Phosphorylation-Mediated Molecular Pathway Changes in Human Pituitary Neuroendocrine Tumors Identified by Quantitative Phosphoproteomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Specimen and Preparation of Protein Samples
2.2. Enzyme Hydrolysis and Peptide Quantification
2.3. TMT Labeling
2.4. TiO2 Enrichment of Phosphopeptides
2.5. LC-MS/MS Analysis of Enriched Phosphopeptides
2.6. Statistical Analysis and Bioinformatics
2.7. Immunoprecipitation and Western Blot Analyses of DPP Calnexin
3. Results
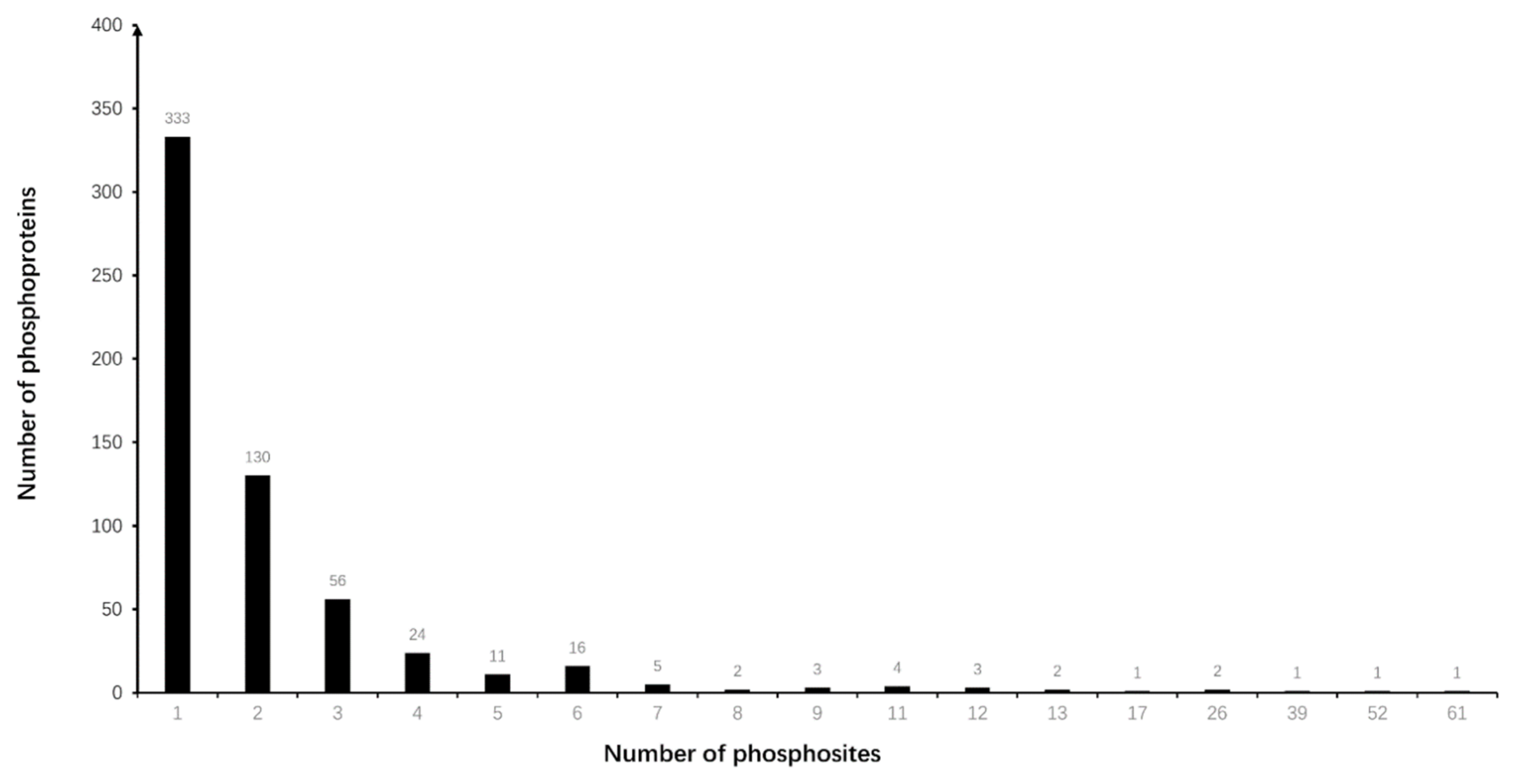
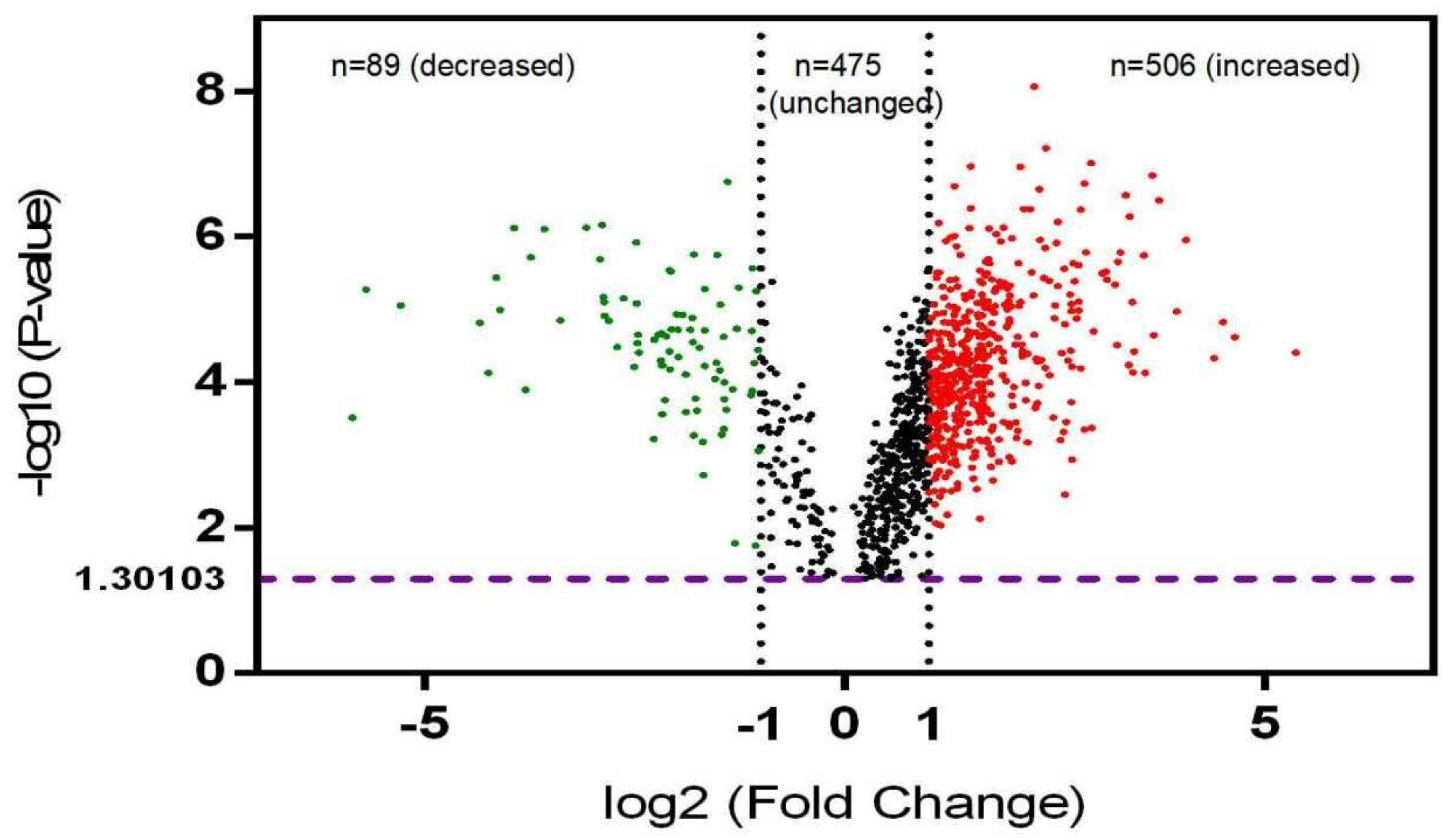
3.1. Differentially Phosphorylated Protein (DPP) Profiling in NF-PitNETs
3.2. Functional Characteristics of DPPs in NF-PitNETs
3.3. Phosphorylation-Involved Signaling Pathway Alterations in NF-PitNETs
3.4. Upstream Kinase Profiling Analysis of DPPs in NF-PitNETs
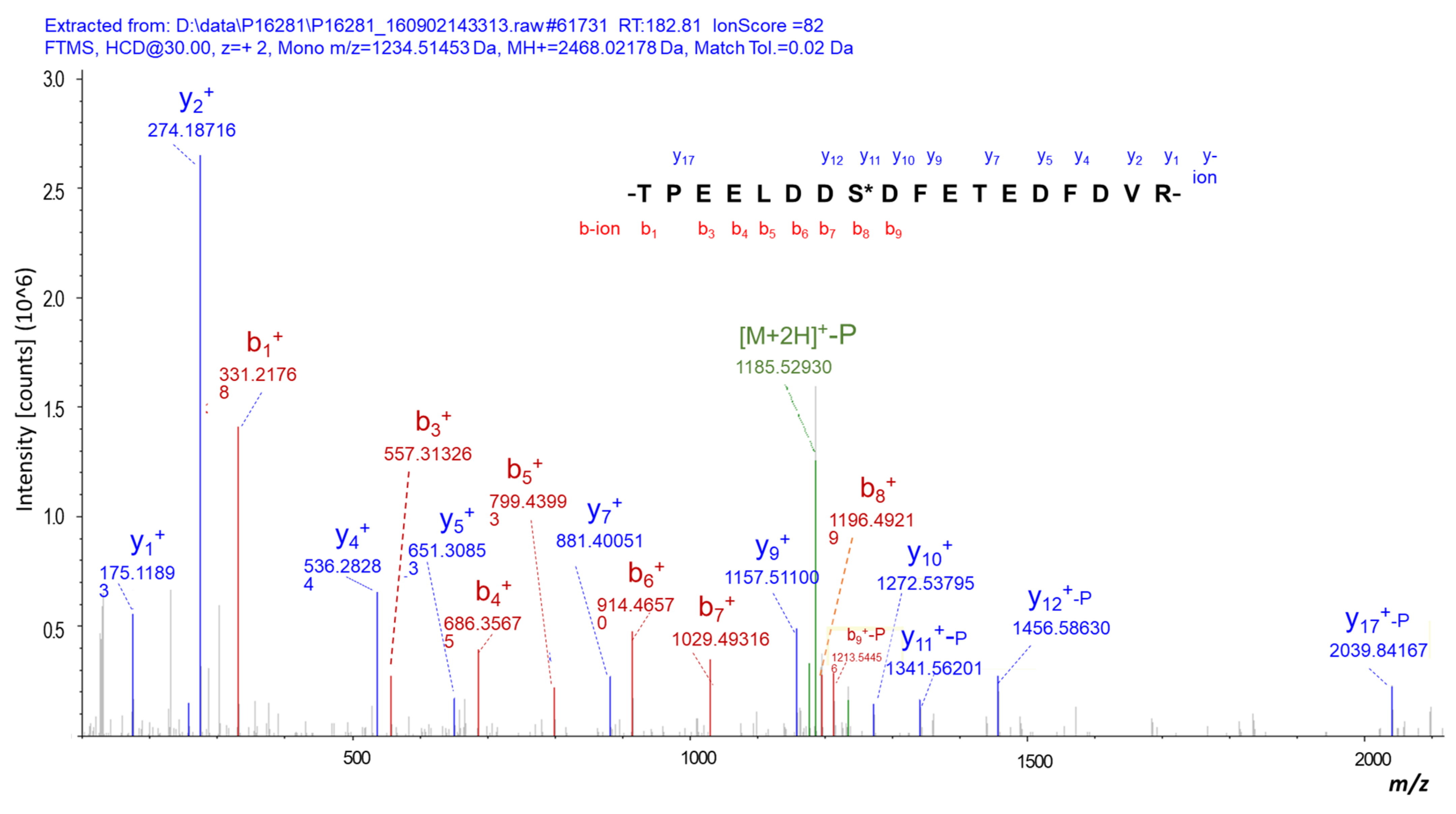
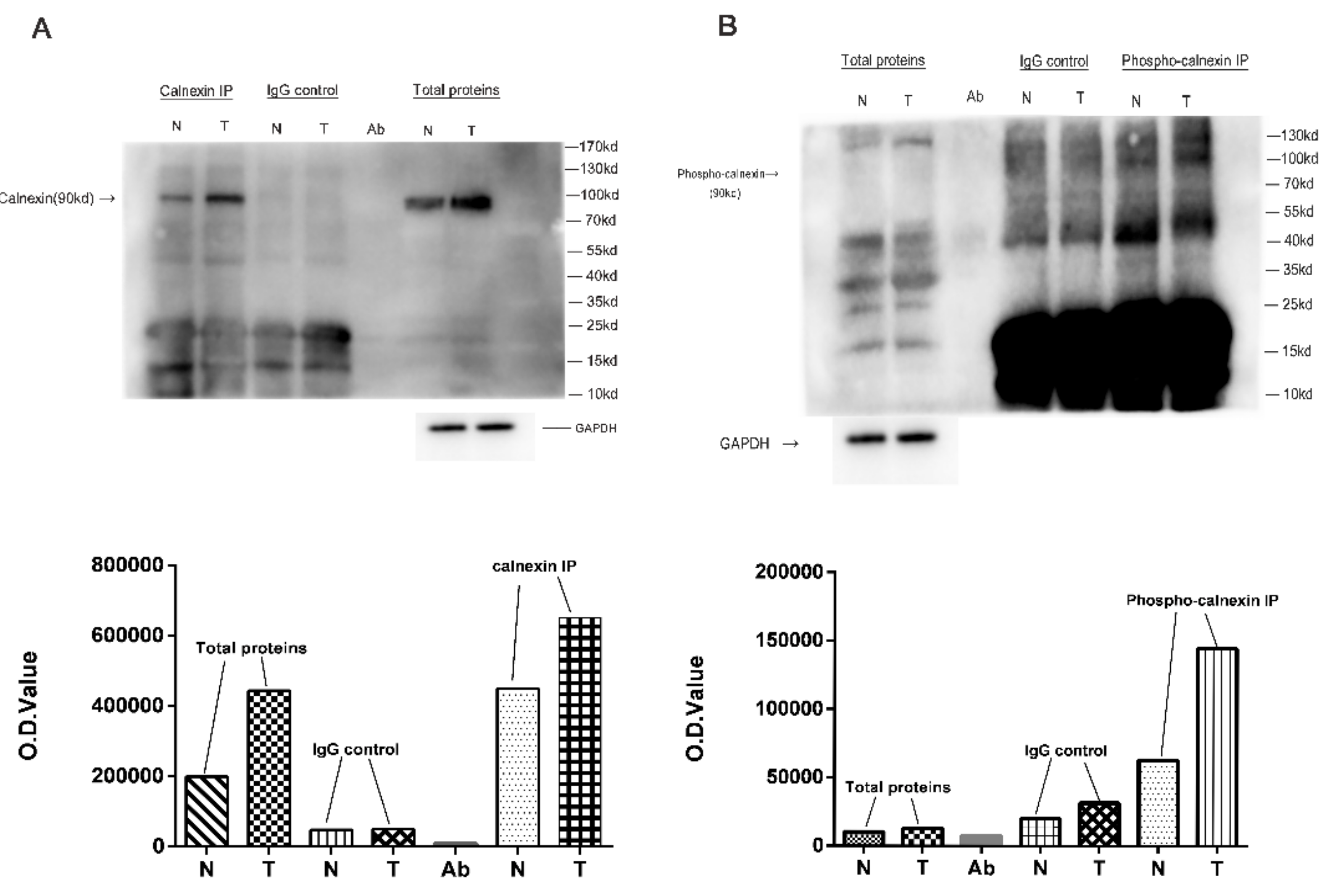
3.5. Verification of DPPs in NF-PitNETs Compared to Controls
4. Discussion
4.1. Phosphorylation-Mediated Biological Processes in NF-PitNETs
4.2. The Functions of Kinases and Their Corresponding Substrates Associated with Quantified Phosphoproteins
4.3. The Phosphorylation of Calnexin in NF-PitNETs
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Zhan, X.; Desiderio, D.M. The use of variations in proteomes to predict, prevent, and personalize treatment for clinically nonfunctional pituitary adenomas. EPMA J. 2010, 1, 439–459. [Google Scholar] [CrossRef][Green Version]
- Zhan, X.; Desiderio, D.M. Comparative proteomics analysis of human pituitary adenomas: Current status and future perspectives. Mass Spectrom. Rev. 2005, 24, 783–813. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Yang, H.; Peng, F.; Li, J.; Mu, Y.; Long, Y.; Cheng, T.; Huang, Y.; Li, Z.; Lu, M.; et al. How many proteins can be identified in a 2DE gel spot within an analysis of a complex human cancer tissue proteome? Electrophoresis 2018, 39, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, M.L.; Li, B.Q.; Zhai, H.L.; Liu, J.J.; Li, S.Y. Prediction of phosphorylation sites based on Krawtchouk image moments. Proteins 2017, 85, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.A.; Murray, B.W. Protein kinase biochemistry and drug discovery. Bioorg. Chem. 2011, 39, 192–210. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Shaytan, A.; Panchenko, A.R. Physicochemical mechanisms of protein regulation by phosphorylation. Front. Genet. 2014, 5, 270. [Google Scholar] [CrossRef]
- Lin, H.J.; Hsieh, F.C.; Song, H.; Lin, J. Elevated phosphorylation and activation of PDK-1/AKT pathway in human breast cancer. Br. J. Cancer 2005, 93, 1372–1381. [Google Scholar] [CrossRef]
- Xie, W.; Liu, C.; Wu, D.; Li, Z.; Li, C.; Zhang, Y. Phosphorylation of kinase insert domain receptor by cyclin-dependent kinase 5 at serine 229 is associated with invasive behavior and poor prognosis in prolactin pituitary adenomas. Oncotarget 2016, 7, 50883–50894. [Google Scholar] [CrossRef][Green Version]
- Zhan, X.; Desiderio, D.M. Editorial: Systems Biological Aspects of Pituitary Tumors. Front. Endocrinol. 2016, 7, 86. [Google Scholar] [CrossRef][Green Version]
- Cheng, S.; Wu, J.; Li, C.; Li, Y.; Liu, C.; Li, G.; Li, W.; Hu, S.; Ying, X.; Zhang, Y. Predicting the regrowth of clinically non-functioning pituitary adenoma with a statistical model. J. Transl. Med. 2019, 17, 164. [Google Scholar] [CrossRef]
- Yamanaka, R.; Abe, E.; Sato, T.; Hayano, A.; Takashima, Y. Secondary Intracranial Tumors Following Radiotherapy for Pituitary Adenomas: A Systematic Review. Cancers 2017, 9, 103. [Google Scholar] [CrossRef]
- Song, Z.J.; Reitman, Z.J.; Ma, Z.Y.; Chen, J.H.; Zhang, Q.L.; Shou, X.F.; Huang, C.X.; Wang, Y.F.; Li, S.Q.; Mao, Y.; et al. The genome-wide mutational landscape of pituitary adenomas. Cell Res. 2016, 26, 1255–1259. [Google Scholar] [CrossRef]
- Välimäki, N.; Schalin-Jäntti, C.; Karppinen, A.; Paetau, A.; Kivipelto, L.; Aaltonen, L.A.; Karhu, A. Genetic and epigenetic characterization of growth hormone-secreting pituitary tumors. Mol. Cancer Res. 2019, 17, 2432–2443. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Reincke, M.; Fassnacht, M.; Komada, M. Decoding the genetic basis of Cushing’s disease: USP8 in the spotlight. Eur. J. Endocrinol. 2015, 173, M73–M83. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Wang, J.; Song, G.; Zhao, Z.; Wang, H.; Wang, W.; Li, H.; Li, Z.; Miao, Y.; et al. Genome-wide analysis of differentially expressed lncRNAs and mRNAs in primary gonadotrophin adenomas by RNA-seq. Oncotarget 2017, 8, 4585–4606. [Google Scholar] [CrossRef]
- Ubersax, J.A.; Ferrell, J.E., Jr. Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Amano, M.; Nishioka, T.; Tsuboi, D.; Kuroda, K.; Funahashi, Y.; Yamahashi, Y.; Kaibuchi, K. Comprehensive analysis of kinase-oriented phospho-signalling pathways. J. Biochem. 2019, 165, 301–307. [Google Scholar] [CrossRef]
- McAlister, G.C.; Huttlin, E.L.; Haas, W.; Ting, L.; Jedrychowski, M.P.; Rogers, J.C.; Kuhn, K.; Pike, I.; Grothe, R.A.; Blethrow, J.D.; et al. Increasing the multiplexing capacity of TMTs using reporter ion isotopologues with isobaric masses. Anal. Chem. 2012, 84, 7469–7478. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.P.; Timms, J.F. Employing TMT Quantification in a Shotgun-MS Platform. In Shotgun Proteomics: Methods and Protocols; Methods in Molecular Biology; Martins, D., Ed.; Humana Press: New York, NY, USA, 2014; Volume 1156, pp. 187–199. [Google Scholar]
- Chahrour, O.; Cobice, D.; Malone, J. Stable isotope labelling methods in mass spectrometry-based quantitative proteomics. J. Pharm. Biomed. Anal. 2015, 113, 2–20. [Google Scholar] [CrossRef]
- Lee, S.C.; Abdel-Wahab, O. Therapeutic targeting of splicing in cancer. Nat. Med. 2016, 22, 976–986. [Google Scholar] [CrossRef]
- Duden, R. ER-to-Golgi transport: COP I and COP II function (Review). Mol. Membr. Biol. 2003, 20, 197–207. [Google Scholar] [CrossRef]
- Wang, J.; Davis, S.; Zhu, M.; Miller, E.A.; Ferro-Novick, S. Autophagosome formation: Where the secretory and autophagy pathways meet. Autophagy 2017, 13, 973–974. [Google Scholar] [CrossRef] [PubMed]
- Errington, W.J.; Bruncsics, B.; Sarkar, C.A. Mechanisms of noncanonical binding dynamics in multivalent protein-protein interactions. Proc. Natl. Acad. Sci. USA 2019, 116, 25659–25667. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, C.; Zhao, Q.; Li, D. Spectrin: Structure, function and disease. Sci. China Life Sci. 2013, 56, 1076–1085. [Google Scholar] [CrossRef]
- Hodges, C.; Kirkland, J.G.; Crabtree, G.R. The Many Roles of BAF (mSWI/SNF) and PBAF Complexes in Cancer. Cold Spring Harb. Perspect. Med. 2016, 6, a026930. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, N.; D’Avino, A.R.; Michel, B.C.; Luo, J.; Pan, J.; Otto, J.E.; Zullow, H.J.; McKenzie, Z.M.; Kubiak, R.L.; St Pierre, R.; et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell 2018, 175, 1272–1288.e20. [Google Scholar] [CrossRef]
- Panwalkar, P.; Pratt, D.; Chung, C.; Dang, D.; Le, P.; Martinez, D.; Bayliss, J.M.; Smith, K.S.; Adam, M.; Potter, S.; et al. SWI/SNF complex heterogeneity is related to polyphenotypic differentiation, prognosis, and immune response in rhabdoid tumors. Neuro Oncol. 2020, 22, 785–796. [Google Scholar] [CrossRef]
- Guo, G.; Sun, X.; Chen, C.; Wu, S.; Huang, P.; Li, Z.; Dean, M.; Huang, Y.; Jia, W.; Zhou, Q.; et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat. Genet. 2013, 45, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Pluhackova, K.; Böckmann, R.A. The Multifaceted Role of SNARE Proteins in Membrane Fusion. Front. Physiol. 2017, 8, 5. [Google Scholar] [CrossRef]
- Hedglin, M.; Benkovic, S.J. Regulation of Rad6/Rad18 Activity during DNA Damage Tolerance. Annu. Rev. Biophys. 2015, 44, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Alcón, P.; Shakeel, S.; Chen, Z.A.; Rappsilber, J.; Patel, K.J.; Passmore, L.A. FANCD2-FANCI is a clamp stabilized on DNA by monoubiquitination of FANCD2 during DNA repair. Nat. Struct. Mol. Biol. 2020, 27, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J. Cell Fate Control by Translation: mRNA Translation Initiation as a Therapeutic Target for Cancer Development and Stem Cell Fate Control. Biomolecules 2019, 9, 665. [Google Scholar] [CrossRef]
- Sriram, A.; Bohlen, J.; Teleman, A.A. Translation acrobatics: How cancer cells exploit alternate modes of translational initiation. EMBO Rep. 2018, 19, e45947. [Google Scholar] [CrossRef]
- Stephens, A.D.; Banigan, E.J.; Marko, J.F. Chromatin’s physical properties shape the nucleus and its functions. Curr. Opin. Cell Biol. 2019, 58, 76–84. [Google Scholar] [CrossRef]
- Viphakone, N.; Sudbery, I.; Griffith, L.; Heath, C.G.; Sims, D.; Wilson, S.A. Co-transcriptional Loading of RNA Export Factors Shapes the Human Transcriptome. Mol. Cell 2019, 75, 310–323.e8. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Tzanakakis, G.N.; Karamanos, N.K. Proteoglycans in health and disease: Novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010, 277, 3904–3923. [Google Scholar] [CrossRef]
- Morath, I.; Hartmann, T.N.; Orian-Rousseau, V. CD44: More than a mere stem cell marker. Int. J. Biochem. Cell Biol. 2016, 81, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Scheller, R.H. SNARE-mediated membrane fusion. Nat. Rev. Mol. Cell Biol. 2001, 2, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Ho-Tin-Noé, B.; Boulaftali, Y.; Camerer, E. Platelets and vascular integrity: How platelets prevent bleeding in inflammation. Blood 2018, 131, 277–288. [Google Scholar] [CrossRef]
- Veiga, E.; Guttman, J.A.; Bonazzi, M.; Boucrot, E.; Toledo-Arana, A.; Lin, A.E.; Enninga, J.; Pizarro-Cerdá, J.; Finlay, B.B.; Kirchhausen, T.; et al. Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell Host Microbe 2007, 2, 340–351. [Google Scholar] [CrossRef]
- Cossart, P.; Veiga, E. Non-classical use of clathrin during bacterial infections. J. Microsc. 2008, 231, 524–528. [Google Scholar] [CrossRef]
- Takano, K.; Kojima, T.; Sawada, N.; Himi, T. Role of tight junctions in signal transduction: An update. EXCLI J. 2014, 13, 1145–1162. [Google Scholar]
- González-Mariscal, L.; Domínguez-Calderón, A.; Raya-Sandino, A.; Ortega-Olvera, J.M.; Vargas-Sierra, O.; Martínez-Revollar, G. Tight junctions and the regulation of gene expression. Semin. Cell Dev. Biol. 2014, 36, 213–223. [Google Scholar] [CrossRef]
- Wier, W.G.; Mauban, J.R.H. Imaging sympathetic neurogenic Ca2+ signaling in blood vessels. Auton. Neurosci. 2017, 207, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef]
- Yu, S.; Sun, L.; Jiao, Y.; Lee, L.T.O. The Role of G Protein-coupled Receptor Kinases in Cancer. Int. J. Biol. Sci. 2018, 14, 189–203. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Xu, J.; Yang, M.; Chen, P.; Xu, W.; Zhao, J.; Geng, L.; Gong, S. PKN2 in colon cancer cells inhibits M2 phenotype polarization of tumor-associated macrophages via regulating DUSP6-Erk1/2 pathway. Mol. Cancer 2018, 17, 13. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Gu, J.; Yin, X.; Jin, N.; Xie, S.; Wang, Y.; Chang, H.; Qian, W.; Shi, J.; et al. Cross talk between PI3K-AKT-GSK-3beta and PP2A pathways determines tau hyperphosphorylation. Neurobiol. Aging 2015, 36, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, X.X.; Wang, J.R.; Yang, T.Y.; Li, X.M.; He, X.S.; Li, Y.; Ye, W.L.; Wu, Y.; Gan, W.J.; et al. TRAF6 inhibits colorectal cancer metastasis through regulating selective autophagic CTNNB1/β-catenin degradation and is targeted for GSK3B/GSK3β-mediated phosphorylation and degradation. Autophagy 2019, 15, 1506–1522. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Hoessli, D.C.; Walker-Nasir, E.; Choudhary, M.I.; Rafik, S.M.; Shakoori, A.R. Phosphorylation and glycosylation interplay: Protein modifications at hydroxy amino acids and prediction of signaling functions of the human β3 integrin family. J. Cell. Biochem. 2006, 99, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Giorgianni, F.; Beranova-Giorgianni, S.; Desiderio, D.M. Identification and characterization of phosphorylated proteins in the human pituitary. Proteomics 2004, 4, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Beranova-Giorgianni, S.; Zhao, Y.; Desiderio, D.M.; Giorgianni, F. Phosphoproteomic analysis of the human pituitary. Pituitary 2006, 9, 109–120. [Google Scholar] [CrossRef]
- Long, Y.; Lu, M.; Cheng, T.; Zhan, X.; Zhan, X. Multiomics-Based Signaling Pathway Network Alterations in Human Non-functional Pituitary Adenomas. Front. Endocrinol. 2019, 10, 835. [Google Scholar] [CrossRef]
- Peverelli, E.; Giardino, E.; Mangili, F.; Treppiedi, D.; Catalano, R.; Ferrante, E.; Sala, E.; Locatelli, M.; Lania, A.G.; Arosio, M.; et al. cAMP/PKA-induced filamin A (FLNA) phosphorylation inhibits SST2 signal transduction in GH-secreting pituitary tumor cells. Cancer Lett. 2018, 435, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Mechanistic insights into precursor messenger RNA splicing by the spliceosome. Nat. Rev. Mol. Cell Biol. 2017, 18, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Sotillo, E.; Barrett, D.M.; Black, K.L.; Bagashev, A.; Oldridge, D.; Wu, G.; Sussman, R.; Lanauze, C.; Ruella, M.; Gazzara, M.R.; et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015, 5, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Borrego, M.C.; Fuentes-Fayos, A.C.; Venegas-Moreno, E.; Rivero-Cortés, E.; Dios, E.; Moreno-Moreno, P.; Madrazo-Atutxa, A.; Remón, P.; Solivera, J.; Wildemberg, L.E.; et al. Splicing machinery is dysregulated in pituitary neuroendocrine tumors and is associated with aggressiveness features. Cancers 2019, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Sipma, W.; Veenhoff, L.M.; Van der Giessen, E.; Onck, P.R. The Effect of FG-Nup Phosphorylation on NPC Selectivity: A One-Bead-Per-Amino-Acid Molecular Dynamics Study. Int. J. Mol. Sci. 2019, 20, 596. [Google Scholar] [CrossRef]
- Buffone, A.; Weaver, V.M. Don’t sugarcoat it: How glycocalyx composition influences cancer progression. J. Cell Biol. 2020, 219, e201910070. [Google Scholar] [CrossRef]
- Tarbell, J.M.; Cancel, L.M. The glycocalyx and its significance in human medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef]
- Kang, H.; Wu, Q.; Sun, A.; Liu, X.; Fan, Y.; Deng, X. Cancer Cell Glycocalyx and Its Significance in Cancer Progression. Int. J. Mol. Sci. 2018, 19, 2484. [Google Scholar] [CrossRef]
- Kavalali, E.T. SNARE interactions in membrane trafficking: A perspective from mammalian central synapses. Bioessays 2002, 24, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Risinger, C.; Bennett, M.K. Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms. J. Neurochem. 1999, 72, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Furuse, M. Tight Junction Structure and Function Revisited. Trends Cell Biol. 2020, 30, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, S.; Gordon, L.; Blechman, J.; Matsuoka, R.L.; Rajamannar, P.; Wircer, E.; Biran, J.; Reuveny, A.; Leshkowitz, D.; Stainier, D.Y.R.; et al. Pituicyte Cues Regulate the Development of Permeable Neuro-Vascular Interfaces. Dev. Cell 2018, 47, 711–726.e5. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Tachibana, K.; Krug, S.M.; Kunisawa, J.; Fromm, M.; Kondoh, M. Potential for Tight Junction Protein-Directed Drug Development Using Claudin Binders and Angubindin-1. Int. J. Mol. Sci. 2019, 20, 4016. [Google Scholar] [CrossRef]
- Spadaro, D.; Le, S.; Laroche, T.; Mean, I.; Jond, L.; Yan, J.; Citi, S. Tension-Dependent Stretching Activates ZO-1 to Control the Junctional Localization of Its Interactors. Curr. Biol. 2017, 27, 3783–3795.e8. [Google Scholar] [CrossRef] [PubMed]
- Bolinger, M.T.; Ramshekar, A.; Waldschmidt, H.V.; Larsen, S.D.; Bewley, M.C.; Flanagan, J.M.; Antonetti, D.A. Occludin S471 Phosphorylation Contributes to Epithelial Monolayer Maturation. Mol. Cell. Biol. 2016, 36, 2051–2066. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.E.; Xu, H.E.; Melcher, K. Structure and Physiological Regulation of AMPK. Int. J. Mol. Sci. 2018, 19, 3534. [Google Scholar] [CrossRef]
- Singh, V.; Ram, M.; Kumar, R.; Prasad, R.; Roy, B.K.; Singh, K.K. Phosphorylation: Implications in Cancer. Protein J. 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Cicenas, J.; Cicenas, E. Multi-kinase inhibitors, AURKs and cancer. Med. Oncol. 2016, 33, 43. [Google Scholar] [CrossRef]
- Xiao, A.; Li, H.; Shechter, D.; Ahn, S.H.; Fabrizio, L.A.; Erdjument-Bromage, H.; Ishibe-Murakami, S.; Wang, B.; Tempst, P.; Hofmann, K.; et al. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature 2009, 457, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Giachino, C. Multiple facets of histone variant H2AX: A DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015, 43, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Ohno, H.; Takase, K.; Ochiai, T.; Saito, T. Cell surface expression of calnexin, a molecular chaperone in the endoplasmic reticulum. J. Biol. Chem. 2000, 275, 35751–35758. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, D.; Wang, X.; Fang, J.; Liu, X.; Song, J.; Li, X.; Ren, X.; Li, Q.; Li, Q.; et al. Calnexin Impairs the Antitumor Immunity of CD4+ and CD8+ T Cells. Cancer Immunol. Res. 2019, 7, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, H.; Inoshita, N. New WHO classification of pituitary adenomas (4th edition): Assessment of pituitary transcription factors and the prognostic histological factors. Brain Tumor Pathol. 2018, 35, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Lopes, M.B. Overview of the 2017 WHO classification of pituitary tumors. Endocr. Pathol. 2017, 28, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Saeger, W.; Wilczak, P.; Luüdecke, D.K.; Buchfelder, M.; Fahlbusch, R. Hormone markers in pituitary adenomas: Changes within last decade resulting from improved method. Endocr. Pathol. 2003, 14, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Saeger, W.; Lüdecke, D.K.; Buchfelder, M.; Fahlbusch, R.; Quabbe, H.J.; Petersenn, S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur. J. Endocrinol. 2007, 156, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, L.B.; Konovalov, P.V.; Krylova, J.S.; Polyakova, V.O.; Kvetnoy, I.M. Plurihormonal cells of normal anterior pituitary: Facts and conclusions. Oncotarget 2017, 8, 29282–29299. [Google Scholar] [CrossRef]
- Zhan, X.; Desiderio, D.M. Heterogeneity analysis of the human pituitaryproteome. Clin. Chem. 2003, 49, 1740–1751. [Google Scholar] [CrossRef]
- Moreno, C.S.; Evans, C.O.; Zhan, X.; Okor, M.; Desiderio, D.M.; Oyesiku, N.M. Novel molecular signaling and classification of human clinically nonfunctional pituitary adenomas identified by gene expression profiling and proteomic analyses. Cancer Res. 2005, 65, 10214–10222. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Wang, X.; Long, Y.; Desiderio, D.M. Heterogeneity analysis of the proteomes in clinically nonfunctional pituitary adenomas. BMC Med. Genet. 2014, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Long, Y.; Zhan, X.; Mu, Y. Consideration of statistical vs. biological significances for omics data-based pathway network analysis. Med. One 2017, 1, e170002. [Google Scholar] [CrossRef]
- Christ-Crain, M. Vasopressin and Copeptin in health and disease. Rev. Endocr. Metab. Disord. 2019, 20, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Klussmann, E.; Maric, K.; Rosenthal, W. The mechanisms of aquaporin control in the renal collecting duct. Rev. Physiol. Biochem. Pharmacol. 2000, 141, 33–95. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontol. 2000 2016, 70, 11–25. [Google Scholar] [CrossRef]

| Category | ID | Count | % | p-Value | Genes | |
|---|---|---|---|---|---|---|
| Annotation Cluster 1 Enrichment Score: 12.9 | ||||||
| GOTERM_CC_DIRECT | GO:0005913 | cell–cell adherens junction | 42 | 7.4 | 5.37 × 10−15 | Q9UHB6, Q9UPN3, P18206, Q9H0B6, A0A087WUZ3, A0A0U4BW16, Q9UDY2, P35221, A0A024R1S8, Q9C0C2, Q15762, P55196, Q6PKG0, Q09666, O60716, C9J6P4, P42166, A0A024R4E5, P07948, E9PRY8, Q9H4G0, Q9ULH1, P35611, Q9H2G2, P21333, Q07960, O00567, Q92522, P35579, Q13813, Q15149, O60763, P08238, O95292, Q9UQN3, Q9BY44, A0A024RAN2, Q16513, O76021, E7EX44, Q92597, P26232, Q14247 |
| GOTERM_MF_DIRECT | GO:0098641 | cadherin binding involved in cell–cell adhesion | 40 | 7.0 | 2.04 × 10−14 | Q9UHB6, Q9UPN3, P18206, Q9H0B6, A0A087WUZ3, Q9UDY2, P35221, A0A0U4BW16, A0A024R1S8, Q9C0C2, P55196, Q6PKG0, Q09666, O60716, C9J6P4, P42166, A0A024R4E5, E9PRY8, Q9H4G0, Q9ULH1, P35611, Q9H2G2, P21333, Q07960, O00567, Q92522, P35579, Q13813, Q15149, O60763, P08238, O95292, Q9UQN3, Q9BY44, A0A024RAN2, Q16513, O76021, E7EX44, Q92597, P26232, Q14247 |
| GOTERM_BP_DIRECT | GO:0098609 | cell–cell adhesion | 34 | 6.0 | 2.25 × 10−11 | Q9UHB6, Q9UPN3, Q9H0B6, A0A087WUZ3, Q9UDY2, A0A024R1S8, Q9C0C2, P55196, Q6PKG0, Q09666, C9J6P4, P42166, A0A024R4E5, E9PRY8, Q9H4G0, Q9ULH1, P35611, Q9H2G2, Q07960, O00567, Q92522, Q13813, Q15149, O60763, P08238, O95292, Q9BY44, Q9UQN3, A0A024RAN2, Q16513, O76021, E7EX44, Q92597, Q14247 |
| Annotation Cluster 2 Enrichment Score: 7.9 | ||||||
| GOTERM_BP_DIRECT | GO:0006405 | RNA export from nucleus | 15 | 2.6 | 9.39 × 10−10 | Q15287, Q13247, O95391, Q96FV9, P52948, A0A0S2Z4Z6, J3KTL2, Q08170, Q16629, O75694, Q05519, Q13242, P35658, P09651, Q01130 |
| GOTERM_BP_DIRECT | GO:0006406 | mRNA export from nucleus | 19 | 3.3 | 1.69 × 10−9 | Q15287, Q9BRD0, Q13247, O75494, O95391, L0R530, Q96FV9, P52948, A0A0S2Z4Z6, J3KTL2, Q08170, P49792, Q16629, O75694, Q05519, Q13242, Q9P2I0, P35658, Q01130 |
| GOTERM_BP_DIRECT | GO:0031124 | mRNA 3’-end processing | 13 | 2.3 | 3.10 × 10−8 | Q15287, Q08170, Q13247, Q16629, Q12996, O95391, Q05519, Q13242, Q96FV9, Q9P2I0, A0A0S2Z4Z6, Q01130, J3KTL2 |
| GOTERM_BP_DIRECT | GO:0006369 | termination of RNA polymerase II transcription | 13 | 2.3 | 5.63 × 10−7 | Q15287, Q08170, Q13247, Q16629, Q12996, O95391, Q05519, Q13242, Q96FV9, Q9P2I0, A0A0S2Z4Z6, Q01130, J3KTL2 |
| Annotation Cluster 3 Enrichment Score: 4.0 | ||||||
| GOTERM_CC_DIRECT | GO:0014731 | spectrin-associated cytoskeleton | 6 | 1.1 | 1.24 × 10−6 | Q12955, Q08495, P16157, A0A087WUZ3, P11171, P11277 |
| GOTERM_CC_DIRECT | GO:0008091 | spectrin | 5 | 0.9 | 8.97 × 10−5 | A0A087WUZ3, P11171, P11277, O43491, Q13813 |
| GOTERM_BP_DIRECT | GO:0051693 | actin filament capping | 4 | 0.7 | 6.84 × 10−3 | Q08495, A0A087WUZ3, P11277, Q13813 |
| Annotation Cluster 4 Enrichment Score: 3.0 | ||||||
| GOTERM_BP_DIRECT | GO:0043044 | ATP-dependent chromatin remodeling | 7 | 1.2 | 5.76 × 10−5 | Q13547, P07910, Q92769, Q14839, B4DY08, Q92922, O96019, F8VXC8 |
| GOTERM_MF_DIRECT | GO:0031492 | nucleosomal DNA binding | 9 | 1.6 | 8.71 × 10−5 | Q13547, P05114, P07910, Q92769, Q14839, B4DY08, Q92922, P49450, O96019, F8VXC8 |
| GOTERM_CC_DIRECT | GO:0000790 | nuclear chromatin | 13 | 2.3 | 1.39 × 10−2 | P51531, Q9H1E3, Q9Y618, P52701, Q92769, O75376, Q14839, O96019, F8VXC8, Q13547, P07910, P16402, B4DY08, Q92922 |
| GOTERM_MF_DIRECT | GO:0000980 | RNA polymerase II distal enhancer sequence-specific DNA binding | 7 | 1.2 | 1.68 × 10−2 | Q13547, P07910, Q92769, Q14839, B4DY08, Q92922, O96019, F8VXC8 |
| Annotation Cluster 5 Enrichment Score: 2.9 | ||||||
| GOTERM_CC_DIRECT | GO:0071564 | npBAF complex | 5 | 0.9 | 3.28 × 10−4 | P51531, Q8WUB8, Q92922, O96019, F8VXC8 |
| GOTERM_CC_DIRECT | GO:0016514 | SWI/SNF complex | 5 | 0.9 | 8.42 × 10−4 | P51531, Q92922, Q8NFD5, O96019, F8VXC8 |
| GOTERM_CC_DIRECT | GO:0071565 | nBAF complex | 4 | 0.7 | 7.65 × 10−3 | P51531, Q92922, Q8NFD5, F8VXC8 |
| Annotation Cluster 6 Enrichment Score: 2.4 | ||||||
| GOTERM_BP_DIRECT | GO:0007064 | mitotic sister chromatid cohesion | 5 | 0.9 | 7.31 × 10−4 | Q9NTI5, Q7Z5K2, Q29RF7, Q6KC79, Q9UQE7 |
| GOTERM_CC_DIRECT | GO:0000775 | chromosome, centromeric region | 7 | 1.2 | 7.06 × 10−3 | Q9NTI5, P83916, Q13185, Q7Z5K2, Q29RF7, P49450, Q9UQE7 |
| GOTERM_BP_DIRECT | GO:0007062 | sister chromatid cohesion | 9 | 1.6 | 1.53 × 10−2 | Q9NTI5, P49792, O75122, Q7Z5K2, Q29RF7, P49450, Q9UQE7, Q8WYP5, P52948 |
| Annotation Cluster 7 Enrichment Score: 2.3 | ||||||
| GOTERM_BP_DIRECT | GO:0061025 | membrane fusion | 9 | 1.6 | 5.61 × 10−5 | O00161, D3DUW5, P63027, Q05193, Q16623, O60763, Q9UNZ2, Q9UQ16, P61266 |
| KEGG_PATHWAY | hsa04130:S | NARE interactions in vesicular transport | 6 | 1.1 | 4.25 × 10−3 | O00161, P63027, Q16623, O75396, P61266, O75379 |
| GOTERM_MF_DIRECT | GO:0005484 | SNAP receptor activity | 6 | 1.1 | 7.42 × 10−3 | O00161, P63027, Q16623, O75396, P61266, O75379 |
| GOTERM_BP_DIRECT | GO:0016192 | vesicle-mediated transport | 12 | 2.1 | 8.19 × 10−3 | P63027, Q16623, O75396, Q13439, O00203, O75131, P61266, P35606, O75379, Q13367, Q9UPT6, Q9UN37 |
| GOTERM_CC_DIRECT | GO:0031201 | SNARE complex | 6 | 1.1 | 2.10 × 10−2 | O00161, P63027, Q16623, O75396, P61266, O75379 |
| GOTERM_BP_DIRECT | GO:0017157 | regulation of exocytosis | 4 | 0.7 | 4.62 × 10−2 | P63027, Q16623, P61266, Q9Y6V0 |
| Annotation Cluster 8 Enrichment Score: 2.3 | ||||||
| GOTERM_BP_DIRECT | GO:0016925 | protein sumoylation | 14 | 2.5 | 7.34 × 10−5 | Q02880, Q12888, Q99502, A0A024R2M8, Q14676, L0R530, Q8NDX5, P52948, P07910, P49792, O75694, B4DY08, Q9UQE7, P35658, P29590 |
| GOTERM_BP_DIRECT | GO:1900034 | regulation of cellular response to heat | 9 | 1.6 | 2.30 × 10−3 | Q96B36, P07900, P08238, P49792, O75694, B3KUY2, L0R530, P35658, P52948 |
| GOTERM_BP_DIRECT | GO:0007077 | mitotic nuclear envelope disassembly | 7 | 1.2 | 2.31 × 10−3 | P02545, P49792, O75694, P17252, L0R530, P35658, P52948 |
| GOTERM_BP_DIRECT | GO:0006409 | tRNA export from nucleus | 5 | 0.9 | 1.69 × 10−2 | P49792, O75694, L0R530, P35658, P52948 |
| GOTERM_BP_DIRECT | GO:0010827 | regulation of glucose transport | 5 | 0.9 | 1.87 × 10−2 | P49792, O75694, L0R530, P35658, P52948 |
| GOTERM_BP_DIRECT | GO:0075733 | intracellular transport of virus | 6 | 1.1 | 2.10 × 10−2 | P49792, O75694, L0R530, P35658, O00505, P52948 |
| GOTERM_CC_DIRECT | GO:0044615 | nuclear pore nuclear basket | 3 | 0.5 | 4.86 × 10−2 | P49792, P35658, P52948 |
| Annotation Cluster 9 Enrichment Score: 2.2 | ||||||
| GOTERM_BP_DIRECT | GO:0031032 | actomyosin structure organization | 7 | 1.2 | 1.52 × 10−4 | P35580, Q9H4G0, Q9Y2J2, Q92614, A0A0U4BW16, P11171, P35579, O43491 |
| GOTERM_CC_DIRECT | GO:0019898 | extrinsic component of membrane | 7 | 1.2 | 3.67 × 10−2 | Q9UEW8, Q9H4G0, Q9Y2J2, Q96C24, P11171, Q9Y4F1, O43491 |
| GOTERM_BP_DIRECT | GO:0030866 | cortical actin cytoskeleton organization | 4 | 0.7 | 3.76 × 10−2 | Q9H4G0, Q9Y2J2, P11171, O43491 |
| Annotation Cluster 10 Enrichment Score: 2.1 | ||||||
| GOTERM_BP_DIRECT | GO:0033523 | histone H2B ubiquitination | 4 | 0.7 | 1.50 × 10−3 | Q5VTR2, Q6PD62, Q8WVC0, Q8N7H5 |
| GOTERM_BP_DIRECT | GO:0010390 | histone monoubiquitination | 4 | 0.7 | 4.13 × 10−3 | Q5VTR2, Q6PD62, Q8WVC0, Q8N7H5 |
| GOTERM_BP_DIRECT | GO:0001711 | endodermal cell fate commitment | 3 | 0.5 | 1.34 × 10−2 | Q6PD62, Q8WVC0, Q8N7H5 |
| GOTERM_CC_DIRECT | GO:0016593 | Cdc73/Paf1 complex | 3 | 0.5 | 1.71 × 10−2 | Q6PD62, Q8WVC0, Q8N7H5 |
| GOTERM_BP_DIRECT | GO:0045638 | negative regulation of myeloid cell differentiation | 4 | 0.7 | 2.02 × 10−2 | Q96T37, Q6PD62, Q8WVC0, Q8N7H5 |
| Annotation Cluster 11 Enrichment Score: 1.5 | ||||||
| GOTERM_BP_DIRECT | GO:0006446 | regulation of translational initiation | 5 | 0.9 | 2.51 × 10−2 | B5ME19, O60841, E7EX17, Q59GJ0, P04792, P23588 |
| GOTERM_BP_DIRECT | GO:0006413 | translational initiation | 10 | 1.8 | 2.79 × 10−2 | Q8NE71, P05387, Q13144, B5ME19, Q6PKG0, Q9BY44, O60841, P05388, E7EX17, Q59GJ0, P23588 |
| GOTERM_MF_DIRECT | GO:0003743 | translation initiation factor activity | 6 | 1.1 | 4.37 × 10−2 | Q13144, B5ME19, Q9BY44, O60841, E7EX17, Q59GJ0, P23588 |
| Annotation Cluster 12 Enrichment Score: 1.4 | ||||||
| GOTERM_BP_DIRECT | GO:1904903 | ESCRT III complex disassembly | 3 | 0.5 | 3.70 × 10−2 | A0A024R2C5, Q9UQN3, Q9UN37 |
| GOTERM_BP_DIRECT | GO:1902188 | positive regulation of viral release from host cell | 3 | 0.5 | 4.44 × 10−2 | A0A024R2C5, Q9UQN3, Q9UN37 |
| GOTERM_BP_DIRECT | GO:0006997 | nucleus organization | 4 | 0.7 | 4.62 × 10−2 | A0A024R2C5, Q9UQN3, Q14980, Q9UN37 |
| Accession | KINASE | GENE | SUB | Description | Coverage | Proteins | Unique Peptides |
|---|---|---|---|---|---|---|---|
| P11021 | GRP78 | HSPA5 | GRP78 | 78 kDa glucose-regulated protein OS = Homo sapiens GN = HSPA5 PE = 1 SV = 2 [GRP78_HUMAN] | 6.57 | 12 | 2 |
| Q9UIG0 | WSTF | BAZ1B | H2AX | Tyrosine-protein kinase BAZ1B OS = Homo sapiens GN = BAZ1B PE = 1 SV = 2 [BAZ1B_HUMAN] | 1.15 | 1 | 1 |
| Q16513 | PKN2 | PKN2 | pyrin | Serine/threonine-protein kinase N2 OS = Homo sapiens GN = PKN2 PE = 1 SV = 1 [PKN2_HUMAN] | 1.42 | 1 | 1 |
| Q13523 | PRP4 | PRPF4B | ELK1 | Serine/threonine-protein kinase PRP4 homolog OS = Homo sapiens GN = PRPF4B PE = 1 SV = 3 [PRP4B_HUMAN] | 5.46 | 2 | 1 |
| O94804 | LOK | STK10 | Radixin, Ezrin, PLK1, Moesin | Serine/threonine-protein kinase 10 OS = Homo sapiens GN = STK10 PE = 1 SV = 1 [STK10_HUMAN] | 1.55 | 1 | 1 |
| Q96PY6 | NEK1 | NEK1 | TAZ, VDAC1, VHL, RAD54L | Serine/threonine-protein kinase Nek1 OS = Homo sapiens GN = NEK1 PE = 1 SV = 2 [NEK1_HUMAN] | 1.27 | 1 | 1 |
| Q13131 | AMPKA1 | PRKAA1 | 5’-AMP-activated protein kinase catalytic subunit alpha-1 OS = Homo sapiens GN = PRKAA1 PE = 1 SV = 4 [AAPK1_HUMAN] | 1.79 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wen, S.; Li, B.; Li, N.; Zhan, X. Phosphorylation-Mediated Molecular Pathway Changes in Human Pituitary Neuroendocrine Tumors Identified by Quantitative Phosphoproteomics. Cells 2021, 10, 2225. https://doi.org/10.3390/cells10092225
Li J, Wen S, Li B, Li N, Zhan X. Phosphorylation-Mediated Molecular Pathway Changes in Human Pituitary Neuroendocrine Tumors Identified by Quantitative Phosphoproteomics. Cells. 2021; 10(9):2225. https://doi.org/10.3390/cells10092225
Chicago/Turabian StyleLi, Jiajia, Siqi Wen, Biao Li, Na Li, and Xianquan Zhan. 2021. "Phosphorylation-Mediated Molecular Pathway Changes in Human Pituitary Neuroendocrine Tumors Identified by Quantitative Phosphoproteomics" Cells 10, no. 9: 2225. https://doi.org/10.3390/cells10092225
APA StyleLi, J., Wen, S., Li, B., Li, N., & Zhan, X. (2021). Phosphorylation-Mediated Molecular Pathway Changes in Human Pituitary Neuroendocrine Tumors Identified by Quantitative Phosphoproteomics. Cells, 10(9), 2225. https://doi.org/10.3390/cells10092225





