Muscle-Related Plectinopathies
Abstract
1. Introduction
2. Human Plectinopathies
3. Clinical Phenotypes and Muscle-Related Disease Manifestations of Human Plectinopathies
3.1. EBS-MD, the Most Common Muscle-Related Plectinopathy
3.2. Other Skeletal Muscle-Associated Plectinopathy Disease Manifestations
3.3. Emerging Cardiac Pathologies in Plectinopathy Patients
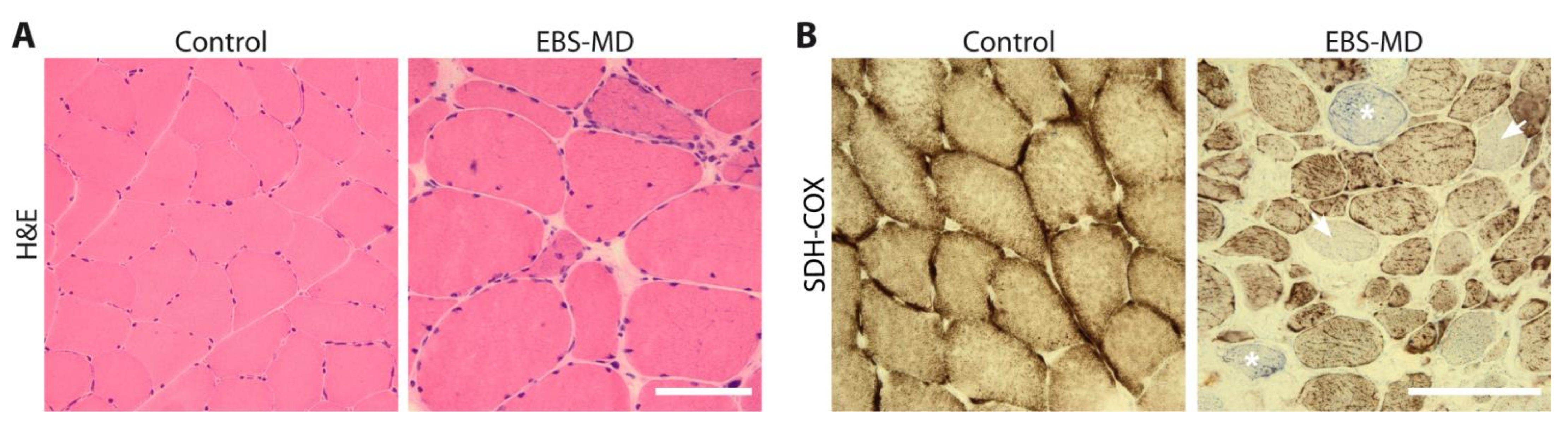
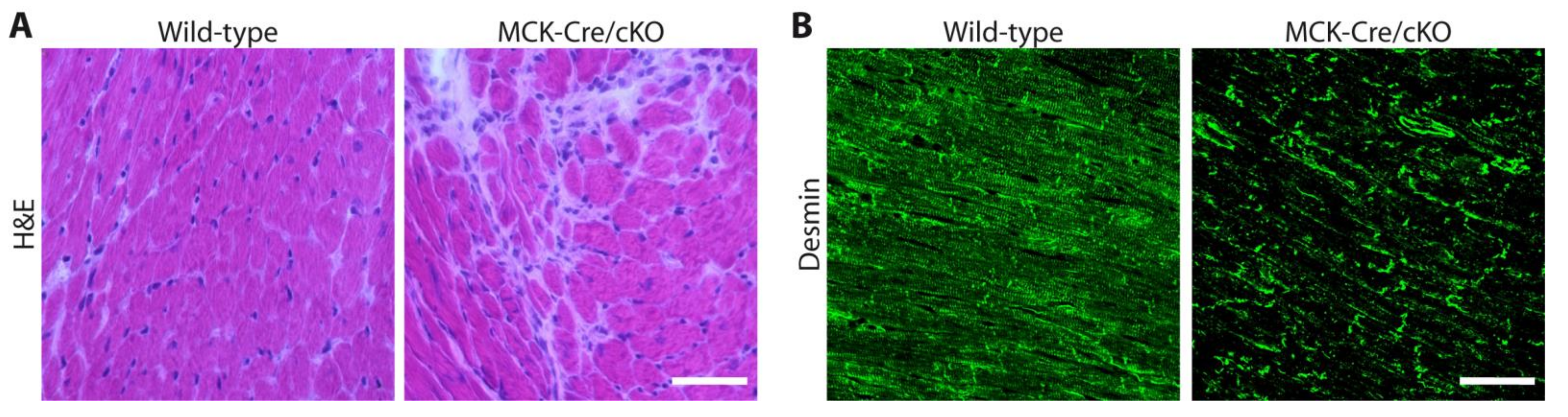
4. Skeletal Muscle Biopsy Findings
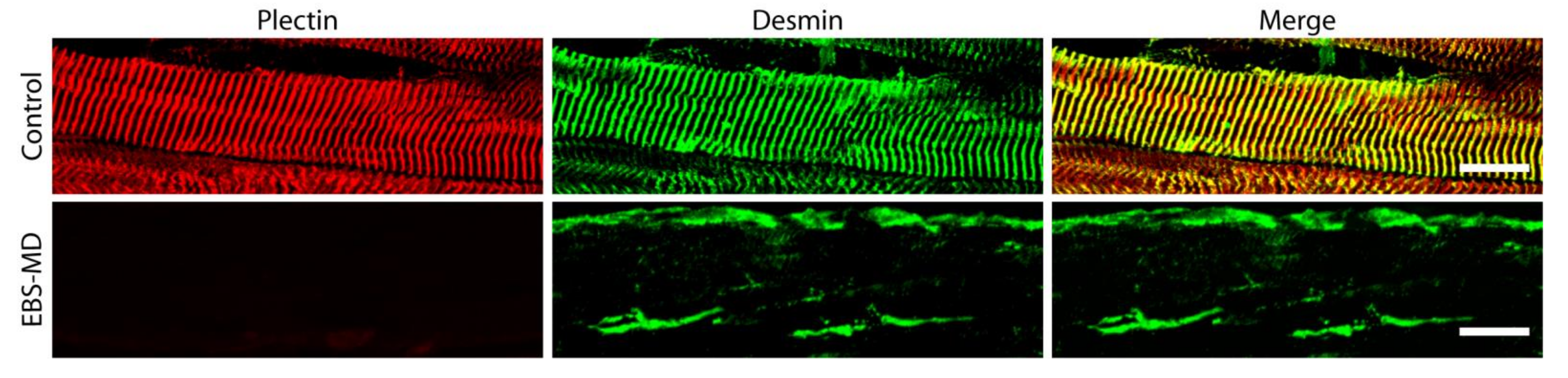
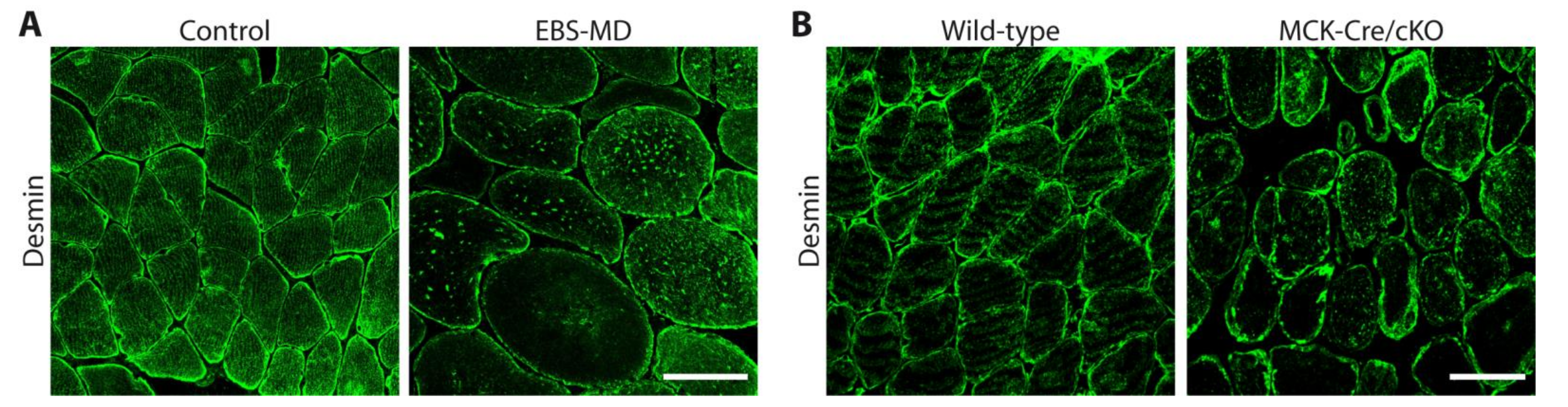
5. Muscle-Related Molecular Pathology
5.1. Downstream Effects of Human PLEC Mutations on Skeletal Muscle Organization
5.2. Muscle-Associated Molecular Pathomechanisms in Plectinopathy Animal and Cell Models
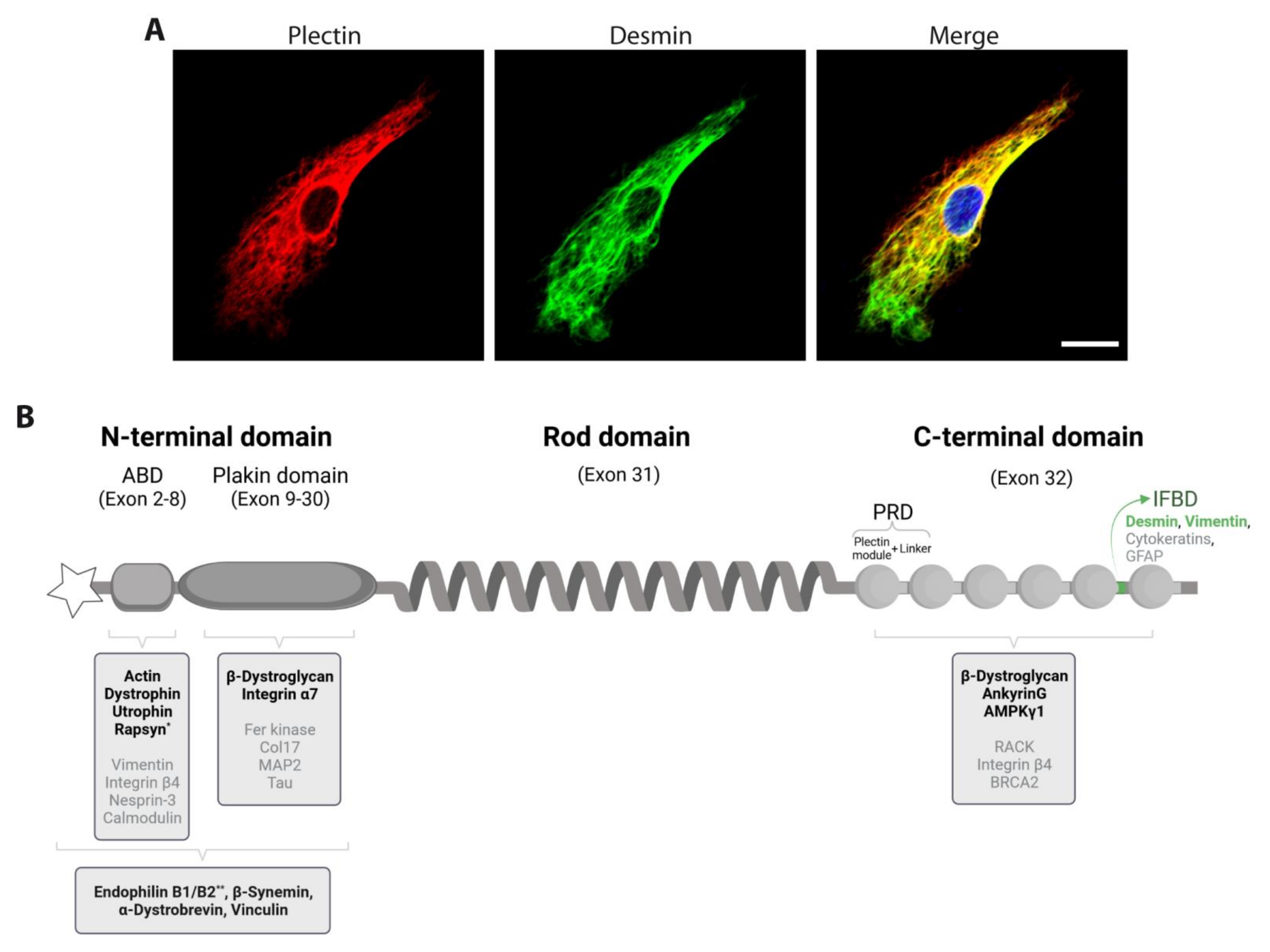
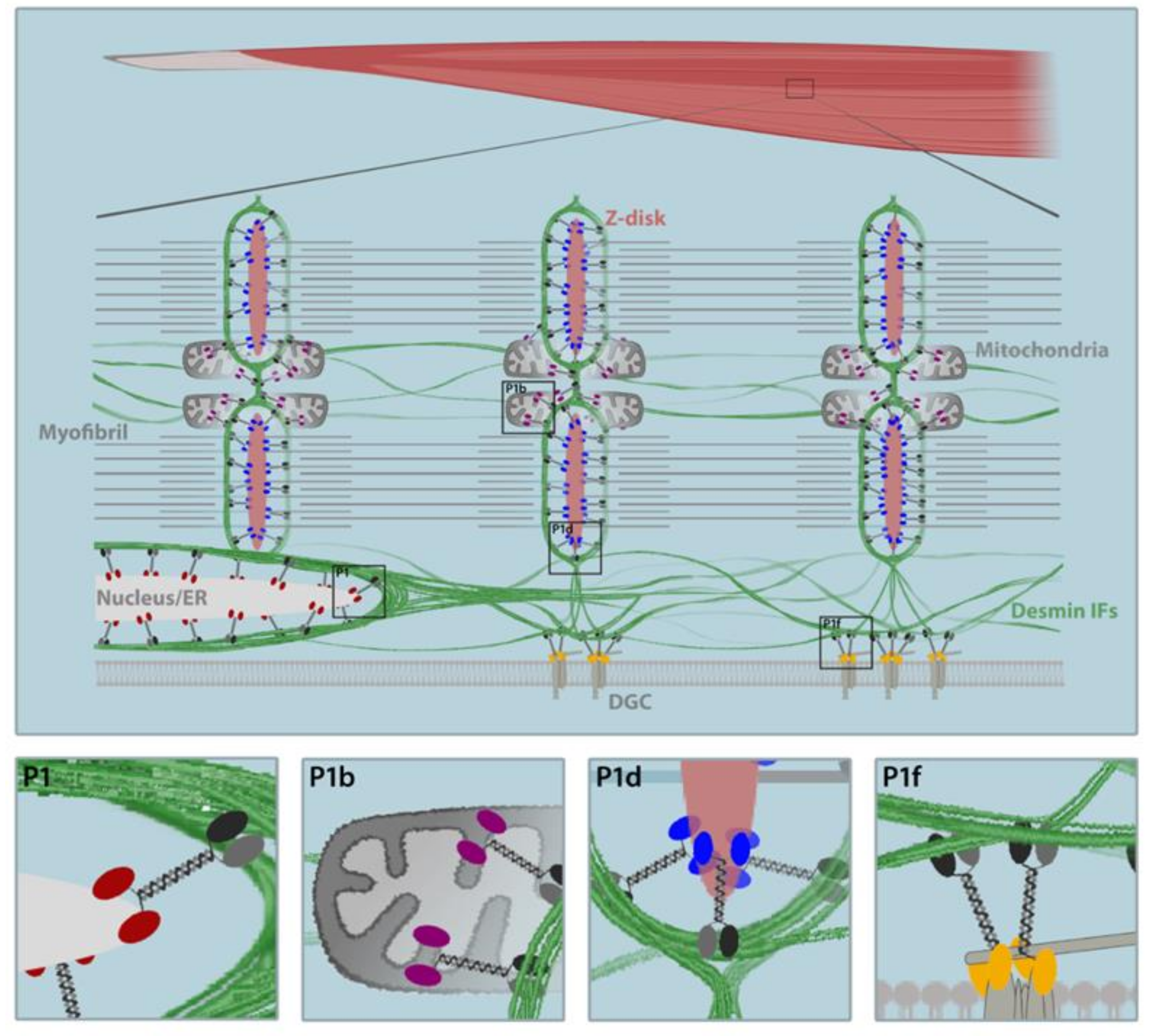
6. Skeletal Muscle-Expressed Pectin Isoforms as the Key to Desmin IF Network Architecture
6.1. P1d Docks Desmin IFs to the Sarcomere
6.2. P1f Tethers Desmin IFs to the Sarcolemma
6.3. P1b Links Desmin IFs to the Mitochondrial Network
6.4. P1 Connects Desmin IFs to the Nucleus/ER Membrane System
7. From Cell and Animal Models to Potential Therapeutic Approaches
8. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Henderson, C.A.; Gomez, C.G.; Novak, S.M.; Mi-Mi, L.; Gregorio, C.C. Overview of the Muscle Cytoskeleton. Compr. Physiol. 2017, 7, 891–944. [Google Scholar] [CrossRef]
- Bouameur, J.E.; Favre, B.; Borradori, L. Plakins, a versatile family of cytolinkers: Roles in skin integrity and in human diseases. J. Investig. Dermatol. 2014, 134, 885–894. [Google Scholar] [CrossRef]
- Wiche, G.; Winter, L. Plectin isoforms as organizers of intermediate filament cytoarchitecture. BioArchitecture 2011, 1, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Wiche, G. The many faces of plectin and plectinopathies: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 77–93. [Google Scholar] [CrossRef]
- Castañón, M.J.; Walko, G.; Winter, L.; Wiche, G. Plectin-intermediate filament partnership in skin, skeletal muscle, and peripheral nerve. Histochem. Cell Biol. 2013, 140, 33–53. [Google Scholar] [CrossRef]
- Fuchs, P.; Zörer, M.; Rezniczek, G.A.; Spazierer, D.; Oehler, S.; Castañón, M.J.; Hauptmann, R.; Wiche, G. Unusual 5’ transcript complexity of plectin isoforms: Novel tissue-specific exons modulate actin binding activity. Hum. Mol. Genet. 1999, 8, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Rezniczek, G.A.; Abrahamsberg, C.; Fuchs, P.; Spazierer, D.; Wiche, G. Plectin 5′-transcript diversity: Short alternative sequences determine stability of gene products, initiation of translation and subcellular localization of isoforms. Hum. Mol. Genet. 2003, 12, 3181–3194. [Google Scholar] [CrossRef]
- Rezniczek, G.A.; Konieczny, P.; Nikolic, B.; Reipert, S.; Schneller, D.; Abrahamsberg, C.; Davies, K.E.; Winder, S.J.; Wiche, G. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J. Cell Biol. 2007, 176, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, P.; Fuchs, P.; Reipert, S.; Kunz, W.S.; Zeöld, A.; Fischer, I.; Paulin, D.; Schröder, R.; Wiche, G. Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J. Cell Biol. 2008, 181, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Koss-Harnes, D.; Hoyheim, B.; Anton-Lamprecht, I.; Gjesti, A.; Jorgensen, R.S.; Jahnsen, F.L.; Olaisen, B.; Wiche, G.; Gedde-Dahl, T., Jr. A site-specific plectin mutation causes dominant epidermolysis bullosa simplex Ogna: Two identical de novo mutations. J. Investig. Dermatol. 2002, 118, 87–93. [Google Scholar] [CrossRef]
- Kiritsi, D.; Pigors, M.; Tantcheva-Poor, I.; Wessel, C.; Arin, M.J.; Kohlhase, J.; Bruckner-Tuderman, L.; Has, C. Epidermolysis bullosa simplex ogna revisited. J. Investig. Dermatol. 2013, 133, 270–273. [Google Scholar] [CrossRef]
- Bolling, M.C.; Jongbloed, J.D.H.; Boven, L.G.; Diercks, G.F.H.; Smith, F.J.D.; Irwin McLean, W.H.; Jonkman, M.F. Plectin mutations underlie epidermolysis bullosa simplex in 8% of patients. J. Investig. Dermatol. 2014, 134, 273–276. [Google Scholar] [CrossRef]
- Straub, V.; Murphy, A.; Udd, B.; LGMD Workshop Study Group. 229th ENMC international workshop: Limb girdle muscular dystrophies-Nomenclature and reformed classification Naarden, The Netherlands, 17–19 March 2017. Neuromuscul. Disord. NMD 2018, 28, 702–710. [Google Scholar] [CrossRef]
- Gundesli, H.; Talim, B.; Korkusuz, P.; Balci-Hayta, B.; Cirak, S.; Akarsu, N.A.; Topaloglu, H.; Dincer, P. Mutation in exon 1f of PLEC, leading to disruption of plectin isoform 1f, causes autosomal-recessive limb-girdle muscular dystrophy. Am. J. Hum. Genet. 2010, 87, 834–841. [Google Scholar] [CrossRef]
- Mroczek, M.; Durmus, H.; Topf, A.; Parman, Y.; Straub, V. Four Individuals with a Homozygous Mutation in Exon 1f of the PLEC Gene and Associated Myasthenic Features. Genes 2020, 11, 716. [Google Scholar] [CrossRef]
- Deev, R.V.; Bardakov, S.N.; Mavlikeev, M.O.; Yakovlev, I.A.; Umakhanova, Z.R.; Akhmedova, P.G.; Magomedova, R.M.; Chekmaryeva, I.A.; Dalgatov, G.D.; Isaev, A.A. Glu20Ter Variant in PLEC 1f Isoform Causes Limb-Girdle Muscle Dystrophy with Lung Injury. Front. Neurol. 2017, 8, 367. [Google Scholar] [CrossRef]
- Gostynska, K.B.; Nijenhuis, M.; Lemmink, H.; Pas, H.H.; Pasmooij, A.M.; Lang, K.K.; Castañón, M.J.; Wiche, G.; Jonkman, M.F. Mutation in exon 1a of PLEC, leading to disruption of plectin isoform 1a, causes autosomal-recessive skin-only epidermolysis bullosa simplex. Hum. Mol. Genet. 2015, 24, 3155–3162. [Google Scholar] [CrossRef]
- Schara, U.; Tucke, J.; Mortier, W.; Nusslein, T.; Rouan, F.; Pfendner, E.; Zillikens, D.; Bruckner-Tuderman, L.; Uitto, J.; Wiche, G.; et al. Severe mucous membrane involvement in epidermolysis bullosa simplex with muscular dystrophy due to a novel plectin gene mutation. Eur. J. Pediatr. 2004, 163, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Yiu, E.M.; Klausegger, A.; Waddell, L.B.; Grasern, N.; Lloyd, L.; Tran, K.; North, K.N.; Bauer, J.W.; McKelvie, P.; Chow, C.W.; et al. Epidermolysis bullosa with late-onset muscular dystrophy and plectin deficiency. Muscle Nerve 2011, 44, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.R.; Ryan, T.D.; Collins, J.J.; Taylor, M.D.; Lucky, A.W.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy associated with epidermolysis bullosa simplex with muscular dystrophy and PLEC1 mutation. Neuromuscul. Disord. NMD 2015, 25, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, V.C.; Penttila, S.T.; Salutto, V.L.; Udd, B.; Mazia, C.G. Epidermolysis bullosa simplex with muscular dystrophy associated with PLEC deletion mutation. Neurol. Genet. 2016, 2, e109. [Google Scholar] [CrossRef] [PubMed]
- Kyrova, J.; Kopeckova, L.; Buckova, H.; Mrazova, L.; Vesely, K.; Hermanova, M.; Oslejskova, H.; Fajkusova, L. Epidermolysis bullosa simplex with muscular dystrophy. Review of the literature and a case report. J. Dermatol. Case Rep. 2016, 10, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R.; Kunz, W.S.; Rouan, F.; Pfendner, E.; Tolksdorf, K.; Kappes-Horn, K.; Altenschmidt-Mehring, M.; Knoblich, R.; van der Ven, P.F.; Reimann, J.; et al. Disorganization of the desmin cytoskeleton and mitochondrial dysfunction in plectin-related epidermolysis bullosa simplex with muscular dystrophy. J. Neuropathol. Exp. Neurol. 2002, 61, 520–530. [Google Scholar] [CrossRef]
- Winter, L.; Turk, M.; Harter, P.N.; Mittelbronn, M.; Kornblum, C.; Norwood, F.; Jungbluth, H.; Thiel, C.T.; Schlotzer-Schrehardt, U.; Schroder, R. Downstream effects of plectin mutations in epidermolysis bullosa simplex with muscular dystrophy. Acta Neuropathol. Commun. 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Gostynska, K.B.; Lemmink, H.; Bremer, J.; Pas, H.H.; Nijenhuis, M.; van den Akker, P.C.; Sinke, R.J.; Jonkman, M.F.; Pasmooij, A.M.G. A PLEC Isoform Identified in Skin, Muscle, and Heart. J. Investig. Dermatol. 2017, 137, 518–522. [Google Scholar] [CrossRef]
- Bauer, J.W.; Rouan, F.; Kofler, B.; Rezniczek, G.A.; Kornacker, I.; Muss, W.; Hametner, R.; Klausegger, A.; Huber, A.; Pohla-Gubo, G.; et al. A compound heterozygous one amino-acid insertion/nonsense mutation in the plectin gene causes epidermolysis bullosa simplex with plectin deficiency. Am. J. Pathol. 2001, 158, 617–625. [Google Scholar] [CrossRef]
- Tu, W.T.; Chen, P.C.; Hou, P.C.; Huang, H.Y.; Wang, J.Y.; Chao, S.C.; Lee, J.Y.; McGrath, J.A.; Natsuga, K.; Hsu, C.K. Plectin Missense Mutation p.Leu319Pro in the Pathogenesis of Autosomal Recessive Epidermolysis Bullosa Simplex. Acta Dermatol. Venereol. 2020, 100, adv00242. [Google Scholar] [CrossRef]
- Bolling, M.C.; Pas, H.H.; de Visser, M.; Aronica, E.; Pfendner, E.G.; van den Berg, M.P.; Diercks, G.F.; Suurmeijer, A.J.; Jonkman, M.F. PLEC1 mutations underlie adult-onset dilated cardiomyopathy in epidermolysis bullosa simplex with muscular dystrophy. J. Investig. Dermatol. 2010, 130, 1178–1181. [Google Scholar] [CrossRef]
- Uitto, J.; Pfendner, E. Compound heterozygosity of unique in-frame insertion and deletion mutation in the plectin gene in a mild case of epidermolysis bullosa with very late onset muscular dystrophy. J. Investig. Dermatol. 2004, 122, A86. [Google Scholar]
- Khan, F.F.; Khan, N.; Rehman, S.; Ejaz, A.; Ali, U.; Erfan, M.; Ahmed, Z.M.; Naeem, M. Identification and Computational Analysis of Novel Pathogenic Variants in Pakistani Families with Diverse Epidermolysis Bullosa Phenotypes. Biomolecules 2021, 11, 620. [Google Scholar] [CrossRef]
- Natsuga, K.; Nishie, W.; Nishimura, M.; Shinkuma, S.; Watanabe, M.; Izumi, K.; Nakamura, H.; Hirako, Y.; Shimizu, H. Loss of interaction between plectin and type XVII collagen results in epidermolysis bullosa simplex. Hum. Mutat. 2017, 38, 1666–1670. [Google Scholar] [CrossRef]
- Pulkkinen, L.; Smith, F.J.; Shimizu, H.; Murata, S.; Yaoita, H.; Hachisuka, H.; Nishikawa, T.; McLean, W.H.; Uitto, J. Homozygous deletion mutations in the plectin gene (PLEC1) in patients with epidermolysis bullosa simplex associated with late-onset muscular dystrophy. Hum. Mol. Genet. 1996, 5, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Masunaga, T.; Kurihara, Y.; Owaribe, K.; Wiche, G.; Pulkkinen, L.; Uitto, J.; Nishikawa, T. Expression of plectin and HD1 epitopes in patients with epidermolysis bullosa simplex associated with muscular dystrophy. Arch. Dermatol. Res. 1999, 291, 531–537. [Google Scholar] [CrossRef]
- Rouan, F.; Pulkkinen, L.; Meneguzzi, G.; Laforgia, S.; Hyde, P.; Kim, D.U.; Richard, G.; Uitto, J. Epidermolysis bullosa: Novel and de novo premature termination codon and deletion mutations in the plectin gene predict late-onset muscular dystrophy. J. Investig. Dermatol. 2000, 114, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, Y.; Shimizu, H.; Rouan, F.; Kawai, M.; Udono, M.; Pulkkinen, L.; Nishikawa, T.; Uitto, J. Four novel plectin gene mutations in Japanese patients with epidermolysis bullosa with muscular dystrophy disclosed by heteroduplex scanning and protein truncation tests. J. Investig. Dermatol. 1999, 112, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Natsuga, K.; Nishie, W.; Akiyama, M.; Nakamura, H.; Shinkuma, S.; McMillan, J.R.; Nagasaki, A.; Has, C.; Ouchi, T.; Ishiko, A.; et al. Plectin expression patterns determine two distinct subtypes of epidermolysis bullosa simplex. Hum. Mutat. 2010, 31, 308–316. [Google Scholar] [CrossRef]
- Charlesworth, A.; Chiaverini, C.; Chevrant-Breton, J.; Delrio, M.; Diociaiuti, A.; Dupuis, R.P.; El Hachem, M.; Le Fiblec, B.; Sankari-Ho, A.M.; Valhquist, A.; et al. Epidermolysis bullosa simplex with PLEC mutations: New phenotypes and new mutations. Br. J. Dermatol. 2013, 168, 808–814. [Google Scholar] [CrossRef]
- Dang, M.; Pulkkinen, L.; Smith, F.J.; McLean, W.H.; Uitto, J. Novel compound heterozygous mutations in the plectin gene in epidermolysis bullosa with muscular dystrophy and the use of protein truncation test for detection of premature termination codon mutations. Lab. Investig. A J. Tech. Methods Pathol. 1998, 78, 195–204. [Google Scholar]
- Sawamura, D.; Goto, M.; Sakai, K.; Nakamura, H.; McMillan, J.R.; Akiyama, M.; Shirado, O.; Oyama, N.; Satoh, M.; Kaneko, F.; et al. Possible involvement of exon 31 alternative splicing in phenotype and severity of epidermolysis bullosa caused by mutations in PLEC1. J. Investig. Dermatol. 2007, 127, 1537–1540. [Google Scholar] [CrossRef]
- Walker, G.D.; Woody, M.; Orrin, E.; Mellerio, J.E.; Levy, M.L. Epidermolysis Bullosa with Pyloric Atresia and Significant Urologic Involvement. Pediatr. Dermatol. 2017, 34, e61–e64. [Google Scholar] [CrossRef]
- Pfendner, E.; Rouan, F.; Uitto, J. Progress in epidermolysis bullosa: The phenotypic spectrum of plectin mutations. Exp. Dermatol. 2005, 14, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mellerio, J.E.; Smith, F.J.; McMillan, J.R.; McLean, W.H.; McGrath, J.A.; Morrison, G.A.; Tierney, P.; Albert, D.M.; Wiche, G.; Leigh, I.M.; et al. Recessive epidermolysis bullosa simplex associated with plectin mutations: Infantile respiratory complications in two unrelated cases. Br. J. Dermatol. 1997, 137, 898–906. [Google Scholar] [CrossRef]
- McLean, W.H.; Pulkkinen, L.; Smith, F.J.; Rugg, E.L.; Lane, E.B.; Bullrich, F.; Burgeson, R.E.; Amano, S.; Hudson, D.L.; Owaribe, K.; et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev. 1996, 10, 1724–1735. [Google Scholar] [CrossRef]
- Kunz, M.; Rouan, F.; Pulkkinen, L.; Hamm, H.; Jeschke, R.; Bruckner-Tuderman, L.; Brocker, E.B.; Wiche, G.; Uitto, J.; Zillikens, D. Mutation reports: Epidermolysis bullosa simplex associated with severe mucous membrane involvement and novel mutations in the plectin gene. J. Investig. Dermatol. 2000, 114, 376–380. [Google Scholar] [CrossRef]
- Yin, J.; Ren, Y.; Lin, Z.; Wang, H.; Zhou, Y.; Yang, Y. Compound heterozygous PLEC mutations in a patient of consanguineous parentage with epidermolysis bullosa simplex with muscular dystrophy and diffuse alopecia. Int J. Dermatol. 2015, 54, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Koss-Harnes, D.; Hoyheim, B.; Jonkman, M.F.; de Groot, W.P.; de Weerdt, C.J.; Nikolic, B.; Wiche, G.; Gedde-Dahl, T., Jr. Life-long course and molecular characterization of the original Dutch family with epidermolysis bullosa simplex with muscular dystrophy due to a homozygous novel plectin point mutation. Acta Dermatol. Venereol. 2004, 84, 124–131. [Google Scholar]
- Chavanas, S.; Pulkkinen, L.; Gache, Y.; Smith, F.J.; McLean, W.H.; Uitto, J.; Ortonne, J.P.; Meneguzzi, G. A homozygous nonsense mutation in the PLEC1 gene in patients with epidermolysis bullosa simplex with muscular dystrophy. J. Clin. Investig. 1996, 98, 2196–2200. [Google Scholar] [CrossRef] [PubMed]
- Gache, Y.; Chavanas, S.; Lacour, J.P.; Wiche, G.; Owaribe, K.; Meneguzzi, G.; Ortonne, J.P. Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J. Clin. Investig. 1996, 97, 2289–2298. [Google Scholar] [CrossRef]
- McMillan, J.R.; Akiyama, M.; Rouan, F.; Mellerio, J.E.; Lane, E.B.; Leigh, I.M.; Owaribe, K.; Wiche, G.; Fujii, N.; Uitto, J.; et al. Plectin defects in epidermolysis bullosa simplex with muscular dystrophy. Muscle Nerve 2007, 35, 24–35. [Google Scholar] [CrossRef]
- Smith, F.J.; Eady, R.A.; Leigh, I.M.; McMillan, J.R.; Rugg, E.L.; Kelsell, D.P.; Bryant, S.P.; Spurr, N.K.; Geddes, J.F.; Kirtschig, G.; et al. Plectin deficiency results in muscular dystrophy with epidermolysis bullosa. Nat. Genet. 1996, 13, 450–457. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, Z.M.; Wang, H.J.; Zhang, J.; Yin, J.H.; Yang, Y. New mutations in the PLEC gene in a Chinese patient with epidermolysis bullosa simplex with muscular dystrophy. Clin. Exp. Dermatol. 2013, 38, 792–794. [Google Scholar] [CrossRef]
- Takahashi, Y.; Rouan, F.; Uitto, J.; Ishida-Yamamoto, A.; Iizuka, H.; Owaribe, K.; Tanigawa, M.; Ishii, N.; Yasumoto, S.; Hashimoto, T. Plectin deficient epidermolysis bullosa simplex with 27-year-history of muscular dystrophy. J. Dermatol. Sci. 2005, 37, 87–93. [Google Scholar] [CrossRef]
- Argyropoulou, Z.; Liu, L.; Ozoemena, L.; Branco, C.C.; Senra, R.; Reis-Rego, A.; Mota-Vieira, L. A novel PLEC nonsense homozygous mutation (c.7159G > T; p.Glu2387*) causes epidermolysis bullosa simplex with muscular dystrophy and diffuse alopecia: A case report. BMC Dermatol. 2018, 18, 1. [Google Scholar] [CrossRef]
- Maccari, M.E.; Speckmann, C.; Heeg, M.; Reimer, A.; Casetti, F.; Has, C.; Ehl, S.; Castro, C.N. Profound immunodeficiency with severe skin disease explained by concomitant novel CARMIL2 and PLEC1 loss-of-function mutations. Clin. Immunol. 2019, 208, 108228. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Shah, K.; Umair, M.; Jan, A.; Irfanullah; Khan, S.; Muhammad, D.; Basit, S.; Wakil, S.M.; Ramzan, K.; et al. Novel autosomal recessive LAMA3 and PLEC variants underlie junctional epidermolysis bullosa generalized intermediate and epidermolysis bullosa simplex with muscular dystrophy in two consanguineous families. Clin. Exp. Dermatol. 2018, 43, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Forrest, K.; Mellerio, J.E.; Robb, S.; Dopping-Hepenstal, P.J.; McGrath, J.A.; Liu, L.; Buk, S.J.; Al-Sarraj, S.; Wraige, E.; Jungbluth, H. Congenital muscular dystrophy, myasthenic symptoms and epidermolysis bullosa simplex (EBS) associated with mutations in the PLEC1 gene encoding plectin. Neuromuscul. Disord. NMD 2010, 20, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Maselli, R.; Arredondo, J.; Cagney, O.; Mozaffar, T.; Skinner, S.; Yousif, S.; Davis, R.; Gregg, J.; Sivak, M.; Konia, T.; et al. Congenital myasthenic syndrome associated with epidermolysis bullosa caused by homozygous mutations in PLEC1 and CHRNE. Clin. Genet. 2010, 80, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Argente-Escrig, H.; Schultheis, D.; Kamm, L.; Schowalter, M.; Thiel, C.; Turk, M.; Clemen, C.S.; Muelas, N.; Castañón, M.J.; Wiche, G.; et al. Plectin-related scapuloperoneal myopathy with treatment-responsive myasthenic syndrome. Neuropathol. Appl. Neurobiol. 2021, 47, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Garcia, A.; Tutmaher, M.S.; Upadhyayula, S.R.; Sanchez Russo, R.; Verma, S. Novel PLEC gene variants causing congenital myasthenic syndrome. Muscle Nerve 2019, 60, E40–E43. [Google Scholar] [CrossRef]
- Banwell, B.L.; Russel, J.; Fukudome, T.; Shen, X.M.; Stilling, G.; Engel, A.G. Myopathy, myasthenic syndrome, and epidermolysis bullosa simplex due to plectin deficiency. J. Neuropathol. Exp. Neurol. 1999, 58, 832–846. [Google Scholar] [CrossRef]
- Selcen, D.; Juel, V.C.; Hobson-Webb, L.D.; Smith, E.C.; Stickler, D.E.; Bite, A.V.; Ohno, K.; Engel, A.G. Myasthenic syndrome caused by plectinopathy. Neurology 2011, 76, 327–336. [Google Scholar] [CrossRef]
- Pfendner, E.; Uitto, J. Plectin gene mutations can cause epidermolysis bullosa with pyloric atresia. J. Investig. Dermatol. 2005, 124, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Sawamura, D.; Goto, M.; McMillan, J.R.; Park, S.; Kono, S.; Hasegawa, S.; Paku, S.; Nakamura, T.; Ogiso, Y.; et al. Epidermolysis bullosa simplex associated with pyloric atresia is a novel clinical subtype caused by mutations in the plectin gene (PLEC1). J. Mol. Diagn. 2005, 7, 28–35. [Google Scholar] [CrossRef]
- Charlesworth, A.; Gagnoux-Palacios, L.; Bonduelle, M.; Ortonne, J.P.; De Raeve, L.; Meneguzzi, G. Identification of a lethal form of epidermolysis bullosa simplex associated with a homozygous genetic mutation in plectin. J. Investig. Dermatol. 2003, 121, 1344–1348. [Google Scholar] [CrossRef][Green Version]
- Valari, M.; Theodoraki, M.; Loukas, I.; Gkantseva-Patsoura, S.; Karavana, G.; Falaina, V.; Lykopoulou, L.; Pons, R.; Athanasiou, I.; Wertheim-Tysarowska, K.; et al. Novel PLEC Variant Causes Mild Skin Fragility, Pyloric Atresia, Muscular Dystrophy and Urological Manifestations. Acta Dermatol. Venereol. 2019, 99, 1309–1310. [Google Scholar] [CrossRef] [PubMed]
- Natsuga, K.; Nishie, W.; Shinkuma, S.; Arita, K.; Nakamura, H.; Ohyama, M.; Osaka, H.; Kambara, T.; Hirako, Y.; Shimizu, H. Plectin deficiency leads to both muscular dystrophy and pyloric atresia in epidermolysis bullosa simplex. Hum. Mutat. 2010, 31, E1687–E1698. [Google Scholar] [CrossRef]
- Fattahi, Z.; Kahrizi, K.; Nafissi, S.; Fadaee, M.; Abedini, S.S.; Kariminejad, A.; Akbari, M.R.; Najmabadi, H. Report of a patient with limb-girdle muscular dystrophy, ptosis and ophthalmoparesis caused by plectinopathy. Arch. Iran. Med. 2015, 18, 60–64. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, G.; Dang, Y.; Liao, H.; Zhang, J.; Lan, D. Novel compound heterozygous PLEC mutations lead to earlyonset limbgirdle muscular dystrophy 2Q. Mol. Med. Rep. 2017, 15, 2760–2764. [Google Scholar] [CrossRef] [PubMed]
- Celik, C.; Uysal, H.; Heper, A.O.; Karaoglan, B. Epidermolysis bullosa simplex associated with muscular dystrophy and cardiac involvement. J. Clin. Neuromuscul. Dis. 2005, 6, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Thorolfsdottir, R.B.; Sveinbjornsson, G.; Sulem, P.; Helgadottir, A.; Gretarsdottir, S.; Benonisdottir, S.; Magnusdottir, A.; Davidsson, O.B.; Rajamani, S.; Roden, D.M.; et al. A Missense Variant in PLEC Increases Risk of Atrial Fibrillation. J. Am. Coll Cardiol. 2017, 70, 2157–2168. [Google Scholar] [CrossRef]
- Milan, D. The Com-PLEC-sity of Atrial Fibrillation Genetics. J. Am. Coll Cardiol. 2017, 70, 2169–2170. [Google Scholar] [CrossRef]
- Elliott, C.E.; Becker, B.; Oehler, S.; Castañón, M.J.; Hauptmann, R.; Wiche, G. Plectin transcript diversity: Identification and tissue distribution of variants with distinct first coding exons and rodless isoforms. Genomics 1997, 42, 115–125. [Google Scholar] [CrossRef]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 529–544. [Google Scholar] [CrossRef]
- Kley, R.A.; Olive, M.; Schroder, R. New aspects of myofibrillar myopathies. Curr. Opin. Neurol. 2016, 29, 628–634. [Google Scholar] [CrossRef]
- Olive, M.; Winter, L.; Furst, D.O.; Schroder, R.; group, E.w.s. 246th ENMC International Workshop: Protein aggregate myopathies 24-26 May 2019, Hoofddorp, The Netherlands. Neuromuscul. Disord. NMD 2021, 31, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Andrä, K.; Lassmann, H.; Bittner, R.; Shorny, S.; Fässler, R.; Propst, F.; Wiche, G. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev. 1997, 11, 3143–3156. [Google Scholar] [CrossRef]
- Reipert, S.; Steinböck, F.; Fischer, I.; Bittner, R.E.; Zeöld, A.; Wiche, G. Association of mitochondria with plectin and desmin intermediate filaments in striated muscle. Exp. Cell Res. 1999, 252, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Ketema, M.; Secades, P.; Kreft, M.; Nahidiazar, L.; Janssen, H.; Jalink, K.; de Pereda, J.M.; Sonnenberg, A. The rod domain is not essential for the function of plectin in maintaining tissue integrity. Mol. Biol. Cell 2015, 26, 2402–2417. [Google Scholar] [CrossRef]
- Winter, L.; Kuznetsov, A.V.; Grimm, M.; Zeöld, A.; Fischer, I.; Wiche, G. Plectin isoform P1b and P1d deficiencies differentially affect mitochondrial morphology and function in skeletal muscle. Hum. Mol. Genet. 2015, 24, 4530–4544. [Google Scholar] [CrossRef]
- Winter, L.; Staszewska, I.; Mihailovska, E.; Fischer, I.; Goldmann, W.H.; Schroder, R.; Wiche, G. Chemical chaperone ameliorates pathological protein aggregation in plectin-deficient muscle. J. Clin. Investig. 2014, 124, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Bonakdar, N.; Schilling, A.; Sporrer, M.; Lennert, P.; Mainka, A.; Winter, L.; Walko, G.; Wiche, G.; Fabry, B.; Goldmann, W.H. Determining the mechanical properties of plectin in mouse myoblasts and keratinocytes. Exp. Cell Res. 2015, 331, 331–337. [Google Scholar] [CrossRef]
- Mihailovska, E.; Raith, M.; Valencia, R.G.; Fischer, I.; Al Banchaabouchi, M.; Herbst, R.; Wiche, G. Neuromuscular synapse integrity requires linkage of acetylcholine receptors to postsynaptic intermediate filament networks via rapsyn-plectin 1f complexes. Mol. Biol. Cell 2014, 25, 4130–4149. [Google Scholar] [CrossRef] [PubMed]
- Burgstaller, G.; Gregor, M.; Winter, L.; Wiche, G. Keeping the vimentin network under control: Cell-matrix adhesion-associated plectin 1f affects cell shape and polarity of fibroblasts. Mol. Biol. Cell 2010, 21, 3362–3375. [Google Scholar] [CrossRef]
- Schröder, R.; Furst, D.O.; Klasen, C.; Reimann, J.; Herrmann, H.; van der Ven, P.F. Association of plectin with Z-discs is a prerequisite for the formation of the intermyofibrillar desmin cytoskeleton. Lab. Investig. A J. Tech. Methods Pathol. 2000, 80, 455–464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schröder, R.; Pacholsky, D.; Reimann, J.; Matten, J.; Wiche, G.; Fürst, D.O.; van der Ven, P.F. Primary longitudinal adhesion structures: Plectin-containing precursors of costameres in differentiating human skeletal muscle cells. Histochem. Cell Biol. 2002, 118, 301–310. [Google Scholar] [CrossRef]
- Winter, L.; Abrahamsberg, C.; Wiche, G. Plectin isoform 1b mediates mitochondrion-intermediate filament network linkage and controls organelle shape. J. Cell Biol. 2008, 181, 903–911. [Google Scholar] [CrossRef]
- Staszewska, I.; Fischer, I.; Wiche, G. Plectin isoform 1-dependent nuclear docking of desmin networks affects myonuclear architecture and expression of mechanotransducers. Hum. Mol. Genet. 2015, 24, 7373–7389. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; MacRae, T.H. The small heat shock proteins and their role in human disease. FEBS J. 2005, 272, 2613–2627. [Google Scholar] [CrossRef]
- de Marco, A. Molecular and chemical chaperones for improving the yields of soluble recombinant proteins. Methods Mol. Biol. 2011, 705, 31–51. [Google Scholar] [CrossRef]
- Spörrer, M.; Prochnicki, A.; Tolle, R.C.; Nystrom, A.; Esser, P.R.; Homberg, M.; Athanasiou, I.; Zingkou, E.; Schilling, A.; Gerum, R.; et al. Treatment of keratinocytes with 4-phenylbutyrate in epidermolysis bullosa: Lessons for therapies in keratin disorders. EBioMedicine 2019, 44, 502–515. [Google Scholar] [CrossRef]
| Ref | Mutation 1 | Mutation 2 | Geno- | MD | Sex | MB | ||
|---|---|---|---|---|---|---|---|---|
| DNA | Protein | DNA | Protein | Type | (Onset) | |||
| EBS-MD | ||||||||
| [25] | 906 + 19_40del * | V303_P313ins11 | 906 + 19_40del * | V303_P313ins11 | hom. | adolescence | F | no |
| [26] | 954_956dupGCT | L319dup | 4222C > T | Q1408X | c.het. | MD not dev. at 4 years; but histological changes | M | yes |
| [26] | 954_956dupGCT | L319dup | 4222C > T | Q1408X | c.het. | N/A | M | no |
| [27] | 956T > C | L319P | 2807G > A | W936X | c.het. | MD not dev. at 18 years | M | no |
| [27] | 956T > C | L319P | 6955C > T | R2319X | c.het. | MD not dev. at 31 years | F | no |
| [28] | 968G > A | R323Q | 4840G > T | E1614X | c.het. | twenties | M | yes |
| [28] | 968G > A | R323Q | 4840G > T | E1614X | c.het. | MD not dev. at 9 years | F | no |
| [29] | 1530_1531ins36 | A510_I511ins12 | 2677_2685del | Q893_A895del | c.het. | 42 years | F | N/A |
| [30] | 1648C > G | R500G | 1648C > G | R500G | hom. | MD not dev. at 2 years | M | no |
| [21] | 2264_2266del | F755del | 2264_2266del | F755del | hom. | twenties | F | n.s. |
| [24] | 2264_2266del | F755del | 3119_3210del | K1040RfsX | c.het. | 27 years | M | yes |
| [31] | 2264_2266del | F755del | 9194dup | S3066EfsX55 | c.het. | MD not dev. at 3 years | M | no |
| [32,33] | 2677_2685del | Q893_A895del | 2677_2685del | Q893_A895del | hom. | early thirties | F | no |
| [32,33] | 2677_2685del | Q893_A895del | 2677_2685del | Q893_A895del | hom. | early thirties | F | no |
| [19] | 2677_2685del | Q893_A895del | 4930C > T | Q1644X | c.het. | 28 years | M | yes |
| [34] | 2694−9_2705del | N/A | 5032delG | V1678WfsX65 | c.het. | MD not dev. at 5 months | F | no |
| [33,35,36] | 3157C > T | Q1053X | 5806C > T | Q1936X | c.het. | infancy | M | no |
| [37] | 3341 + 1G > T | N/A | 6955C > T | R2319X | c.het. | MD not dev. at 1.5 years | - | no |
| [37] | 4126−4A > G | N/A | 7804C > T | Q2602X | c.het. | 18 years | - | no |
| [37] | 4216C > T | Q1421X | 4216C > T | Q1421X | hom. | teens | - | no |
| [38] | 4294_4306dup | V1436GfsX40 | 4365delC | S1456RfsX93 | c.het. | 20 years | M | N/A |
| [36,39] | 4348C > T | Q1450X | 4348C > T | Q1450X | hom. | 19 years | F | no |
| [40] | 4549C > T | R1517X | 4549C > T | R1517X | hom. | MD not dev. at 24 years | M | no |
| [36] | 4643_4667dup | K1558GfsX89 | 7120C > T | Q2374X | c.het. | MD not dev. at 7 years | M | no |
| [41] | 4840G > T | E1614X | 4840G > T | E1614X | hom. | teens | - | no |
| [24,42] | 5018_5036del | L1673RfsX64 | 5018_5036del | L1673RfsX64 | hom. | MD not dev. at 5 years | F | yes |
| [43] | 5105_5112del | R1702QfsX14 | 5105_5112del | R1702QfsX14 | hom. | 10 years | M | n.s. |
| [44] | 5137C > T | Q1713X | 7051C > T | R2351X | c.het. | MD not dev. at 4 years | M | no |
| [45] | 5254C > T | Q1752X | 7285C > T | R2429X | c.het. | adolescence | F | n.s. |
| [18] | 5257dupG | E1753GfsX17 | 5257dupG | E1753GfsX17 | hom. | MD not dev. at 3 years | F | no |
| [46] | 5410G > T | E1804X | 5410G > T | E1804X | hom. | 17 years | M | no |
| [46] | 5410G > T | E1804X | 5410G > T | E1804X | hom. | 15 years | M | n.s. |
| [47,48] | 5728C > T | Q1910X | 5728C > T | Q1910X | hom. | infancy | F | yes |
| [47,48] | 5728C > T | Q1910X | 5728C > T | Q1910X | hom. | infancy | F | n.s. |
| [37] | 5770C > T | Q1924X | N/A | N/A | N/A | 30 years | - | no |
| [32,33,36,49] | 5815delC | L1939WfsX6 | 5815delC | L1939WfsX6 | hom. | late twenties | F | yes |
| [50] | 5849_5856dup | E1953WfsX8 | 5849_5856dup | E1953WfsX8 | hom. | infancy | M | n.s. |
| [42,49] | 5854_5855del | E1952GfsX60 | 5854_5855del | E1952GfsX60 | hom. | MD not dev. at 3 years | F | yes |
| [22] | 5902_5093del | K1968GfsX44 | 9109_9125del | V3037CfsX78 | c.het. | 8 years | M | n.s. |
| [34] | 6013G > T | E2005X | 13378A > T | K4460X | c.het. | MD not dev. at 6 months | M | no |
| [51] | 6622C > T | Q2208X | 8119C > T | Q2707X | c.het. | 6 years | M | no |
| [36] | 6549_6582del | L2184RfsX21 | 13040dupG | I4348HfsX8 | c.het. | 10 years | F | no |
| [37] | 6682C > T | Q2228X | 10456C > T | Q3486X | c.het. | 5 years | - | no |
| [52] | 6955C > T | R2319X | 6955C > T | R2319X | hom. | 25 years | F | no |
| [20] | 7100C > T | R2351X | 7100C > T | R2351X | hom. | teens | M | no |
| [53] | 7159G < T | E2387X | 7159G < T | E2387X | hom. | adolescence | F | no |
| [33,35,36] | 7261C > T | R2421X | 12578_12581dup | Y4195DfsX41 | c.het. | 5 years | M | no |
| [41] | 7261C > T | R2421X | N/A | N/A | N/A | childhood | - | no |
| [41,49,50] | 7393C > T | R2465X | 7393C > T | R2465X | hom. | early childhood | M | yes |
| [54] | 7468C > T | Q2490X | 7468C > T | Q2490X | hom. | MD not dev. at 4 years | M | no |
| [55] | 10909C > T | R3637C | 10909C > T | R3637C | hom. | yes (onset N/A) | M | no |
| [55] | 10909C > T | R3637C | 10909C > T | R3637C | hom. | yes (onset N/A) | M | no |
| [23,24] | 13459_13474dup | E4492GfsX48 | 13459_13474dup | E4492GfsX48 | hom. | 4 years | F | yes |
| EBS-MD-MyS | ||||||||
| [56] | IVS11 + 2T > G | N/A | 10187_10190del | K3395GfsX11 | c.het. | birth | M | yes |
| [57] | 1500_1501ins36 | R500_V501ins12 | 1500_1501ins36 | R500_V501ins12 | hom. | childhood | F | yes |
| [57] | 1500_1501ins36 | R500_V501ins12 | 1500_1501ins36 | R500_V501ins12 | hom. | childhood | M | no |
| [58] | 2539−2A > G | N/A | 11737delC | R3913VfsX30 | c.het. | 25 years | M | yes |
| [59] | 3086G > A | R1029H | 9679_9766del | D3229VfsX21 | c.het. | N/A | F | no |
| [59] | 3086G > A | R1029H | 9679_9766del | D3229VfsX21 | c.het. | N/A | M | no |
| [59] | 3086G > A | R1029H | 9679_9766del | D3229VfsX21 | c.het. | N/A | M | no |
| [60,61] | 6169C > T | Q2057X | 12043dupG | E4015GfsX69 | c.het. | 9 years | F | yes |
| [61] | 6955C > T | R2319X | 12043dupG | E4015GfsX69 | c.het. | 3 years | M | yes |
| EBS-PA | ||||||||
| [62] | 913C > T | Q305X | 913C > T | Q305X | hom. | N/A | M | no |
| [63] | 913C > T | Q305X | 1344G > A | N/A | c.het. | N/A | M | no |
| [62] | 1563_1567del | G522WfsX11 | 1563_1567del | G522WfsX11 | hom. | N/A | F | no |
| [64] | 2680_2693del | E894AfsX84 | 2680_2693del | E894AfsX84 | hom. | N/A | F | no |
| [62] | 2769_2788del | W923CfsX53 | 2769_2788del | W923CfsX53 | hom. | N/A | M | no |
| [40] | 2888dupT | F963PfsX19 | N/A | Q2367X | c.het. | MD not dev. at 6 years | F | no |
| [37] | 3342−2A > G | N/A | 3902_3903del | Q1301LfsX8 | c.het. | N/A | - | no |
| [63] | 3565C > T | R1189X | 3565C > T7612C > T | R1189XQ2538X | hom.& c.het | N/A | F | no |
| [37] | 4119_4120del | N/A | 12499C > T | R4167X | c.het. | MD not dev. at 12 years | - | no |
| [39] | 7396C > T | Q2466X | 7633C > T | Q2545X | c.het. | N/A | M | no |
| [62] | 9085C > T | R3029X | 9085C > T | R3029X | hom. | N/A | F | no |
| [65] | 11912del | K3971Ter | 12499C > T | R4167X | c.het. | birth | M | no |
| [66] | 10984C > T | E3662X | 11453_11462del | I3818RfsX90 | c.het. | birth | M | no |
| LGMDR17 (P1f mutation) | ||||||||
| [14] | 1_9del ** | - | 1_9del ** | - | hom. | 3 years | M | yes |
| [14] | 1_9del ** | - | 1_9del ** | - | hom. | early childhood | M | no |
| [14] | 1_9del ** | - | 1_9del ** | - | hom. | early childhood | F | no |
| [14] | 1_9del ** | - | 1_9del ** | - | hom. | early childhood | F | no |
| [14] | 1_9del ** | - | 1_9del ** | - | hom. | 2 years | M | yes |
| [14] | 1_9del ** | - | 1_9del ** | - | hom. | early childhood | M | n.s. |
| [15] | 1_9del ** | - | 1_9del ** | - | hom. | 6 years | F | n.s. |
| [15] | 1_9del ** | - | 1_9del ** | - | hom. | 26 years | F | n.s. |
| [15] | 1_9del ** | - | 1_9del ** | - | hom. | early childhood | F | n.s. |
| [15] | 1_9del ** | - | 1_9del ** | - | hom. | early childhood | F | yes |
| [16] | 58G > T ** | E20X | 58G > T ** | E20X | hom. | early childhood | M | yes |
| [16] | 58G > T ** | E20X | 58G > T ** | E20X | hom. | N/A | M | no |
| [16] | 58G > T ** | E20X | 58G > T ** | E20X | hom. | N/A | F | no |
| Other MD-related plectinopathy reports | ||||||||
| [67] | 3064C > T | Q1022Ter | 11503G > A | G3835S | c.het. | 4 years | F | no |
| [67] | 3064C > T | Q1022Ter | 11503G > A | G3835S | c.het. | 16 years | F | no |
| [68] | 6118C > T | R2040W | 10063T > A | F3355I | c.het. | 2 years | M | yes |
| Ref | Mutation | Histological Analysis | Des | Ultrastructural Analysis |
|---|---|---|---|---|
| EBS-MD | ||||
| [26] | 954_956dupGCT 4222C > T | irregular distribution of nuclei, variation in fiber size and shape | N/A | Z- and I-band alterations, disoriented fibers, misplaced/degenerating mitochondria, slightly altered postsynaptic cleft system |
| [28] | 968G > A 4840G > T | cytoplasmatic nuclei, variation in fiber size, and widened interfiber spaces | + | N/A |
| [24] | 2264_2266del 3119_3210del | myopathic pattern, fibers with rubbed-out lesions | + | degenerating myofibrils, autophagic vacuoles |
| [19] | 2677_2685del 4930C > T | variation in fiber size, atrophic fibers, occasional de-/regenerating fibers, internal nuclei, sarcolemmal nuclear aggregates | + | subsarcolemmal clustering of mitochondria (dumbbell-shaped), paracrystalline inclusions |
| [24] | 5018_5036del | myopathic pattern, fibers with rubbed-out lesions, COX-negative fibers | + | N/A |
| [48] | 5728C > T | variation in fiber size, hypertrophic and atrophic fibers | + | disorganization in myofibrils and sarcomeres |
| [49] | 5815delC | variation in fiber size, very small vacuoles at the edge of occasional fibers | N/A | loss of sarcomere organization and Z-line-plasma membrane anchorage |
| [49] | 5854_5855del | very mild variation in fiber size | N/A | myocyte disorganization, space between the membrane and the plasma membrane is enlarged |
| [22] | 5902_5093del 9109_9125del | fiber necrosis and regeneration, variation in fiber size, atrophic fibers, increased amounts of connective and fatty tissue | N/A | increased space between sarcolemma and sarcomere, myofibrillar disorganization, sarcomere disorganization, glycogen inclusions |
| [49] | 7393C > T | variation in fiber size, atrophic and split fibers, central nuclei, increased amount of connective tissue | N/A | loss of normal sarcomere organization and Z-line-plasma membrane anchorage |
| [23,24] | 13459_13474dup | myopathic pattern, rimmed vacuoles, fibers with rubbed-out lesions | + | degenerating myofibrils, cytoplasmic bodies, abnormally shaped mitochondria with paracrystalline inclusions |
| EBS-MD-MyS | ||||
| [56] | IVS11 + 2T > G 10187_10190del | variation in fiber size, mild increase in fat and connective tissue, inflammatory infiltrates, predominance of type 2 fibers | N/A | N/A |
| [57] | 1500_1501ins36 | necrotic fibers, endomysial fibrosis, variation in fiber size, splitting of hypertrophied fibers | N/A | fragmentation of endplates, postsynaptic simplification, thread-like mitochondria |
| [58] | 2539−2A > G 11737delC | variation in fiber size, internalized myonuclei, COX-negative fibers, rubbed-out lesions, increase in connective tissue | N/A | N/A |
| [60,61] | 6169C > T 12043dupG | variation in fiber size, necrotic and regenerating fibers, increased fibrous and fatty tissue | N/A | apoptotic nuclei, nemaline rods, disarrayed myofibrils, thick-filament loss, vacuolar change, endplate degeneration of the junctional folds |
| [61] | 6955C > T 12043dupG | necrotic and regenerating fibers, variation in fiber size, endomysial fibrosis, clusters of large nuclei at periphery | N/A | large nuclei, clusters of mitochondria, aberrant and disrupted myofibrils, NMJ destruction of the junctional folds |
| LGMDR17 (P1f mutation) | ||||
| [14] | 1_9del * | variation in fiber size, internal nuclei, scattered basophilic and few necrotic fibers, mild endomysial fibrosis, predominance of type 2 fibers | N/A | membrane duplications, enlarged space between the membrane and sarcomere, misaligned Z-disks, glycogen inclusions |
| [15] | 1_9del * | myopathic changes, internal nuclei, angular atrophic fibers | N/A | N/A |
| [16] | 58G > T* | variation in fiber size, central nuclei, fibrosis, swollen endomysium | + | Z-line disorganization, enlarged space between the membrane and sarcomere, clusters of mitochondria |
| Other MD-related plectinopathy reports | ||||
| [68] | 6118C > T 10063T > A | dystrophic features, variation in fiber size, scattered necrotic fibers and mild endomysial fibrosis | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zrelski, M.M.; Kustermann, M.; Winter, L. Muscle-Related Plectinopathies. Cells 2021, 10, 2480. https://doi.org/10.3390/cells10092480
Zrelski MM, Kustermann M, Winter L. Muscle-Related Plectinopathies. Cells. 2021; 10(9):2480. https://doi.org/10.3390/cells10092480
Chicago/Turabian StyleZrelski, Michaela M., Monika Kustermann, and Lilli Winter. 2021. "Muscle-Related Plectinopathies" Cells 10, no. 9: 2480. https://doi.org/10.3390/cells10092480
APA StyleZrelski, M. M., Kustermann, M., & Winter, L. (2021). Muscle-Related Plectinopathies. Cells, 10(9), 2480. https://doi.org/10.3390/cells10092480







