression by regulating hexokinase 2 phosphorylation. Cell Cycle 2016, 15, 1620–1630. [Google Scholar] [CrossRef][Green Version]
- Vilaça, R.; Barros, I.; Matmati, N.; Silva, E.; Martins, T.; Teixeira, V.; Hannun, Y.A.; Costa, V. The ceramide activated protein phosphatase Sit4 impairs sphingolipid dynamics, mitochondrial function and lifespan in a yeast model of Niemann-Pick type C1. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 79–88. [Google Scholar] [CrossRef]
- Aguilar-Toral, R.; Fernández-Quintero, M.; Ortiz-Avila, O.; de la Paz, L.H.; Calderón-Cortés, E.; Rodríguez-Orozco, A.R.; Saavedra-Molina, A.; Calderón-Torres, M.; Cortés-Rojo, C. Characterization of the effects of a polyunsaturated fatty acid (PUFA) on mitochondrial bioenergetics of chronologically aged yeast. J. Bioenerg. Biomembr. 2014, 46, 205–220. [Google Scholar] [CrossRef]
- Beach, A.; Richard, V.R.; Leonov, A.; Burstein, M.T.; Bourque, S.D.; Koupaki, O.; Juneau, M.; Feldman, R.; Iouk, T.; Titorenko, V.I. Mitochondrial membrane lipidome defines yeast longevity. Aging 2013, 5, 551–574. [Google Scholar] [CrossRef]
- Burstein, M.T.; Titorenko, V.I. A mitochondrially targeted compound delays aging in yeast through a mechanism linking mitochondrial membrane lipid metabolism to mitochondrial redox biology. Redox Biol. 2014, 2, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Medkour, Y.; Mohammad, K.; Arlia-Ciommo, A.; Svistkova, V.; Dakik, P.; Mitrofanova, D.; Rodriguez, M.E.L.; Junio, J.A.B.; Taifour, T.; Escudero, P.; et al. Mechanisms by which PE21, an extract from the white willow Salix alba, delays chronological aging in budding yeast. Oncotarget 2019, 10, 5780–5816. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirisola, M.G.; Longo, V.D. Yeast Chronological Lifespan: Longevity Regulatory Genes and Mechanisms. Cells 2022, 11, 1714. https://doi.org/10.3390/cells11101714
Mirisola MG, Longo VD. Yeast Chronological Lifespan: Longevity Regulatory Genes and Mechanisms. Cells. 2022; 11(10):1714. https://doi.org/10.3390/cells11101714
Chicago/Turabian StyleMirisola, Mario G., and Valter D. Longo. 2022. "Yeast Chronological Lifespan: Longevity Regulatory Genes and Mechanisms" Cells 11, no. 10: 1714. https://doi.org/10.3390/cells11101714
APA StyleMirisola, M. G., & Longo, V. D. (2022). Yeast Chronological Lifespan: Longevity Regulatory Genes and Mechanisms. Cells, 11(10), 1714. https://doi.org/10.3390/cells11101714






