Abstract
Adaptive cellular immunity plays a major role in clearing microbial invasion of mucosal tissues in mammals. Following the clearance of primary pathogens, memory lymphocytes are established both systemically and locally at pathogen entry sites. Recently, resident memory CD8 T and B cells (TRM and BRM respectively), which are parked mainly in non-lymphoid mucosal tissues, were characterized and demonstrated to be essential for protection against secondary microbial invasion. Here we reviewed the current understanding of the cellular and molecular cues regulating CD8 TRM and BRM development, maintenance and function. We focused particularly on elucidating the role of a novel tissue-resident helper T (TRH) cell population in assisting TRM and BRM responses in the respiratory mucosa following viral infection. Finally, we argue that the promotion of TRH responses by future mucosal vaccines would be key to the development of successful universal influenza or coronavirus vaccines, providing long-lasting immunity against a broad spectrum of viral strains.
1. Introduction
A cardinal feature of the adaptive immune system is the ability to develop immunological memory following primary antigenic encounter. Upon microbial invasion, naïve T cells, primed by antigen-presenting cells (APCs), rapidly undergo massive expansion and effector T cell differentiation to generate a large pool of antigen-specific effector T cells for the clearance of invading pathogens. Following pathogen clearance, effector T cells go through a contraction phase, in which majority of the effector T cells undergo apoptosis. The surviving effector cells or memory precursor cells convert into long-term memory T cells (including both CD4 and CD8 memory) after the contraction phase. Based on their trafficking properties, CD8 memory T cells can be further categorized into different subsets including central memory T (TCM), effector memory T (TEM), peripheral memory T (TPM) and tissue resident memory T (TRM) cells [1,2,3,4,5]. TCM cells re-circulate through secondary lymphoid organs, TEM cells have more broad capacity of mobility between blood and non-lymphoid tissues, while TPM cells mainly patrol the blood vessels [4]. In contrast, TRM cells are parked in non-lymphoid mucosal tissues and have an impaired capacity to enter re-circulation [6,7,8,9,10]. Following secondary infection with the same virus or viruses bearing conserved T cell epitopes, memory T cells are rapidly activated, undergo secondary effector T cell expansion and differentiation, and mediate prompt pathogen clearance before their systemic dissemination.
Similarly, naïve B cells can be activated and differentiated into extrafollicular plasmablasts (PBs) or germinal center (GC) B cells in secondary lymphoid organs. The primary extrafollicular PBs have been identified as short-lived antibody secreting cells and may provide a significant source of protective antibodies during microbial infections [11]. GCs are anatomically distinguished into two regions, the dark zone and the light zone [12]. Proliferation and somatic hypermutation of antigen-specific GC B cells occur in the dark zone, following which mutated B cell clones move into the light zone to terminate their differentiation. Within the light zone, B cells internalize antigens which are presented by follicular DCs (FDCs) and interact with follicular helper T (TFH) cells, a major CD4 T helper subset facilitating B cell-help [13,14]. GC B cells can further differentiate into long-lived plasma cells (LLPCs) or memory B cells (MBCs). LLPCs mediate long-term antibody secretion following primary infection, while MBCs can respond to secondary infection to either differentiate into PBs or re-enter GCs to undergo further affinity maturation [15,16,17]. Like memory T cells, MBCs can be divided into circulating and tissue-resident (BRM) populations, which reside in mucosal tissues [18].
Despite intensive studies on the cellular and molecular programming of memory lymphocyte generation over the past two decades, our understanding of the mechanisms of memory T and B cell maintenance and function, particularly in the mucosal tissues remains limited. Furthermore, little is known regarding the cellular and molecular pathways that may be targeted to simultaneously promote both BRM and TRM cell responses in the mucosal tissues. Here we will review the current understanding of mechanisms maintaining long-term immunological memory in mucosal tissues, with a focus on the roles of a subset of CD4 T helper cells, tissue-resident T helper cells (TRH), in coordinating respiratory mucosal CD8 and B cell memory responses following viral infection.
2. Tissue-Resident Memory T (TRM) and B (BRM) Cells
2.1. Generation of CD8 TRM Cells
CD8 TRM cell development is initiated when dendritic cells (DCs), either resident within lymph nodes or following migration from peripheral tissues, prime naïve T cells into effector CD8 T cells in draining lymph nodes. Antigen-experienced effector CD8 T cells subsequently infiltrate infected tissue to mediate pathogen clearance, after which a subset of CD8 T cells persists to become TRM cells. Interestingly, migrating DCs can also precondition naïve T cells towards a resident memory T cell fate via the activation and presentation of transforming growth factor (TGF)-β [19]. Furthermore, distinct DC subsets appear to differentially program lymph node homing TCM, mucosal tissue-homing effector and resident memory T cells following influenza infection [20]. Therefore, TRM cell development may start before the entry of effector T cells into peripheral organs. Such a notion is supported by recent genetic tracing (using retroviral barcoding) and single cell RNA-seq experiments demonstrating the existence of TRM precursors in the circulating effector T cell pool [21].
After their activation in lymphoid organs, effector CD8 T cells enter the circulation and migrate into nonlymphoid tissues to combat invading pathogens. While CD8 TRM fate determination can be trained in lymphoid organs, local environment and antigenic re-encounter in the tissue further promote TRM development and/or maturation. The differentiation of TRM cells can occur independently of local antigen recognition in the peripheral tissue [22,23,24]; however, local antigenic re-stimulation of effector CD8 T cells in nonlymphoid tissue greatly enhances TRM formation [25,26,27]. Following the formation of TRM cells, it is believed that TRM maintenance is largely antigen or TCR signaling independent [28]. However, chronic low levels of TCR stimulation due to persistence of antigen following influenza virus infection or following immunization with an adenoviral vector facilitates the accumulation of a protective population of CD69+ CD8 TRM cells [29,30].
CD69 is a key tissue retention signal of TRM cells functioning via the interference of sphingosine-1-phosphate receptor (S1pr1) activity [31,32], thereby restricting cell egress out of the tissue [31,33]. Local antigen-restimulation enhances the expression of CD69, and concomitantly suppresses S1pr1 and Krüppel-like Factor 2 (KLF2) expression [31,34]. TRM cells located within the epithelium further express CD103 (Integrin, alpha E), which binds to E-cadherin expressed on epithelial cells, supporting the accumulation and retention of TRM cells in tissues [22,35,36]. In the absence of TGF-β signaling, the migrated tissue effector CD8 T cells fail to develop into CD103+ TRM cells due to lack of CD103 expression [35,37,38]. Interleukin (IL)-15 has been reported to provide a survival signal to memory T cells [39]. Soluble IL-15/IL-15Rα complexes in local tissue do not directly induce CXCR3 production in effector cells, but promote the recruitment of CXCR3+ antigen-specific effector T cells to the mucosal area through the downregulation of KLF2 [40]. IL-7 is another important cytokine able to maintain memory T cell homeostasis through Stat5 signaling [41,42]. Interestingly, while IL-7 and IL-15 can both promote CD8 TRM cell maintenance, the homeostatic persistence of skin CD4 TRM cells seems mainly dependent on IL-7 produced from hair follicle of skin [43]. TGF-β has also been reported to facilitate CD8 TRM cell development via down-regulation of T-box transcription factor Eomes and T-bet expression, but residual T-bet activity is critical to maintain responsiveness to IL-15 for CD8 TRM cell survival [44]. In the lymphocytic choriomeningitis virus (LCMV) infection model, combination of cytokines including TGF-β, IL-33 and tumor necrosis factor (TNF) enhances the establishment of CD8 TRM cells via the downregulation of S1pr1 [45]. Additionally, proinflammatory cytokines including type I interferons and IL-12 can facilitate the differentiation and accumulation of CD103− TRM cells in the intestine following bacterial infection [46]. Taken together, CD8 TRM generation is constantly modulated by a variety of antigenic and environmental factors at various steps of the T cell life cycle following infection.
2.2. Transcriptional Regulation of CD8 TRM Development and Persistence
TRM cells exhibit distinct transcriptional profiles when compared to circulating memory T cells. Several transcription factors have been demonstrated to play important roles in TRM cell generation and/or maintenance in peripheral tissues. The two related transcription factors, Blimp-1 and Hobit, cooperatively instruct a tissue-residency transcriptional program in TRM cells [10]. Hobit and Blimp-1 directly bind to Klf2 and Tcf7 loci, and then downregulate the expression of Ccr7 and S1pr1. Therefore, they prevent the egress of TRM cells from tissues to blood circulation [10,45]. Runx3 promotes the expression of CD103 and tissue residency-associated gene programs in TRM cells, while simultaneously repressing signature genes associated with circulating memory [47]. Runx3 has been reported to enhance the accessibility of the Blimp1 binding region and may facilitate the accessibility of the Hobit binding motif as well [48]. Notch signaling through RBP-jκ is also important for the formation of lung TRM cells following influenza virus infection [49]. Of note, TGF-β and Notch signaling can be integrated by direct protein–protein interactions of Smad3 and the intracellular domain of Notch (NICD), potentially providing a mechanism underlying the dual requirement of TGF-β and Notch in TRM formation [50,51]. Compared to circulating memory T cells, TRM cells highly express the transcription factor Bhlhe40 and its-associated molecules. Bhlhe40 deficiency caused diminished expression of TRM tissue residency-associated genes and molecules involved with TRM effector function, largely due to impaired mitochondria fitness and function in Bhlhe40-deficient TRM cells [52]. Additionally, the expression of Ahr and NR4A1 is upregulated in TRM cells compared to circulating memory CD8 T cells, and their function is required for the maintenance of CD8 TRM cells [37,53,54].
2.3. Development and Maintenance of BRM Cells
Long-term humoral immunity is generally maintained by LLPCs in the bone marrow (BM) [55], while resting MBCs provide rapid and augmented antibody (Ab) responses upon recognition of same/conserved antigens following secondary infections [56]. The development of both cell types is initiated within the GC structure following help from CD4 T cells [57,58]. Analogous to memory T cells, MBCs can be divided into circulating and tissue-resident (BRM) populations. BRM cells, mainly residing in non-lymphoid peripheral tissues, express similar tissue-homing and retention molecules as TRM cells. For instance, influenza hemagglutinin-specific lung-residing memory B cells [59] highly express CXCR3 and CD69, which are believed to mediate their tissue-homing and residency respectively [59]. In humans, a large number of CD19+CD27+CD45RB+CD69+ BRM cells have been identified in the gut and tonsil but not in blood and BM [60].
Compared to circulating memory B cells, BRM cells facilitate rapid recall responses in the tissue and may exhibit unique phenotypical and functional markers. For instance, mouse lung BRM expresses lower CD73 than those MBCs in the blood or spleen [18]. Furthermore, respiratory BRM cells reside in specific niches; in the upper respiratory tract, these are located in the nasal-associated lymphoid tissue (NALT) whereas they are found in the inducible bronchus associated lymphoid tissue (iBALT) in the lower respiratory tract [61,62,63]. Like TRM cell development, BRM cells are thought to be initiated following CD4 T cell help in secondary lymphoid organs, but the full establishment of BRM cells in peripheral tissue requires local antigenic re-encounter [18]. Additionally, GC B cells of the lung, particularly those developed early post infection, may also supply BRM precursors following influenza virus infection [64,65]. Thus, optimal BRM development is subject to both distal regulation in the lymphoid organs and local regulation inside the peripheral tissue.
The functions of BRM cells have not been fully elucidated yet. However, BRM cells are thought to provide immediate and rapid responses against pathogen entry at mucosal tissues [66]. MBCs re-activated by pathogens have been reported to either differentiate into antibody secreting cells (ASCs) or undergo expansion and affinity maturation through re-entry of GC [67,68,69]. Lung influenza-specific BRM cells are reported to directly differentiate into ASCs upon recognition of the same viral antigen during influenza reinfection, but do not re-enter the GC structure, thereby facilitating viral clearance in the respiratory mucosa [18]. Interestingly, influenza-specific lung BRM cells developed from local GC responses possess high cross-reactivity to viral escape mutants [64,65], and thus they may exert broadly protective function against distinct viral strains. Furthermore, BRM cells located in tertiary lymphoid structures are also considered potential APCs and may facilitate T cell responses in the respiratory mucosa [66,70]. In addition to influenza infection, antigen-specific BRM cells are also developed following pneumococcal infection, although tertiary lymphoid organ (i.e., iBALT) formation was not observed. Importantly, depletion of PD-L2+ lung BRM cells caused diminished bacterial clearance and reduction of pneumococcus-reactive antibodies in the lung upon pneumococcal reinfection, suggesting that lung BRM cells are vital for pulmonary antibacterial immunity [71].
3. Characteristic Tissue-Resident CD4 T Cells
3.1. Heterogeneity of Tissue-Resident CD4 T Cells
The mechanisms underlying CD4 TRM cell formation and maintenance are relatively less well-studied compared to those of CD8 TRM cells. Similar to CD8 TRM cells, activated CD4 T cells migrate into peripheral tissues and survive long term to form CD4 TRM cells. CD4 TRM cells share tissue-residency markers like CD69 and CXCR6 with CD8 TRM cells [72,73]. Like mouse TRM cells, human CD4 TRM cells also express high level of CD69 while circulating CD4 memory T cells do not [73,74]. Unlike effector CD8 T cells, which mainly produce type 1 cytokines such as IFN-γ and TNF, effector CD4 T cells can be subdivided into distinct subtypes based on their cytokine production including IFN-γ producing T helper type 1 (TH1), IL-4/5/13-producing T helper type 2 (TH2), IL-17 producing T helper type 17 (TH17) and IL-21 producing follicular helper T (TFH) cells [75,76]. In the murine model, tissue-resident TH1 (TH1 TRM) cells have been reported following respiratory infection. Lung TH1 TRM cells developed following influenza virus infection rapidly produce IFN-γ and contribute to host protection upon secondary infection [77,78]. TH1 TRM cells have also been identified in the skin and gut following Leishmania and Listeria infections, respectively [79,80,81]. TH1 TRM cells generated by tuberculosis are characterized by high expression of CXCR3 and low expression of KLRG1 [82,83]. For long-term maintenance in tissues, TH1 TRM cells express high levels of CD11a and VLA-1 to promote their retention and survival in the tissue niche [84]. TH2 TRM are usually generated during allergic responses or parasitic infections. In a house dust mite (HDM)-induced allergic asthma model, it was shown that lung TRM cells and circulating TH2 memory cells cooperatively induce allergic inflammation in the lung [85]. During Heligmosomoides polygyrus infection, TH2 TRM cells are formed and persist in the lamina propria and peritoneal cavity (PC). Interestingly, TH2 TRM cells in both sites produce general TH2 cytokines like IL-4, IL-5 and IL-13 following TCR restimulation, but only TH2 TRM in PC can respond with IL-33 and IL-7 upon TCR-independent restimulation [86]. TH17 TRM can be generated following Candida albicans (C. albicans) infection in both mouse and human subjects. Both circulating TH17 memory cells and TH17 TRM cells are important to clear the C. albicans upon rechallenge, but TH17 TRM cells are more effective in rapidly clearing the pathogens [87]. In Mycobacterium tuberculosis (M. tuberculosis)-infected patients, lung CD4 TRM cells produce IL-17 following antigenic stimulation, which along with IL-17 and IL-2 produced by TH17 TRM cells suppress the growth of M. tuberculosis in 3D culture system [88].
Like effector TRM subsets, Foxp-3 expressing regulatory T cells (TREG) cells in the tissue can express CD69 and possess tissue-residency features. Importantly, tissue-resident TREG cells may provide an essential check point for the pathogenic activities of TRM cells. Chronic exposure of Aspergillus fumigatus induces the formation of CD69hiCD103loCD4 TRM cells, which contribute to pulmonary fibrosis. At the same time, CD69hiCD103hiFoxp3+ TREG cells constrain the effects of pathogenic CD103lo CD4 TRM cells and limit their fibrogenic potential [89]. Furthermore, lung tissue TREG cells produce amphiregulin (Areg), an epidermal growth factor receptor ligand, to repair tissue damage following influenza virus infection [90].
Common gamma-chain cytokines such as IL-2, IL-15 and IL-7 are essential to develop or maintain memory CD4 T cells. The cytokines have recently been reported to be essential in the formation of CD4 TRM cells. Autocrine IL-2 signaling in infiltrating tissue CD4 T cells is critical for the generation of TH1 TRM cells in the lung [91]. In the absence of IL-2R signaling, TH1 TRM cells induced by intranasal LCMV infection or allergic TH2 TRM cells generated following HDM administration fail to be maintained over the long-term within the lung [92,93]. High levels of IL-7 receptor are expressed by lung TRM cells compared to circulating memory T cells, and IL-7 treatment in vivo induces the infiltration of circulating CD4 T cells into the lung to form TRM cells [94]. Similarly, IL-15 supports the development of lung CD4 TRM post influenza virus infection [95].
3.2. Niches of Local CD4 T Cells
Tissue-resident CD4 T cells are typically located under epithelial layers and inside the ectopic lymphoid structures with stromal cells or APCs [96]. In the skin, particularly the dermis, lymphoid structures are generated around hair follicles with CD4 T cells and CD11b+DCs [97,98]. CCL5, IL-7 and IL-15 which are produced in the local environment, promote the maintenance of CD4 TRM clusters in skin [43,97]. The female reproductive tract (FRT) is a mucosal tissue that consists of two different areas, including the upper FRT and the lower FRT. Mucosa-associated lymphoid tissues (MALT) composed of B cells and CD4 TRM cells are typically developed in the lamina propria (LP) of the upper FRT. Furthermore, CD4 T cells can migrate to upper FRT following skin infection with Chlamydia to form a cluster with B and CD8 TRM cells [99,100]. In the lower FRT, there are no MALT at steady state, but CD4 TRM cells along with B cells, DC and macrophages form clusters during the clearance of an intravaginal HSV-2 infection. APCs including B cells and DCs facilitate CD4 TRM maintenance in the lower FRT area [101,102].
The respiratory tract is an entry site for many viruses including influenza, respiratory syncytial virus (RSV) or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The respiratory tract can also be divided into two compartments, the upper respiratory tract (URT) and the lower respiratory tract (LRT). Nasal-associated lymphoid tissues (NALT) localized in URT contain CD4 TRM cells [96]. iBALTs within the LRT are the primary niches for lung CD4 TRM cells [64,103,104] following influenza infection, which is in contrast to those influenza-specific CD8 TRM cells that are primarily localized to the site of regeneration in the lung parenchyma following tissue injury [33,105].
4. CD4 Help and Memory B and CD8 T Cell Responses
4.1. CD4 Help and Memory B Cell Generation
CXCR5 and PD-1 expressing CD4 helper T cells that localize in the GC are termed TFH cells [106]. TFH cells express the transcription factor BCL6, the cytokine IL-21 and provide CD4 T cell help to B cells in the GC. Therefore, TFH cells are important in the development of long-term humoral immunity mediated by MBCs and plasma cells (PCs), which are mainly derived from GC B cells. TFH cell formation goes through two sequential steps [58], including T cell priming first at the T cell zone by DCs followed by subsequent maturation in the B cell zone through interactions with B cells via ICOS-ICOS-L and MHC II-TCR. Cytokines including IL-6, IL-12 and IL-21 can promote TFH formation, while IL-2 and type I IFNs potently suppress TFH cell generation [58,107,108,109,110].
BCL6loCD69hi GC-B cells that express high levels of IRF4 favor the differentiation into PCs [111], while CCR6 has been reported as a marker for memory B cell precursors [112]. Recently, IL-9 producing TFH cells have been reported to support the development of GC-derived memory precursor B cells and subsequent optimal formation of memory B cells [113]. Interestingly, the strength of the interaction between GC B and TFH cells affects the formation of memory B cells. Cells prone to enter the memory B cell pool typically exhibit lower B cell receptor affinity and express high levels of Bach2, which has been found to be inversely correlated with the strength of help provided by TFH cells [114].
4.2. CD4 T Cell Help and CD8 Memory T Cell Responses
CD4 T cell help plays an indispensable role in the primary CD8 T cell response in certain infection and/or immunization models [115,116,117]. CD4 help is critical for licensing APCs to support optimal CD8 T cell activation and differentiation [118]. In this case, interaction of CD4 T cells with APCs promotes the expression of key co-stimulatory molecules and pro-inflammatory cytokines required for maximal CD8 T cell activation [119,120]. Additionally, cytokines produced by CD4 T cells such as IL-2 can facilitate CD8 T cell expansion and effector generation [121]. However, in infectious models that generate strong inflammatory responses such as influenza infection, the primary CD8 T cell responses are largely independent of CD4 T cell help, potentially due to the direct activation of DCs by robust TLR signaling [122].
In contrast to the context-dependent roles of CD4 T cells in helping primary CD8 T cell responses, CD4 T cell help is uniformly required for the generation, maintenance and/or recall responses of memory CD8 T cell responses. To this end, CD4 T cell-derived IL-2 has been linked to promote secondary CD8 T cell responses [123,124]. Furthermore, CD4 T cells could license APCs to produce IL-15, a key cytokine involved in memory CD8 T cell formation and/or maintenance [120]. Additionally, TREG cell-derived IL-10 has been shown to promote memory CD8 T cell maturation during the contraction phase via the suppression of pro-inflammatory cytokine production by DCs [125]. Lastly, activated CD4 T cells may directly interact with effector CD8 T cells via CD40-CD40L to facilitate memory CD8 T cell differentiation [126]. Regardless of the molecular cues provided by CD4 T cells for CD8 memory T cell generation, un-helped CD8 T cells are prone to apoptosis and could undergo activation-induced cell death, possibly through the induction of TRAIL expression [116,127].
Besides the role of CD4 help in the generation of circulating effector and memory T cells, recent advances have suggested that CD4 T cell help is important in mucosal T cell responses and the induction of CD8 TRM responses. During HSV-2 infection, CD4 T cells control the migration of CTL through the secretion of IFN-γ and induction of local chemokine secretion in the infected tissue [128]. During influenza infection, CD4 T cell help occurs at the priming phase of T cell responses, which is critical for the development of CD8 TRM cells in the lung. In the absence of CD4 T cell help, CD8 T cells failed to properly localize to the lung niches supporting optimal TRM development. Furthermore, un-helped CD8 T cells exhibited high levels of T-bet expression, which interferes with CD103 expression through the modulation of TGF-β responsiveness [129]. These results demonstrate the importance of CD4 helper T cells in the formation of CD8 TRM cell precursors, while the role of CD4 T cell help in CD8 TRM cell maintenance has not been elucidated.
5. CD4 Tissue-Resident Helper Cells Coordinate TRM and BRM Responses in the Respiratory Mucosa
5.1. TFH-Like Cells in Non-Lymphoid Tissues
Although TFH cells are generally localized in secondary lymphoid organs, TFH-like cells can be found in circulation or in non-lymphoid tissues [130,131]. In human joint tissue from patients with rheumatoid arthritis, a subset of TFH-like peripheral helper CD4 T cells (TPH) that exhibits potent B cell help activities was recently identified [132]. Phenotypically, TPH cells share key signatures with TFH cells including high levels of PD-1 expression, production of IL-21, expression of BCL6 and the lack of expression of other T helper lineage cytokines and/or transcription factors. However, TPH cells have distinct features from TFH cells, including the lack or low expression of CXCR5 expression and the high expression of Blimp1. TPH or TPH-like cells have been observed in tertiary lymphoid structures developed in various inflammatory conditions such as rheumatoid arthritis (RA), Crohn’s disease and malignancy [133,134,135,136].
Tissue-infiltrating TFH-like cells have also been reported in animal models, particularly in inflammatory lung conditions [130,137,138]. In an HDM-induced allergic model, IL-21 producing TFH-like cells, which lack expression of CXCR5, were found in the inflamed lung [137]. Furthermore, IL-21 produced by those TFH-like cells promotes lung TH2 responses, eosinophil recruitment and HDM-specific IgG1 production [137]. Like human TPH cells, antigen-specific TFH-like cells expressed high levels of IL-21, PD-1 and ICOS, but lower BCL6, CXCR5 than lymph node TFH cells. In a model of LPS-adjuvanted airway immunization model, lung-infiltrating T cells were identified to exhibit follicular helper-like properties including the potential to provide help to naive B cells. These TFH-like cells did not express classical TFH markers, CXCR5 and PD-1, but expressed molecules involved in B cell help including CD40L and IL-21. As such, these TFH-like cells supported the generation of GC B cells in situ within the lung [138]. Together, these data demonstrate the presence of a TFH-like CD4 helper population in non-lymphoid tissues, but the roles of these cells in regulating local B and CD8 memory T cells have not been examined. Furthermore, the cellular and molecular cues regulating the development of TFH-like cells in peripheral tissue are still unknown.
5.2. Identification of Tissue-Resident Helper T Cells in the Lung
As stated above, many questions remain unanswered regarding the nature and function of TFH-like cells in non-lymphoid tissues. First, are the TFH-like cells tissue-resident in non-lymphoid tissues? Second, what are the mechanisms underlying the phenotypic similarity and difference between conventional TFH cells in secondary lymphoid organs and TFH-like cells in the non-lymphoid tissues? Third, what are the physiological functions of TFH-like cells in regulating local tissue immunity?
Using single-cell RNA sequencing, our group and the group of Carolyn King recently found that lung parenchyma CD4 T cells exhibit marked heterogeneity following primary influenza virus infection. In addition to traditional TH1-like TRM, TH17-like TRM cells and tissue TREG cells, lung parenchyma CD4 T cell compartment contains a TFH-like CD4 T cell population [64,104]. This TFH-like cell population appeared around two weeks after infection, following clearance of infectious virus and persisted through the memory phase, i.e., more than two months after infection. The TFH-like cells expressed modest BCL6 and CXCR5, and high levels of PD-1, IL-21 and FR4, thus exhibiting key TFH phenotypic markers. Compared to splenic TFH cells, these TFH-like cells had higher levels of the tissue residency gene program, the transcription factor Bhlhe40 and peripheral homing marker CXCR6, thus exhibiting TRM features. Indeed, using parabiosis, we demonstrated that these tissue TFH-like cells were tissue-resident. Furthermore, the optimal responses of these lung TFH-like cells required the presence of both BCL6 and Bhlhe40, demonstrating the dual requirement of TFH and TRM gene programs for its development [52]. Thus, these tissue TFH-like cells appear to be a “hybrid” population of TFH and TRM cells. Based on the transcriptional, phenotypic and non-migratory characteristics, we termed these cells tissue-resident helper T cells (TRH).
5.3. Promotion of Local B Cell Immunity by TRH Cells
Like TFH cells, TRH cells express key B cell helping molecules including ICOS, CD40L and/or IL-21. As such, TRH ablation severely impaired lung GC B cell responses and iBALT formation (Figure 1).
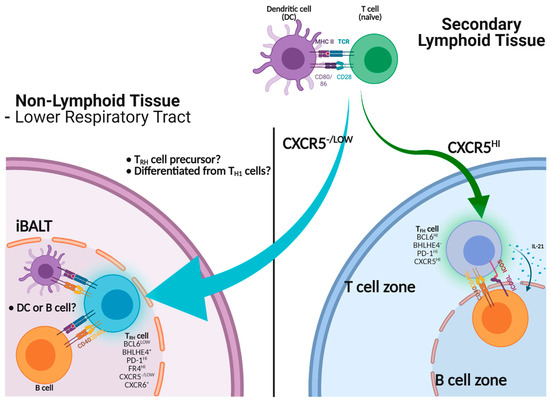
Figure 1.
Help of B cell immunity by TRH or TFH cells. Activated CD4 T cells migrate into the B cell zone to become mature CXCR5hi TFH cells to help B cells via CD40-CD40L, ICOS-ICOS-L interactions and cytokines including IL-21. TRH precursors, which express low levels of CXCR5, can infiltrate into non-lymphoid tissues such as the lung. TRH precursors adapt to the lung environment to become mature TRH cells, thereby assisting B cell immunity in situ through the expression of CD40L.
As discussed above, lung GC contributes to respiratory BRM development and iBALT is likely a niche for lung BRM cells. Consequently, we found that TRH ablation impaired influenza-specific lung BRM responses but not systemic B cell memory. Those BRM cells that are cross-reactive to viral escape mutants were also diminished following TRH depletion, suggesting that TRH cells may be key for the development of broadly reactive memory B cells against heterologous influenza strains [64]. In a subsequent study, Swarnalekha et al. further showed that lung antigen-specific ASCs were significantly decreased in TRH cell-ablated mice following influenza virus rechallenge [104], demonstrating the importance of TRH cells in mediating memory B cell recall responses.
These studies have established the critical roles of TRH in assisting the development local effector and memory B cell responses during both primary and recall responses following influenza virus infection. Swarnalekha et al. also showed that TRH cells are localized within the iBALT structure, while TH1 TRM cells are more concentrated outside or at the border of the iBALT, suggesting that TRH cells may lend their help to B cells within the tertiary lymphoid organ. Similar to TFH cells, TRH development requires B cells and is facilitated by prolonged antigen presentation [104]. Interestingly, we found that the B cell helper function of TRH cells was dependent on the CD40-CD40L interaction, but not IL-21, which is required for TFH-mediated B cell help. Thus, TRH and TFH cells may have both common and distinct B cell help mechanisms.
5.4. TRH Cells and the Maintenance of CD8 TRM Cells
During experiments dissecting the physiologic function of TRH cells, perhaps the biggest surprise came from the observation that TRH depletion selectively diminished a population of CD8 TRM cells specific to the influenza Nucleoprotein peptide 366–374 (NP366–374) [64]. Previously, we found that NP366–374 specific CD8 TRM cells receive persistent low-levels of antigenic stimulation at the memory stage, due to the delayed clearance of the NP antigen [29]. These NP366–374 TRM cells expressed high levels of PD-1 but low levels of CD103 and possessed features of both memory and exhausted-like T cells, compared to those of conventional CD69+CD103+ TRM cells [29]. Importantly, the NP366–374 TRM cells offered critical protective function against secondary heterologous viral infection. Conversely, the blockade of PD-1 activity at the memory stage selectively expanded these TRM cells and promoted the lung pathological responses [29]. Thus, those PD-1Hi exhausted-like TRM cells are important in maintaining the balance between TRM-mediated protection and pathology. When TRH cells were ablated, we found that the quantity of NP366–374 TRM cells were significantly decreased, but not those of conventional TRM cells restricted to other peptides such as the influenza polymerase peptide 224–233 (PA224–233). Consequently, TRM-mediated protective immunity against heterologous viral reinfection was diminished following TRH ablation. These data suggest that TRH cells are vital for maintaining protective TRM responses following influenza infection.
As discussed above, BCL6-expressing TRH cells are located inside the iBALT [104]. Previous results have shown that CD8 TRM cells are found outside the iBLAT (nearby border area), most of which are particularly localized near repair associated memory depots (RAMD) [105]. It is thus intriguing that TRH cells facilitate NP366–374 TRM maintenance in a contact-independent way, as TRH and TRM are likely localized in different lung niches. IL-21 has been showed to be a critical molecule mediating CD4 T cell help for optimal CD8 T cell responses during chronic viral infection [64]. Since NP366–374 TRM cells exhibit features of exhausted CD8 T cells from chronic viral infection, we hypothesized that IL-21 produced by TRH cells is critical to sustain NP366–374 TRM responses (Figure 2).
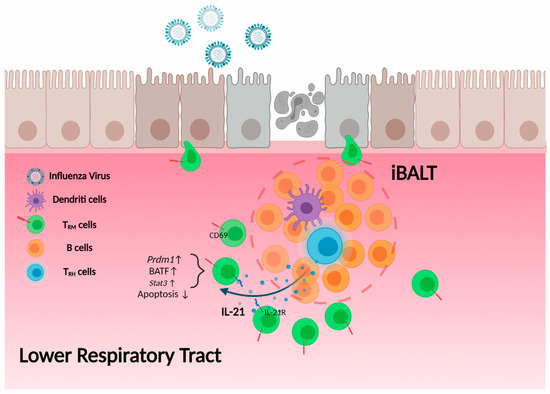
Figure 2.
TRH cell help to CD8 TRM cells. TRH cells, which localize within the iBALT, secrete IL-21. Influenza NP366–374-specific CD8 TRM cells are located outside but near the border of iBALT structure and express high levels of IL-21 receptor. IL-21 secreted from TRH cells promotes the expression of Blimp-1 (Prdm1), BATF and other molecules in CD8 TRM cells, thereby maintaining NP366–374-specific TRM cell retention and survival in the lung.
Indeed, IL-21R blockade following influenza viral clearance led to diminished NP366–374 TRM responses. Furthermore, IL-21 is mainly produced by TRH cells in the lung following influenza infection [64]. These data suggest that TRH cells maintain optimal TRM-mediated protective immunity through IL-21, which may act over a relatively longer range to provide help to CD8 TRM cells compared to their help to B cells, which is mediated by cell surface molecule CD40L. Consistent with our observations, antigen-specific IL-21 producing CD4 T cells have also been identified in the brain of mice after polyomavirus (MuPyV) infection. The IL-21-producing CD4 T cells express high-affinity TCRs and TFH cell markers like PD-1 and CXCR5. Importantly, in the absence of IL-21 signaling, brain CD8 T cells failed to differentiate into TRM cells with sufficient CD103 expression [139]. Although it is not known whether these brain TFH-like cells had TRM features (thus being equivalent to brain TRH cells), these data do suggest that tissue TFH-like cells may be required for sustaining maximal CD8 TRM responses in a broad spectrum of nonlymphoid tissues.
6. TRH cells as a Potential Target for Mucosal Vaccine against Respiratory Viral Infection
Lower respiratory tract viral infections represent a major public health challenge and economic burden worldwide. In a matter of months, SARS-CoV2 infection completely altered societal norms, stagnated economies, and overwhelmed healthcare infrastructures across the globe. Annual influenza epidemics cause up to 500,000 deaths globally and there exists an ever-present threat of the emergence of a pandemic influenza strain in the future [140]. Vaccination still remains the best approach to mitigate disease burden caused by respiratory viral infection. Current vaccines against influenza and SARS-CoV2 infections are mainly administered via the systemic route, which induces strong systemic but typically weak mucosal immune responses [141,142,143,144].
The efficacy of the current influenza vaccines in providing protection against infection is still relatively limited, even in the years when the predicted vaccine strains perfectly match circulating strains. Furthermore, due to escape mutants generated by the rapid mutation of influenza virus, the current influenza vaccines require an annual update. To this end, the “holy grail” of influenza vaccine development is to create a universal influenza vaccine that can provide long-lasting and cross-reactive immunity against a broad-spectrum of influenza viral strains. It is argued that an “all-inclusive” approach, i.e., the induction of concerted immune responses, including both strong memory B and T cell responses, is needed to provide protective immunity against a wide ranges of influenza viruses [145]. Due to the nature of mucosal BRM and TRM responses, a mucosal vaccine that can induce strong cross-reactive BRM and TRM responses is more than likely a viable strategy for universal vaccine development. Since TRH cells are able to provide local “help” for the development and/or maintenance of robust mucosal B and CD8 T cell responses, we argue that the promotion of TRH responses by future mucosal vaccines will be key to develop successful universal influenza vaccines.
The current mRNA vaccines against SARS-CoV2 infection induce robust systemic immunity, thereby providing strong protection against viral infection and severe disease development [146]. It is still unknown whether SARS-CoV2 mRNA vaccines are able to induce effective mucosal antibody and/or B/T cell responses in humans. However, based on data generated using mouse immunization models, it seems unlikely that systemic immunization of mRNA-encoded antigens would generate strong mucosal memory T and B cells against SARS-CoV2 infection. Despite the current success of SARS-CoV2 mRNA vaccines, emerging data have suggested that the mRNA vaccine induced protective immunity may be dampened against newer variants of the SARS-CoV2 virus, particularly the delta strain [147,148]. Thus, there is still room for improvement with the development of better and broader protective SARS-CoV2 vaccines. To this end, a SARS-CoV2 vaccine capable of inducing both strong humoral and cellular (i.e., BRM and/or TRM development) immunity in the respiratory mucosa may ultimately meet the needs for long-lasting protection against a variety of SARS-CoV2 strains. In this case, the induction of robust respiratory TRH responses again may be a pre-requirement for the success of such a vaccine.
7. Concluding Remarks
Memory lymphocytes establish tissue residency in the respiratory tract following pulmonary viral infection and/or mucosal vaccination. Here we reviewed the developmental cues and molecular mechanisms regulating the formation of CD4 and CD8 TRM and BRM cells in mucosal tissues. We put forward a TRH-centric model, modulating the concerted development of mucosal B and T cell memory responses following mucosal infection and immunization. We believe that the induction of a strong TRH response is key to protective mucosal immunity generated by future universal vaccine candidates. Conversely, dysregulated TRH responses may contribute to the development pulmonary inflammation in various disease conditions including asthma or long-term chronic sequelae following respiratory viral infections [149,150].
Author Contributions
Y.M.S. and J.S. designed the contents and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the U.S. NIH R01s AI112844, AI147394, AI154598 and AG047156 (to J.S.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Harish Narasimhan for critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Farber, D.L.; Ahmadzadeh, M. Dissecting the complexity of the memory T cell response. Immunol. Res. 2002, 25, 247–259. [Google Scholar] [CrossRef]
- Mueller, S.N.; Gebhardt, T. Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 2013, 31, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Badovinac, V.P. Defining Memory CD8 T Cell. Front. Immunol. 2018, 9, 2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlach, C.; Moseman, E.A.; Loughhead, S.M.; Alvarez, D.; Zwijnenburg, A.J.; Waanders, L.; Garg, R.; de la Torre, J.C.; von Andrian, U.H. The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity 2016, 45, 1270–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masopust, D.; Soerens, A.G. Tissue-Resident T Cells and Other Resident Leukocytes. Annu. Rev. Immunol. 2019, 37, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, T.; Wakim, L.M.; Eidsmo, L.; Reading, P.C.; Heath, W.R.; Carbone, F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009, 10, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, K.; Vincenti, I.; Merkler, D. Resident-Memory T Cells in Tissue-Restricted Immune Responses. For Better or Worse? Front. Immunol. 2018, 9, 2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grau-Expósito, J.; Sánchez-Gaona, N.; Massana, N.; Suppi, M.; Astorga-Gamaza, A.; Perea, D.; Rosado, J.; Falcó, A.; Kirkegaard, C.; Torrella, A.; et al. Peripheral and lung resident memory T cell responses against SARS-CoV-2. Nat. Commun. 2021, 12, 3010. [Google Scholar] [CrossRef]
- Mackay, L.K.; Minnich, M.; Kragten, N.A.; Liao, Y.; Nota, B.; Seillet, C.; Zaid, A.; Man, K.; Preston, S.; Freestone, D.; et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 2016, 352, 459–463. [Google Scholar] [CrossRef] [Green Version]
- Blanchard-Rohner, G.; Pulickal, A.S.; Jol-van der Zijde, C.M.; Snape, M.D.; Pollard, A.J. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood 2009, 114, 4998–5002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Silva, N.S.; Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 2015, 15, 137–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Xu, H.; Shih, C.; Wan, Z.; Ma, X.; Ma, W.; Luo, D.; Qi, H. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature 2015, 517, 214–218. [Google Scholar] [CrossRef]
- Han, S.; Hathcock, K.; Zheng, B.; Kepler, T.B.; Hodes, R.; Kelsoe, G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 1995, 155, 556–567. [Google Scholar]
- Zuccarino-Catania, G.V.; Sadanand, S.; Weisel, F.J.; Tomayko, M.M.; Meng, H.; Kleinstein, S.H.; Good-Jacobson, K.L.; Shlomchik, M.J. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat. Immunol. 2014, 15, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Dugan, H.L.; Guthmiller, J.J.; Arevalo, P.; Huang, M.; Chen, Y.Q.; Neu, K.E.; Henry, C.; Zheng, N.Y.; Lan, L.Y.; Tepora, M.E.; et al. Preexisting immunity shapes distinct antibody landscapes after influenza virus infection and vaccination in humans. Sci. Transl. Med. 2020, 12, eabd3601. [Google Scholar] [CrossRef]
- Mesin, L.; Schiepers, A.; Ersching, J.; Barbulescu, A.; Cavazzoni, C.B.; Angelini, A.; Okada, T.; Kurosaki, T.; Victora, G.D. Restricted Clonality and Limited Germinal Center Reentry Characterize Memory B Cell Reactivation by Boosting. Cell 2020, 180, 92–106.e11. [Google Scholar] [CrossRef] [Green Version]
- Allie, S.R.; Bradley, J.E.; Mudunuru, U.; Schultz, M.D.; Graf, B.A.; Lund, F.E.; Randall, T.D. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 2019, 20, 97–108. [Google Scholar] [CrossRef]
- Mani, V.; Bromley, S.K.; Äijö, T.; Mora-Buch, R.; Carrizosa, E.; Warner, R.D.; Hamze, M.; Sen, D.R.; Chasse, A.Y.; Lorant, A.; et al. Migratory DCs activate TGF-β to precondition naïve CD8(+) T cells for tissue-resident memory fate. Science 2019, 366, eaav5728. [Google Scholar] [CrossRef]
- Kim, T.S.; Gorski, S.A.; Hahn, S.; Murphy, K.M.; Braciale, T.J. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8(+) T cell differentiation by a CD24-dependent mechanism. Immunity 2014, 40, 400–413. [Google Scholar] [CrossRef] [Green Version]
- Kok, L.; Dijkgraaf, F.E.; Urbanus, J.; Bresser, K.; Vredevoogd, D.W.; Cardoso, R.F.; Perié, L.; Beltman, J.B.; Schumacher, T.N. A committed tissue-resident memory T cell precursor within the circulating CD8+ effector T cell pool. J. Exp. Med. 2020, 217, e20191711. [Google Scholar] [CrossRef]
- Casey, K.A.; Fraser, K.A.; Schenkel, J.M.; Moran, A.; Abt, M.C.; Beura, L.K.; Lucas, P.J.; Artis, D.; Wherry, E.J.; Hogquist, K.; et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012, 188, 4866–4875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, L.K.; Stock, A.T.; Ma, J.Z.; Jones, C.M.; Kent, S.J.; Mueller, S.N.; Heath, W.R.; Carbone, F.R.; Gebhardt, T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. USA 2012, 109, 7037–7042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holz, L.E.; Prier, J.E.; Freestone, D.; Steiner, T.M.; English, K.; Johnson, D.N.; Mollard, V.; Cozijnsen, A.; Davey, G.M.; Godfrey, D.I.; et al. CD8(+) T Cell Activation Leads to Constitutive Formation of Liver Tissue-Resident Memory T Cells that Seed a Large and Flexible Niche in the Liver. Cell Rep. 2018, 25, 68–79.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.N.; Mooster, J.L.; Kilgore, A.M.; Osborn, J.F.; Nolz, J.C. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J. Exp. Med. 2016, 213, 951–966. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, D.; Ng, W.Y.; Holz, L.E.; Ma, J.Z.; Zaid, A.; Wong, Y.C.; Lau, L.S.; Mollard, V.; Cozijnsen, A.; Collins, N.; et al. Liver-Resident Memory CD8(+) T Cells Form a Front-Line Defense against Malaria Liver-Stage Infection. Immunity 2016, 45, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Wakim, L.M.; Woodward-Davis, A.; Bevan, M.J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA 2010, 107, 17872–17879. [Google Scholar] [CrossRef] [Green Version]
- Wijeyesinghe, S.; Beura, L.K.; Pierson, M.J.; Stolley, J.M.; Adam, O.A.; Ruscher, R.; Steinert, E.M.; Rosato, P.C.; Vezys, V.; Masopust, D. Expansible residence decentralizes immune homeostasis. Nature 2021, 592, 457–462. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Goplen, N.P.; Li, C.; Cheon, I.S.; Dai, Q.; Huang, S.; Shan, J.; Ma, C.; Ye, Z.; et al. PD-1(hi) CD8(+) resident memory T cells balance immunity and fibrotic sequelae. Sci. Immunol. 2019, 4, eaaw1217. [Google Scholar] [CrossRef]
- Uddbäck, I.; Cartwright, E.K.; Schøller, A.S.; Wein, A.N.; Hayward, S.L.; Lobby, J.; Takamura, S.; Thomsen, A.R.; Kohlmeier, J.E.; Christensen, J.P. Long-term maintenance of lung resident memory T cells is mediated by persistent antigen. Mucosal Immunol. 2021, 14, 92–99. [Google Scholar] [CrossRef]
- Mackay, L.K.; Braun, A.; Macleod, B.L.; Collins, N.; Tebartz, C.; Bedoui, S.; Carbone, F.R.; Gebhardt, T. Cutting edge. CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015, 194, 2059–2063. [Google Scholar] [CrossRef] [Green Version]
- Shiow, L.R.; Rosen, D.B.; Brdicková, N.; Xu, Y.; An, J.; Lanier, L.L.; Cyster, J.G.; Matloubian, M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 2006, 440, 540–544. [Google Scholar] [CrossRef]
- Takamura, S.; Yagi, H.; Hakata, Y.; Motozono, C.; McMaster, S.R.; Masumoto, T.; Fujisawa, M.; Chikaishi, T.; Komeda, J.; Itoh, J.; et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J. Exp. Med. 2016, 213, 3057–3073. [Google Scholar] [CrossRef]
- Carlson, C.M.; Endrizzi, B.T.; Wu, J.; Ding, X.; Weinreich, M.A.; Walsh, E.R.; Wani, M.A.; Lingrel, J.B.; Hogquist, K.A.; Jameson, S.C. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 2006, 442, 299–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.T.; Suarez-Ramirez, J.E.; Wu, T.; Redman, J.M.; Bouchard, K.; Hadley, G.A.; Cauley, L.S. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J. Virol. 2011, 85, 4085–4094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thom, J.T.; Weber, T.C.; Walton, S.M.; Torti, N.; Oxenius, A. The Salivary Gland Acts as a Sink for Tissue-Resident Memory CD8(+) T Cells, Facilitating Protection from Local Cytomegalovirus Infection. Cell Rep. 2015, 13, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Mackay, L.K.; Rahimpour, A.; Ma, J.Z.; Collins, N.; Stock, A.T.; Hafon, M.L.; Vega-Ramos, J.; Lauzurica, P.; Mueller, S.N.; Stefanovic, T.; et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013, 14, 1294–1301. [Google Scholar] [CrossRef]
- Sheridan, B.S.; Pham, Q.M.; Lee, Y.T.; Cauley, L.S.; Puddington, L.; Lefrançois, L. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity 2014, 40, 747–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schluns, K.S.; Williams, K.; Ma, A.; Zheng, X.X.; Lefrançois, L. Cutting edge: Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002, 168, 4827–4831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowell, R.T.; Goldufsky, J.W.; Rogozinska, M.; Quiles, Z.; Cao, Y.; Castillo, E.F.; Finnegan, A.; Marzo, A.L. IL-15 Complexes Induce Migration of Resting Memory CD8 T Cells into Mucosal Tissues. J. Immunol. 2017, 199, 2536–2546. [Google Scholar] [CrossRef] [Green Version]
- Burchill, M.A.; Goetz, C.A.; Prlic, M.; O’Neil, J.J.; Harmon, I.R.; Bensinger, S.J.; Turka, L.A.; Brennan, P.; Jameson, S.C.; Farrar, M.A. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: Development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J. Immunol. 2003, 171, 5853–5864. [Google Scholar] [CrossRef] [Green Version]
- Hand, T.W.; Cui, W.; Jung, Y.W.; Sefik, E.; Joshi, N.S.; Chandele, A.; Liu, Y.; Kaech, S.M. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc. Natl. Acad. Sci. USA 2010, 107, 16601–16606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, T.; Kobayashi, T.; Sugihara, E.; Yamada, T.; Ikuta, K.; Pittaluga, S.; Saya, H.; Amagai, M.; Nagao, K. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 2015, 21, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Mackay, L.K.; Wynne-Jones, E.; Freestone, D.; Pellicci, D.G.; Mielke, L.A.; Newman, D.M.; Braun, A.; Masson, F.; Kallies, A.; Belz, G.T.; et al. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 2015, 43, 1101–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skon, C.N.; Lee, J.Y.; Anderson, K.G.; Masopust, D.; Hogquist, K.A.; Jameson, S.C. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013, 14, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Bergsbaken, T.; Bevan, M.J.; Fink, P.J. Local Inflammatory Cues Regulate Differentiation and Persistence of CD8(+) Tissue-Resident Memory T Cells. Cell Rep. 2017, 19, 114–124. [Google Scholar] [CrossRef]
- Grueter, B.; Petter, M.; Egawa, T.; Laule-Kilian, K.; Aldrian, C.J.; Wuerch, A.; Ludwig, Y.; Fukuyama, H.; Wardemann, H.; Waldschuetz, R.; et al. Runx3 regulates integrin alpha E/CD103 and CD4 expression during development of CD4-/CD8+ T cells. J. Immunol. 2005, 175, 1694–1705. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Diao, H.; Getzler, A.J.; Rogal, W.; Frederick, M.A.; Milner, J.; Yu, B.; Crotty, S.; Goldrath, A.W.; Pipkin, M.E. The Transcription Factor Runx3 Establishes Chromatin Accessibility of cis-Regulatory Landscapes that Drive Memory Cytotoxic T Lymphocyte Formation. Immunity 2018, 48, 659–674.e6. [Google Scholar] [CrossRef] [Green Version]
- Hombrink, P.; Helbig, C.; Backer, R.A.; Piet, B.; Oja, A.E.; Stark, R.; Brasser, G.; Jongejan, A.; Jonkers, R.E.; Nota, B.; et al. Programs for the persistence, vigilance and control of human CD8(+) lung-resident memory T cells. Nat. Immunol. 2016, 17, 1467–1478. [Google Scholar] [CrossRef]
- Elyaman, W.; Bassil, R.; Bradshaw, E.M.; Orent, W.; Lahoud, Y.; Zhu, B.; Radtke, F.; Yagita, H.; Khoury, S.J. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity 2012, 36, 623–634. [Google Scholar] [CrossRef] [Green Version]
- Blokzijl, A.; Dahlqvist, C.; Reissmann, E.; Falk, A.; Moliner, A.; Lendahl, U.; Ibáñez, C.F. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J. Cell Biol. 2003, 163, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, B.; Son, Y.M.; Wang, Z.; Jiang, L.; Xiang, M.; Ye, Z.; Beckermann, K.E.; Wu, Y.; Jenkins, J.W.; et al. The Transcription Factor Bhlhe40 Programs Mitochondrial Regulation of Resident CD8(+) T Cell Fitness and Functionality. Immunity 2019, 51, 491–507.e7. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Mackay, L.K.; Rahimpour, A.; Braun, A.; Veldhoen, M.; Carbone, F.R.; Manton, J.H.; Heath, W.R.; Mueller, S.N. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl. Acad. Sci. USA 2014, 111, 5307–5312. [Google Scholar] [CrossRef] [Green Version]
- Boddupalli, C.S.; Nair, S.; Gray, S.M.; Nowyhed, H.N.; Verma, R.; Gibson, J.A.; Abraham, C.; Narayan, D.; Vasquez, J.; Hedrick, C.C.; et al. ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells. J. Clin. Investig. 2016, 126, 3905–3916. [Google Scholar] [CrossRef] [Green Version]
- Halliley, J.L.; Tipton, C.M.; Liesveld, J.; Rosenberg, A.F.; Darce, J.; Gregoretti, I.V.; Popova, L.; Kaminiski, D.; Fucile, C.F.; Albizua, I.; et al. Long-Lived Plasma Cells Are Contained within the CD19(-)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 2015, 43, 132–145. [Google Scholar] [CrossRef] [Green Version]
- Tarlinton, D.; Good-Jacobson, K. Diversity among memory B cells: Origin, consequences, and utility. Science 2013, 341, 1205–1211. [Google Scholar] [CrossRef]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology. A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Takahashi, Y.; Yokoi, Y.; Ato, M.; Kodama, Y.; Hachimura, S.; Kurosaki, T.; Kobayashi, K. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc. Natl. Acad. Sci. USA 2012, 109, 2485–2490. [Google Scholar] [CrossRef] [Green Version]
- Weisel, N.M.; Weisel, F.J.; Farber, D.L.; Borghesi, L.A.; Shen, Y.; Ma, W.; Luning Prak, E.T.; Shlomchik, M.J. Comprehensive analyses of B-cell compartments across the human body reveal novel subsets and a gut-resident memory phenotype. Blood 2020, 136, 2774–2785. [Google Scholar] [CrossRef] [PubMed]
- Boyden, A.W.; Legge, K.L.; Waldschmidt, T.J. Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses. PLoS ONE 2012, 7, e40733. [Google Scholar]
- Allie, S.R.; Randall, T.D. Pulmonary immunity to viruses. Clin. Sci. 2017, 131, 1737–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyron-Quiroz, J.E.; Rangel-Moreno, J.; Hartson, L.; Kusser, K.; Tighe, M.P.; Klonowski, K.D.; Lefrançois, L.; Cauley, L.S.; Harmsen, A.G.; Lund, F.E.; et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 2006, 25, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.M.; Cheon, I.S.; Wu, Y.; Li, C.; Wang, Z.; Gao, X.; Chen, Y.; Takahashi, Y.; Fu, Y.X.; Dent, A.L.; et al. Tissue-resident CD4(+) T helper cells assist the development of protective respiratory B and CD8(+) T cell memory responses. Sci. Immunol. 2021, 6, eabb6852. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Onodera, T.; Yamada, Y.; Daio, R.; Tsuiji, M.; Inoue, T.; Kobayashi, K.; Kurosaki, T.; Ato, M.; Takahashi, Y. Distinct germinal center selection at local sites shapes memory B cell response to viral escape. J. Exp. Med. 2015, 212, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Allie, S.R.; Randall, T.D. Resident Memory B Cells. Viral Immunol. 2020, 33, 282–293. [Google Scholar] [CrossRef]
- Dogan, I.; Bertocci, B.; Vilmont, V.; Delbos, F.; Mégret, J.; Storck, S.; Reynaud, C.A.; Weill, J.C. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 2009, 10, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- McHeyzer-Williams, L.J.; Milpied, P.J.; Okitsu, S.L.; McHeyzer-Williams, M.G. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat. Immunol. 2015, 16, 296–305. [Google Scholar] [CrossRef]
- Pape, K.A.; Taylor, J.J.; Maul, R.W.; Gearhart, P.J.; Jenkins, M.K. Different B cell populations mediate early and late memory during an endogenous immune response. Science 2011, 331, 1203–1207. [Google Scholar] [CrossRef] [Green Version]
- Tarlinton, D. Antigen presentation by memory B cells: The sting is in the tail. Science 1997, 276, 374–375. [Google Scholar] [CrossRef]
- Barker, K.A.; Etesami, N.S.; Shenoy, A.T.; Arafa, E.I.; Lyon de Ana, C.; Smith, N.M.; Martin, I.M.; Goltry, W.N.; Barron, A.M.; Browning, J.L.; et al. Lung-resident memory B cells protect against bacterial pneumonia. J. Clin. Investig. 2021, 131, e141810. [Google Scholar] [CrossRef]
- Beura, L.K.; Fares-Frederickson, N.J.; Steinert, E.M.; Scott, M.C.; Thompson, E.A.; Fraser, K.A.; Schenkel, J.M.; Vezys, V.; Masopust, D. CD4(+) resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 2019, 216, 1214–1229. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.V.; Ma, W.; Miron, M.; Granot, T.; Guyer, R.S.; Carpenter, D.J.; Senda, T.; Sun, X.; Ho, S.H.; Lerner, H.; et al. Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep. 2017, 20, 2921–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oja, A.E.; Piet, B.; Helbig, C.; Stark, R.; van der Zwan, D.; Blaauwgeers, H.; Remmerswaal, E.B.M.; Amsen, D.; Jonkers, R.E.; Moerland, P.D.; et al. Trigger-happy resident memory CD4(+) T cells inhabit the human lungs. Mucosal Immunol. 2018, 11, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, D.; King, C.G. CD4+ Memory T Cells at Home in the Tissue: Mechanisms for Health and Disease. Front. Immunol. 2018, 9, 2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruner, K.B.; Pepper, M. Local memory CD4 T cell niches in respiratory viral infection. J. Exp. Med. 2021, 218, e20201733. [Google Scholar] [CrossRef]
- Turner, D.L.; Bickham, K.L.; Thome, J.J.; Kim, C.Y.; D’Ovidio, F.; Wherry, E.J.; Farber, D.L. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014, 7, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Teijaro, J.R.; Turner, D.; Pham, Q.; Wherry, E.J.; Lefrançois, L.; Farber, D.L. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011, 187, 5510–5514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glennie, N.D.; Volk, S.W.; Scott, P. Skin-resident CD4+ T cells protect against Leishmania major by recruiting and activating inflammatory monocytes. PLoS Pathog. 2017, 13, e1006349. [Google Scholar] [CrossRef] [PubMed]
- Glennie, N.D.; Yeramilli, V.A.; Beiting, D.P.; Volk, S.W.; Weaver, C.T.; Scott, P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med. 2015, 212, 1405–1414. [Google Scholar] [CrossRef]
- Romagnoli, P.A.; Fu, H.H.; Qiu, Z.; Khairallah, C.; Pham, Q.M.; Puddington, L.; Khanna, K.M.; Lefrançois, L.; Sheridan, B.S. Differentiation of distinct long-lived memory CD4 T cells in intestinal tissues after oral Listeria monocytogenes infection. Mucosal Immunol. 2017, 10, 520–530. [Google Scholar] [CrossRef]
- Sakai, S.; Kauffman, K.D.; Schenkel, J.M.; McBerry, C.C.; Mayer-Barber, K.D.; Masopust, D.; Barber, D.L. Cutting edge: Control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J. Immunol. 2014, 192, 2965–2969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallin, M.A.; Sakai, S.; Kauffman, K.D.; Young, H.A.; Zhu, J.; Barber, D.L. Th1 Differentiation Drives the Accumulation of Intravascular, Non-protective CD4 T Cells during Tuberculosis. Cell Rep. 2017, 18, 3091–3104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, T.J.; Topham, D.J. Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. J. Immunol. 2010, 184, 3841–3849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimi, R.A.; Nepal, K.; Cetinbas, M.; Sadreyev, R.I.; Luster, A.D. Distinct functions of tissue-resident and circulating memory Th2 cells in allergic airway disease. J. Exp. Med. 2020, 217, e20190865. [Google Scholar] [CrossRef] [PubMed]
- Steinfelder, S.; Rausch, S.; Michael, D.; Kühl, A.A.; Hartmann, S. Intestinal helminth infection induces highly functional resident memory CD4(+) T cells in mice. Eur. J. Immunol. 2017, 47, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Park, C.O.; Fu, X.; Jiang, X.; Pan, Y.; Teague, J.E.; Collins, N.; Tian, T.; O’Malley, J.T.; Emerson, R.O.; Kim, J.H.; et al. Staged development of long-lived T-cell receptor alphabeta TH17 resident memory T-cell population to Candida albicans after skin infection. J. Allergy Clin. Immunol. 2018, 142, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Ogongo, P.; Tezera, L.B.; Ardain, A.; Nhamoyebonde, S.; Ramsuran, D.; Singh, A.; Ng’oepe, A.; Karim, F.; Naidoo, T.; Khan, K.; et al. Tissue-resident-like CD4+ T cells secreting IL-17 control Mycobacterium tuberculosis in the human lung. J. Clin. Investig. 2021, 131, e142014. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hirahara, K.; Kokubo, K.; Kiuchi, M.; Aoki, A.; Morimoto, Y.; Kumagai, J.; Onodera, A.; Mato, N.; Tumes, D.J.; et al. CD103(hi) T(reg) cells constrain lung fibrosis induced by CD103(lo) tissue-resident pathogenic CD4 T cells. Nat. Immunol. 2019, 20, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Green, J.A.; Moltedo, B.; Arvey, A.; Hemmers, S.; Yuan, S.; Treuting, P.M.; Rudensky, A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015, 162, 1078–1089. [Google Scholar] [CrossRef] [Green Version]
- McKinstry, K.K.; Strutt, T.M.; Bautista, B.; Zhang, W.; Kuang, Y.; Cooper, A.M.; Swain, S.L. Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2. Nat. Commun. 2014, 5, 5377. [Google Scholar] [CrossRef] [Green Version]
- Hondowicz, B.D.; An, D.; Schenkel, J.M.; Kim, K.S.; Steach, H.R.; Krishnamurty, A.T.; Keitany, G.J.; Garza, E.N.; Fraser, K.A.; Moon, J.J.; et al. Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity 2016, 44, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Hondowicz, B.D.; Kim, K.S.; Ruterbusch, M.J.; Keitany, G.J.; Pepper, M. IL-2 is required for the generation of viral-specific CD4(+) Th1 tissue-resident memory cells and B cells are essential for maintenance in the lung. Eur. J. Immunol. 2018, 48, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.C.; Choi, D.H.; Choi, Y.W.; Park, S.J.; Namkoong, H.; Park, K.S.; Ahn, S.S.; Surh, C.D.; Yoon, S.W.; Kim, D.J.; et al. Intranasal Introduction of Fc-Fused Interleukin-7 Provides Long-Lasting Prophylaxis against Lethal Influenza Virus Infection. J. Virol. 2015, 90, 2273–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strutt, T.M.; Dhume, K.; Finn, C.M.; Hwang, J.H.; Castonguay, C.; Swain, S.L.; McKinstry, K.K. IL-15 supports the generation of protective lung-resident memory CD4 T cells. Mucosal Immunol. 2018, 11, 668–680. [Google Scholar] [CrossRef] [Green Version]
- Takamura, S. Niches for the Long-Term Maintenance of Tissue-Resident Memory T Cells. Front. Immunol. 2018, 9, 1214. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.; Jiang, X.; Zaid, A.; Macleod, B.L.; Li, J.; Park, C.O.; Haque, A.; Bedoui, S.; Heath, W.R.; Mueller, S.N.; et al. Skin CD4(+) memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat. Commun. 2016, 7, 11514. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, T.; Whitney, P.G.; Zaid, A.; Mackay, L.K.; Brooks, A.G.; Heath, W.R.; Carbone, F.R.; Mueller, S.N. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 2011, 477, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Gondek, D.C.; Olive, A.J.; Stary, G.; Starnbach, M.N. CD4+ T cells are necessary and sufficient to confer protection against Chlamydia trachomatis infection in the murine upper genital tract. J. Immunol. 2012, 189, 2441–2449. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.M.; Brunham, R.C. Tissue-Resident T Cells as the Central Paradigm of Chlamydia Immunity. Infect. Immun. 2016, 84, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.M.; Yu, H.; Strank, N.O.; Karunakaran, K.; Zhu, Y.; Brunham, R.C. B Cell Presentation of Chlamydia Antigen Selects Out Protective CD4γ13 T Cells: Implications for Genital Tract Tissue-Resident Memory Lymphocyte Clusters. Infect. Immun. 2018, 86, e00614–e00617. [Google Scholar] [CrossRef] [Green Version]
- Iijima, N.; Linehan, M.M.; Zamora, M.; Butkus, D.; Dunn, R.; Kehry, M.R.; Laufer, T.M.; Iwasaki, A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J. Exp. Med. 2008, 205, 3041–3052. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Randall, T.D.; Silva-Sanchez, A. Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front. Immunol. 2016, 7, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swarnalekha, N.; Schreiner, D.; Litzler, L.C.; Iftikhar, S.; Kirchmeier, D.; Kunzli, M.; Son, Y.M.; Sun, J.; Moreira, E.A.; King, C.G. T resident helper cells promote humoral responses in the lung. Sci. Immunol. 2021, 6, eabb6808. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A.; Bi, K.; Keskin, D.B.; Zhang, G.; Reinhold, B.; Reinherz, E.L. TCR-pMHC encounter differentially regulates transcriptomes of tissue-resident CD8 T cells. Eur. J. Immunol. 2018, 48, 128–150. [Google Scholar] [CrossRef] [Green Version]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- Schmitt, N.; Bustamante, J.; Bourdery, L.; Bentebibel, S.E.; Boisson-Dupuis, S.; Hamlin, F.; Tran, M.V.; Blankenship, D.; Pascual, V.; Savino, D.A.; et al. IL-12 receptor β1 deficiency alters in vivo T follicular helper cell response in humans. Blood 2013, 121, 3375–3385. [Google Scholar] [CrossRef] [Green Version]
- Johnston, R.J.; Choi, Y.S.; Diamond, J.A.; Yang, J.A.; Crotty, S. STAT5 is a potent negative regulator of TFH cell differentiation. J. Exp. Med. 2012, 209, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros-Tato, A.; Leon, B.; Graf, B.A.; Moquin, A.; Adams, P.S.; Lund, F.E.; Randall, T.D. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 2012, 36, 847–856. [Google Scholar] [CrossRef] [Green Version]
- DiToro, D.; Winstead, C.J.; Pham, D.; Witte, S.; Andargachew, R.; Singer, J.R.; Wilson, C.G.; Zindl, C.L.; Luther, R.J.; Silberger, D.J.; et al. Differential IL-2 expression defines developmental fates of follicular versus nonfollicular helper T cells. Science 2018, 361, eaao2933. [Google Scholar] [CrossRef]
- Ise, W.; Fujii, K.; Shiroguchi, K.; Ito, A.; Kometani, K.; Takeda, K.; Kawakami, E.; Yamashita, K.; Suzuki, K.; Okada, T.; et al. T Follicular Helper Cell-Germinal Center B Cell Interaction Strength Regulates Entry into Plasma Cell or Recycling Germinal Center Cell Fate. Immunity 2018, 48, 702–715.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suan, D.; Kräutler, N.J.; Maag, J.L.V.; Butt, D.; Bourne, K.; Hermes, J.R.; Avery, D.T.; Young, C.; Statham, A.; Elliott, M.; et al. CCR6 Defines Memory B Cell Precursors in Mouse and Human Germinal Centers, Revealing Light-Zone Location and Predominant Low Antigen Affinity. Immunity 2017, 47, 1142–1153.e4. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shi, J.; Yan, J.; Xiao, Z.; Hou, X.; Lu, P.; Hou, S.; Mao, T.; Liu, W.; Ma, Y.; et al. Germinal-center development of memory B cells driven by IL-9 from follicular helper T cells. Nat. Immunol. 2017, 18, 921–930. [Google Scholar] [CrossRef]
- Shinnakasu, R.; Inoue, T.; Kometani, K.; Moriyama, S.; Adachi, Y.; Nakayama, M.; Takahashi, Y.; Fukuyama, H.; Okada, T.; Kurosaki, T. Regulated selection of germinal-center cells into the memory B cell compartment. Nat. Immunol. 2016, 17, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Phares, T.W.; Stohlman, S.A.; Hwang, M.; Min, B.; Hinton, D.R.; Bergmann, C.C. CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis. J. Virol. 2012, 86, 2416–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, E.M.; Droin, N.M.; Lemmens, E.E.; Pinkoski, M.J.; Bensinger, S.J.; Ehst, B.D.; Griffith, T.S.; Green, D.R.; Schoenberger, S.P. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 2005, 434, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Bevan, M.J. Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 2004, 4, 595–602. [Google Scholar] [CrossRef]
- Smith, C.M.; Wilson, N.S.; Waithman, J.; Villadangos, J.A.; Carbone, F.R.; Heath, W.R.; Belz, G.T. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat. Immunol. 2004, 5, 1143–1148. [Google Scholar] [CrossRef]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Perera, L.P.; Terabe, M.; Ni, L.; Waldmann, T.A.; Berzofsky, J.A. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 5201–5206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.A.; Tyznik, A.J.; Bevan, M.J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 2006, 441, 890–893. [Google Scholar] [CrossRef]
- Johnson, S.; Zhan, Y.; Sutherland, R.M.; Mount, A.M.; Bedoui, S.; Brady, J.L.; Carrington, E.M.; Brown, L.E.; Belz, G.T.; Heath, W.R.; et al. Selected Toll-like receptor ligands and viruses promote helper-independent cytotoxic T cell priming by upregulating CD40L on dendritic cells. Immunity 2009, 30, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.M.; Ravkov, E.V.; Williams, M.A. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J. Immunol. 2010, 184, 6719–6730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalia, V.; Sarkar, S. Regulation of Effector and Memory CD8 T Cell Differentiation by IL-2-A Balancing Act. Front. Immunol. 2018, 9, 2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laidlaw, B.J.; Cui, W.; Amezquita, R.A.; Gray, S.M.; Guan, T.; Lu, Y.; Kobayashi, Y.; Flavell, R.A.; Kleinstein, S.H.; Craft, J.; et al. Production of IL-10 by CD4(+) regulatory T cells during the resolution of infection promotes the maturation of memory CD8(+) T cells. Nat. Immunol. 2015, 16, 871–879. [Google Scholar] [CrossRef]
- Bourgeois, C.; Rocha, B.; Tanchot, C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 2002, 297, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Badovinac, V.P.; Messingham, K.A.; Griffith, T.S.; Harty, J.T. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J. Immunol. 2006, 177, 999–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, Y.; Lu, B.; Gerard, C.; Iwasaki, A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature 2009, 462, 510–513. [Google Scholar] [CrossRef] [Green Version]
- Laidlaw, B.J.; Zhang, N.; Marshall, H.D.; Staron, M.M.; Guan, T.; Hu, Y.; Cauley, L.S.; Craft, J.; Kaech, S.M. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 2014, 41, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Hutloff, A. T Follicular Helper-Like Cells in Inflamed Non-Lymphoid Tissues. Front. Immunol. 2018, 9, 1707. [Google Scholar] [CrossRef] [Green Version]
- Yoshitomi, H.; Ueno, H. Shared and distinct roles of T peripheral helper and T follicular helper cells in human diseases. Cell Mol. Immunol. 2021, 18, 523–527. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Manzo, A.; Vitolo, B.; Humby, F.; Caporali, R.; Jarrossay, D.; Dell’accio, F.; Ciardelli, L.; Uguccioni, M.; Montecucco, C.; Pitzalis, C. Mature antigen-experienced T helper cells synthesize and secrete the B cell chemoattractant CXCL13 in the inflammatory environment of the rheumatoid joint. Arthritis Rheum. 2008, 58, 3377–3387. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murata, K.; Shibuya, H.; Morita, M.; Ishikawa, M.; Furu, M.; Ito, H.; Ito, J.; Matsuda, S.; Watanabe, T.; et al. A distinct human CD4+ T cell subset that secretes CXCL13 in rheumatoid synovium. Arthritis Rheum. 2013, 65, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Rubin, S.J.S.; Bai, L.; Haileselassie, Y.; Garay, G.; Yun, C.; Becker, L.; Streett, S.E.; Sinha, S.R.; Habtezion, A. Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases. Nat. Commun. 2019, 10, 2686. [Google Scholar] [CrossRef]
- Pitzalis, C.; Jones, G.W.; Bombardieri, M.; Jones, S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat. Rev. Immunol. 2014, 14, 447–462. [Google Scholar] [CrossRef]
- Coquet, J.M.; Schuijs, M.J.; Smyth, M.J.; Deswarte, K.; Beyaert, R.; Braun, H.; Boon, L.; Karlsson Hedestam, G.B.; Nutt, S.L.; Hammad, H.; et al. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity 2015, 43, 318–330. [Google Scholar] [CrossRef] [Green Version]
- Vu Van, D.; Beier, K.C.; Pietzke, L.J.; Al Baz, M.S.; Feist, R.K.; Gurka, S.; Hamelmann, E.; Kroczek, R.A.; Hutloff, A. Local T/B cooperation in inflamed tissues is supported by T follicular helper-like cells. Nat. Commun. 2016, 7, 10875. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.M.; Kolawole, E.M.; Ren, M.; Jin, G.; Netherby-Winslow, C.S.; Wade, Q.; Shwetank Rahman, Z.S.M.; Evavold, B.D.; Lukacher, A.E. IL-21 from high-affinity CD4 T cells drives differentiation of brain-resident CD8 T cells during persistent viral infection. Sci. Immunol. 2020, 5, eabb5590. [Google Scholar] [CrossRef]
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza virus and SARS-CoV-2. pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef]
- Sano, K.; Ainai, A.; Suzuki, T.; Hasegawa, H. The road to a more effective influenza vaccine: Up to date studies and future prospects. Vaccine 2017, 35, 5388–5395. [Google Scholar] [CrossRef]
- Hellfritzsch, M.; Scherließ, R. Mucosal Vaccination via the Respiratory Tract. Pharmaceutics 2019, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Pilkington, E.H.; Suys, E.J.A.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Wang, R.; Liu, M.; Liu, J. The Association between Influenza Vaccination and COVID-19 and Its Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Vaccines 2021, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.D.; Schultz-Cherry, S. Development of a Universal Influenza Vaccine. J. Immunol. 2019, 202, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Sandbrink, J.B.; Shattock, R.J. RNA Vaccines: A Suitable Platform for Tackling Emerging Pandemics? Front. Immunol. 2020, 11, 608460. [Google Scholar] [CrossRef]
- Liu, C.; Ginn, H.M.; Dejnirattisai, W.; Supasa, P.; Wang, B.; Tuekprakhon, A.; Nutalai, R.; Zhou, D.; Mentzer, A.J.; Zhao, Y.; et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell 2021, 184, 4220–4236.e13. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Goplen, N.P.; Sun, J. Aging and respiratory viral infection: From acute morbidity to chronic sequelae. Cell Biosci. 2021, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Goplen, N.P.; Wu, Y.; Son, Y.M.; Li, C.; Wang, Z.; Cheon, I.S.; Jiang, L.; Zhu, B.; Ayasoufi, K.; Chini, E.N.; et al. Tissue-resident CD8(+) T cells drive age-associated chronic lung sequelae after viral pneumonia. Sci. Immunol. 2020, 5, eabc4557. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).