Abstract
Immune checkpoint inhibitors (ICIs) are reshaping the landscape of cancer treatment, redefining the prognosis of several tumors. They act by restoring the cytotoxic activity of tumor-specific T lymphocytes that are in a condition of immune exhaustion. The same condition has been widely described in chronic HIV infection. In this review, we dissect the role of ICIs in people living with HIV/AIDS (PLWHIV). First, we provide an overview of the immunologic scenario. Second, we discuss the possible use of ICIs as adjuvant treatment of HIV to achieve elimination of the viral reservoir. Third, we examine the influence of HIV infection on ICI safety and effectiveness. Finally, we describe how the administration of ICIs impacts opportunistic infections.
1. Introduction
The landscape of oncologic treatments is undergoing an epochal change of perspective since the advent of therapeutic regimens based on immune checkpoint inhibitors (ICIs). These molecules act on the immune system, re-establishing the activity of cytotoxic T lymphocytes (CTLs) against neoplastic cells. Therefore, they are defined as cancer immunotherapies, and the daily-growing field where they are employed has been called immuno-oncology.
The first approved ICI was ipilimumab, an anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), for the treatment of unresectable or metastatic melanoma in 2011 [1]. Since then several other drugs of this class have been approved by regulatory agencies, all targeting the programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway: atezolizumab [2], avelumab [3], cemiplimab [4], dostarlimab [5], durvalumab [6], nivolumab [7], and pembrolizumab [8]. ICIs currently approved by the Food and Drug Administration (FDA) with their indications are shown in Table 1.

Table 1.
Currently approved ICIs with their indications. (CTLA-4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; FDA, Food and Drug Administration).
The reason for their widespread application resides in their innovative therapeutic approach, which has guaranteed excellent results against cancers once considered less susceptible to treatment. For example, 20 years ago the average life expectancy for patients with advanced melanoma was 6/7 months, whereas recent studies have shown an overall survival at 5 years in 52% of patients treated with nivolumab-plus-ipilimumab and 44% of patients treated with nivolumab alone [9]. Similar excellent results have been described for non-small-cell lung cancer and advanced urothelial carcinoma [10,11,12].
Their own mechanism of action, which has led to their success in certain malignancies, has hampered their use for cancer treatment among people living with HIV/AIDS (PLWHIV). Indeed, the alteration of T cell immune response described during chronic HIV infection resembles the immune exhaustion observed among oncologic patients. Several doubts arose about the risk of eliciting an immune response against host cells infected by HIV. Consequently, PLWHIV were and still are excluded from many randomized controlled trials (RCT) investigating ICIs for neoplastic conditions [13]. Table 2 reports the studies registered on Clinicaltrial.gov assessing the efficacy of ICIs for treatment of cancers specifically in PLWHIV.

Table 2.
Studies registered on Clinicaltrial.gov assessing ICIs for treatment of cancers in PLWHIV. Search was performed on 24 April 2021. (NSCLC: Non-small-cell lung carcinoma; cART: combination antiretroviral therapy; AEs: adverse events.).
The aim of this review is to dissect the role of ICIs in PLWHIV. First, we provide an overview of the immunologic background. Second, we discuss the possible use of ICIs as adjuvant treatment of HIV to achieve elimination of the viral reservoir. Third, we examine the influence of HIV infection on ICIs safety and effectiveness. Finally, we describe how the administration of ICIs impacts opportunistic infections.
2. The Immunologic Background
T cells exhaustion is a condition that occurs in chronic infections and in several cancers. Initially it was identified and described in a murine model of infection by lymphocytic choriomeningitis virus [14,15,16], but subsequently was depicted also in other infections [17] and many tumors [18]. Several mechanisms lead to T cell exhaustion: cell-to-cell signals including prolonged T cell receptor (TCR) engagement and co-stimulatory and/or co-inhibitory signals, soluble factors such as excessive levels of inflammatory cytokines, and tissue and microenvironmental influences [19]. As a consequence of these stimuli, T cells progressively develop a state of exhaustion characterized by the inability to elaborate the arrays of effector functions associated with typical effector and memory T cells. This dysfunction can lead to clonal deletion of antigen-specific T cells [20]. The result is the inability of specific T cells to control and eliminate infected or neoplastic cells. The prolonged and/or high expression of multiple inhibitory receptors is a key feature of T cell exhaustion. Among these, CTLA-4 and PD-1 are the inhibitory receptors more involved in T cell exhaustion. CTLA-4 and PD-1 propagate inhibitory signals that converge on Akt to limit cellular metabolism. Whereas PD-1 disrupts the intracellular accumulation of 3-phosphorylated phosphatidylinositol lipids, CTLA-4 targets downstream effectors of PI3K through activation of the serine/threonine phosphatase PP2A [21]. The administration of molecules able to block these receptors or their ligands has been shown to be able to restore antiviral or antitumoral activities in several experimental models [22].
Progression of HIV infection occurs in most patients in the presence of persistently high viremia and is associated with a loss of immune control of viral replication. Virus-specific CD8 T cells partially suppress HIV viral replication in the initial stages of infection [23], but, with persistently high levels of viral antigens, HIV-specific T cells become exhausted and lose their capacity to efficiently kill the infected cells. In addition to high levels of circulating viral antigens, the strong pro-inflammatory immune activation during HIV infection and the T-cell subpopulation imbalance during HIV infection contribute to the development of T-cell exhaustion [24,25].
Combination antiretroviral therapy (cART) has a dramatic effect on HIV infection. After treatment start, the majority of patients experience a significant decline in viral load; the aim is reaching undetectability, and, with time, the count of CD4+ T cells can increase. Unfortunately the drugs currently available cannot eradicate the infection, but only control it, which is associated with significant clinical benefit [26]. Patients receiving efficacious cART display a progressive reduction of ICI expression on T cells, while their values remain higher than those observed in HIV-uninfected individuals [23]. The best current strategy for fully successful immune restoration is early cART initiation, which can prevent acquired immunodeficiency syndrome (AIDS)-associated events and restrict cell subset imbalances and dysfunction, while preserving the structural integrity of lymphoid tissues [27].
PD-1 is a crucial immune checkpoint in chronic HIV infection and is significantly upregulated on HIV-specific CD8 T-cells in patients naïve to antiretroviral therapy. Its expression correlates with impaired HIV-specific CD8 T-cell function as well as predictors of disease progression positively with plasma viral load and inversely with CD4 T-cell count [28]. Importantly, the level of PD-1 surface expression is the primary determinant of apoptosis sensitivity of HIV-specific CD8+ T-cells. Interestingly, cytomegalovirus (CMV)-specific CD8+ T cells from the same individuals whose HIV-specific CD8+ T-cells upregulate PD-1, do not upregulate PD-1 and maintain the production of high levels of cytokines, suggesting the specific impact of HIV [29]. Likewise, PD-1 expression on CD4+ T cells is directly correlated with viral load and inversely correlated with CD4+ T-cell count. The blockade of the PD-1/PD-L1 pathway can restore both HIV-specific CD4+ and CD8+ T-cell function, suggesting that this pathway is operative during persistent viral infection and is a reversible defect in HIV-specific T-cell function [30].
Similarly to PD-1, several pieces of evidence highlight an essential role also for CTLA-4 in driving HIV-specific T cells toward exhaustion. Initial works identified how CTLA-4 is more highly expressed by unstimulated blood CD4+ T cells from HIV patients than by control T cells [31]. In their pivotal work, Kaufmann and colleagues confirmed how CTLA-4 is selectively upregulated in HIV–specific CD4+ T cells but not in CD8+ T cells in various categories of HIV-infected subjects (untreated HIV-infected subjects, viremic controllers, untreated chronically infected subjects, subjects with acute untreated HIV infection), excluding elite controllers. CTLA-4 expression correlates positively with disease progression and negatively with the capacity of CD4+ T cells to produce interleukin 2 in response to viral antigen, and consequently to clear the infection. Most HIV-specific CD4+ T cells co-express CTLA-4 alongside PD-1. Interestingly, in vitro blockade of CTLA-4 augmented HIV-specific CD4+ T cell function [32].
Alongside PD-1 and CTLA-4, other immune checkpoints associated with T cell immune exhaustion in PLWHIV are T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), lymphocyte-activation gene 3 (LAG-3), and T cell immunoreceptor with Ig and ITIM domains (TIGIT). Levels of TIM-3 expression on T cells from HIV-infected individuals correlate positively with HIV-1 viral load and inversely with CD4+ T cell count. In progressive HIV infection, TIM-3 expression is upregulated on HIV-specific CD8+ T cells. TIM-3-expressing T cells do not produce cytokine or proliferate in response to antigen and exhibit impaired Stat5, Erk1/2, and p38 signaling. The blockade of the TIM-3 signaling pathway can restore proliferation and enhance cytokine production in HIV-specific T cells, suggesting how this inhibitory receptor is central in the immune response against HIV [33]. Moreover, the downregulation of TIM-3 is associated with restoration of CD4+ T cell counts in PLWHIV receiving cART with suppressed viremia [34]. Similarly, HIV-1 infection results in a significant increase in LAG-3 expression in both the peripheral blood and the lymph nodes, and this upregulation is dramatically manifested on both CD4+ and CD8+ T cells and correlates with disease progression. Prolonged cART reduces the expression of LAG-3 on both CD4+ and CD8+ T cells, and the ex vivo blockade of LAG-3 significantly augments HIV-specific CD4+ and CD8+ T cell responses, whereas the overexpression of LAG-3 in T cells or the stimulation of LAG-3 on T cells leads to the reduction of T cell responses. Overall, these data show how the LAG-3/MHC class II pathway plays an immunoregulatory role, thereby providing an important target for enhancing HIV-specific responses in infected patients [35]. During HIV infection, CD8+ T cells exhibit higher levels also of TIGIT. Increased frequencies of TIGIT+ and TIGIT+/PD-1+/CD8+ T cells correlate with parameters of HIV disease progression. TIGIT remain elevated despite viral suppression both in individuals with pharmacological antiretroviral control and in elite controllers. Ex-vivo single or combinational antibody blockade of TIGIT is able to restore viral-specific CD8+ T cell effector responses. The frequency of TIGIT+/CD4+ T cells correlates with CD4+ T cell total HIV DNA. Overall, these findings identify TIGIT as another marker of dysfunctional HIV-specific T cells and suggest how TIGIT along with other checkpoint receptors may be a new possible target in HIV treatment to reverse T cell exhaustion [36].
Other than immune checkpoints, inflammatory cytokines also participate in the process of immune exhaustion. Among them, IL-10 has a crucial role. IL-10 mRNA expression is increased in the setting of chronic uncontrolled HIV infection and correlates with plasma viremia in infected persons. IL-10Rα blockade restores not only HIV-specific CD4 cell proliferation but also antigen-specific CD8 T-cell proliferation. IL-10 mRNA expression and IL-10 plasma levels are reduced through successful antiretroviral treatment, indicating a direct effect of viral antigen load on IL-10 production. Overall, IL-10 contributes to a reversible T-cell dysfunction in HIV infected persons, and its levels are directly correlated to viral antigen levels [37]. Figure 1 provides a summary of the immune exhaustion process occurring in PLWHIV.
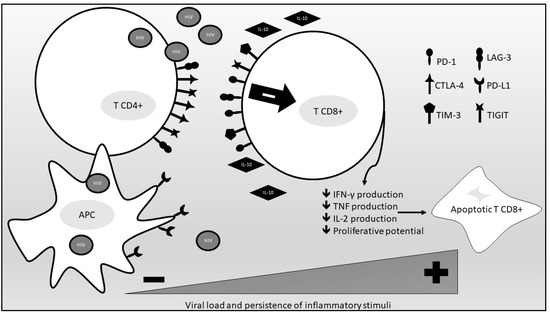
Figure 1.
An overview of the immune exhaustion process occurring in PLWHIV. (PD-1, programmed death cell protein-1; CTLA-4, anti-cytotoxic T-lymphocyte-associated protein 4; TIM-3, T cell immunoglobulin and mucin domain-containing protein 3; TIGIT, T cell immunoreceptor with Ig and ITIM domains; LAG-3, lymphocyte-activation gene 3; PD-L1, PD-1 ligand 1; APC, antigen-presenting cell; IL-10, interleukin 10; IFN-γ, interferon gamma; TNF, tumor necrosis factor; IL-2 interleukin 2).
3. The Impact of ICIs on HIV Replication
PLWHIV and patients with other chronic infections were excluded from the first trials that validated the use of ICIs in clinical practice, based on the concern that immune checkpoint inhibition could lead to a surge of immune response against the virus. In the following years, several studies investigated this hypothesis, as viral reactivation could both represent a severe adverse reaction and, on the other hand, prove pivotal for future eradication strategies.
ICIs, namely ipilimumab for the treatment of metastatic melanoma, were first prescribed to a patient living with HIV in 2011, as described by Burke et al. [38]. Since then, several case series, retrospective cohort studies, and systematic reviews have accumulated. Six years later, PLWHIV were first included into a prospective trial with an ICI-based intervention: two HIV patients received nivolumab for squamous cell carcinoma of the anal canal [39]. Neither of the participants reported serious immunologic or virologic events after exposure. Since 2017, other trials—some of them still ongoing—have been opened to HIV-positive participants. In 2019, in a systematic review of PLWHIV with cancer undergoing treatment with ICIs, Kim and Cook concluded that PLWHIV treated with ICIs achieve therapeutic outcomes similar to HIV-negative patients and did not report significant virologic or immunologic events [40]. A French multicenter retrospective observational study of PLWHIV with various oncologic conditions (2014–2019) collected data on 23 participants with undetectable HIV RNA treated with pembrolizumab and nivolumab (mostly for NSCLC): all patients who continued receiving cART during treatment did not experience loss of viremia control [41]. In conclusion, treatment with ICIs does not seem to have deleterious effects on HIV infection. Randomized controlled trials are needed to further validate such observations and optimize cART during therapy with ICIs [42].
Another line of research involving PLWHIV and ICIs explores the use of the latter for treatment strategies aimed at viral eradication. As of today, strategies allowing viral control in the absence of cART are not available in clinical practice. Upon diagnosis, PLWHIV therefore become candidates for life-long chronic treatment, posing adherence, toxicity, availability, and cost issues. On this basis, vast effort has been put into investigating functional cure strategies. The main obstacle to permanent viral suppression is posed by the HIV reservoir, constituted by cells latently infected with integrated proviral genomes [43]. Various pre-clinical studies have shown that inducible, replication competent HIV genomes are preferably harbored in CD4+ cells over-expressing immune checkpoints, and that immune checkpoint expression correlates with total HIV DNA [44,45]. In vitro, ICIs have been shown to restore HIV-specific CD8+ cells and to increase HIV production from reservoir cells [29].
Due to these findings, ICIs have been recognized as candidates for the shock and kill strategy, that is, using certain drugs to activate the latent reservoir (“shock”) to enable HIV-specific immunity to recognize and eliminate (“kill”) the cells that harbored latent HIV, effectively purging the reservoir [46]. Despite promising results in preclinical studies and animal models, the impact of this strategy on the human host is yet to be fully defined. In 2015, a case report on ipilimumab used against melanoma in an HIV-positive patient was published, and for the first time in the literature, the effect of ICI treatment on the HIV reservoir was described [47]. A second case reported a decrease in HIV DNA levels and a simultaneous plasmatic HIV RNA peak after the use of nivolumab (used for NSCLC) in an HIV-positive patient [48]. Abbar and collaborators performed a systematic review of the available literature in 2020, rounding up 176 PLWHIV subjected to ICI treatment, some of whom had HIV DNA and/or HIV-specific CD8+ responses measured [49]. Among individuals with both molecular and immunological variables available, no effect was observed in more than a half, and only one patient displayed both an HIV-specific CD8+ cell increase and HIV DNA decrease. Despite the underlying safety viro-immunologic profile of ICIs, the authors concluded that such drugs have a limited impact on the HIV reservoir. However, according to various ongoing trials, ICIs remain suitable candidates as combination drugs in synergistic strategies towards HIV cure. Probably these strategies will employ latency reversing agents, and preliminary results suggest the ability of anti-PD1 molecules to potentiate HIV latency reversal in cART suppressed PLWHIV [50].
4. Safety and Efficacy of ICIs in PLWHIV
Even though PLWHIV are at increased risk of developing several cancers, this population has been historically excluded from clinical trials assessing the efficacy of ICIs, thus inhibiting broad implementation of these therapies among PLWHIV with cancer [51]. As stated above, this exclusion was due to the fear of side effects related to reestablishing the immunity against HIV and to the concern that this population lacks a sufficient underlying T-cell immunity to benefit from therapy. In the last few years, encouraging trends in the inclusion of PLWHIV in ICI trials have been identified [51,52]. These data have already been collected in a small number of reviews [40,41,42] assessing the safety and efficacy of ICIs in this subgroup of patients with advanced cancer. In PLWHIV, ICIs have been employed for the treatment of both AIDS-defining malignancies [52] and non-AIDS defining malignancies.
In their systematic review, Kim and Cook [40] described 73 PLWHIV treated with ICIs. Most patients were reported to have non-AIDS defining malignancies: 25 patients had non-small cell lung cancer (NSCLC) and 16 patients had melanoma, whereas nine patients had Kaposi sarcoma. Pre-treatment and post-treatment HIV loads were available in 34 of 73 patients. Twenty-eight individuals had undetectable viral load. HIV remained suppressed in 26 of the 28 (93%) with undetectable HIV load, and their CD4 cell counts increased (mean [SD] change, 12.3 [28.5]/μL). This data could suggest that ICI treatment does not impact HIV control, maintaining undetectable HIV viral load and not causing CD4 T cell counts to decrease. Unfortunately, data on HIV DNA were not available to assess the impact of ICIs on the viral reservoir. Immune checkpoint inhibitor therapy was generally well tolerated, with grade 3 or higher immune-related adverse events noted in six of 70 patients (8.6%), a value similar to that observed in patients without HIV infection [40].
Uldrick et al. [53] conducted an open-label study to assess the safety of pembrolizumab in PLWHIV with advanced cancer. Eligibility criteria were HIV infection, a concomitant advanced cancer, a CD4+ T-cell count greater than or equal to 100 cells/μL, cART for 4 or more weeks, and an HIV viral load of less than 200 copies/mL. Overall, 30 PLWHIV were treated with at least one cycle of pembrolizumab, monitoring viro-immunological status, adverse events, and disease progression. The majority of treatment-emergent adverse events were grade 1 or 2 and included anemia, fatigue, nausea, and hypothyroidism. Based on this study, the authors concluded that pembrolizumab has a similar safety profile in PLWHIV with suppressed HIV or low-level viremia on cART and advanced cancer to that observed in the general population. Interestingly, one participant with detectable Kaposi sarcoma (KS) herpesvirus (KSHV) viremia before receiving pembrolizumab developed a polyclonal KSHV-associated B-cell lymphoproliferative disorder and died, suggesting the need for additional monitoring in patients with this active infection while receiving ICIs. Regarding viro-immunological status, pembrolizumab does not appear to influence CD4+ T-cell counts or HIV viral load. The impact on viro-immunological status reported by Uldrik et al. is in accordance with data previously reported in the literature. In a case series [54] of three patients with HIV infection affected by Merkel-cell carcinoma, pembrolizumab administration seemed not to modify viro-immunological status, with all patients having an HIV viral load consistently undetectable at baseline and after treatment, maintaining stable CD4+ T cell counts during treatment with ICI. Similarly, in a retrospective study [55] that collected data from nine patients with HIV infection and Kaposi sarcoma treated with ICIs, eight patients received nivolumab and one pembrolizumab. At baseline, all patients were receiving antiretroviral therapy, with well-controlled HIV viral load in seven of the nine patients. Seven patients (78%) during ICI treatment experienced an improvement in CD4+ T cell counts.
Regarding efficacy of ICIs in PLWHIV, Uldrik et al. [53] documented a protocol-defined clinical benefit in 17% of participants affected by different type of cancers, suggesting a comparable efficacy to the HIV-negative population with advanced cancer. In agreement with this preliminary data, Bari et al. [56] performed a retrospective analysis of 17 HIV patients treated with one of the PD-1/PD-L1 inhibitors for advanced cancer: 10 patients had lung cancer, two hepatocellular cancer, two anal cancers, one kidney cancer, one non-Hodgkin’s lymphoma, and one advanced basal cell carcinoma. Ten patients responded to treatment, of whom five had partial response and five had stable disease. Matching retrospectively reported evidence and Uldrik’s prospective study, anti-PD1/PD-L1 therapy appears to be safe and effective in HIV patients with cancer. However, these data are based on small samples. Further prospective studies on the use of ICIs in PLWHIV with advanced cancer are currently ongoing [57,58,59] to better assess safety and efficacy and to promote broader use of this immunotherapy in PLWHIV with stable immunovirological status.
5. ICIs and Opportunistic Infections
The use of ICIs is associated with immune-related adverse effects (irAEs) related to the upregulated immune system. These toxicities can affect a variety of organs including lung (pneumonitis), gastrointestinal tract (colitis), liver (hepatitis), skin (rash), pancreas (pancreatitis), endocrine (hypophysitis, thyroiditis), and kidneys (nephritis). Standard management algorithms from scientific societies [60] recommend the use of immunosuppressive medications such as steroids or, in case of refractory disease, tumor necrosis factor alpha (TNF-α) inhibitors to manage these immune-related adverse events. The immunosuppression deriving from the use of these drugs has been reported to be a risk factor for opportunistic infections [61,62].
Several cases of opportunistic infections among patients with melanoma receiving the CTLA-4 inhibitor ipilimumab have been published, including invasive aspergillosis [63], cytomegalovirus-induced hepatitis [64], and pneumocystis pneumonia (PJP) [65].
One retrospective study has systemically evaluated the risk of infection in 748 melanoma patients receiving ICIs (CTLA-4, PD-1, and/or PD-L1) [66]: 658 (73.2%) received ipilimumab, 52 (5.7%) received nivolumab, 83 (9.2%) received pembrolizumab, and 105 (11.7%) received a combination therapy. Serious infections developed in 54 patients (7.3%), including bacterial pneumonia, intra-abdominal infection, invasive pulmonary aspergillosis, pneumocystis pneumonia, disseminated herpes zoster (HZ), cytomegalovirus colitis, and Strongyloides stercoralis hyperinfestation syndrome. The major risk factors for infection were use of corticosteroids and/or infliximab and a combination of ipilimumab with nivolumab.
Another retrospective study [67] analyzed 167 patients affected by non-small cell lung carcinoma (NSCLC) treated with nivolumab. Thirty-two (19.2%) out of 167 patients developed serious infections, most of them bacterial (78.1%), while 18.8% were viral and 6.3% fungal; a case of pulmonary invasive aspergillosis and two cases of herpes zoster reactivation have been reported.
Various cases of reactivation of latent tuberculosis (LTBI) among patients treated with nivolumab or pembrolizumab have been described in literature in patients not receiving further immunosuppressive therapies for irAEs. In 2016 Lee et al. [68] described the first case of LTBI reactivation in a patient undergoing immunotherapy with pembrolizumab in Hodgkin’s lymphoma. In some recent reviews [69,70], reactivations or primary infections by M. tuberculosis have been described in patients treated with ICIs for various neoplastic diseases (non-small cell lung cancer, melanoma, Hodgkin’s lymphoma, nasopharyngeal cancer, squamous cell carcinoma of the oral cavity, Merkel cell carcinoma). The risk of primary infection or reactivation during ICI treatments could be influenced by the underlying malignant disease causing local and systemic immune dysregulation. Particularly, immune checkpoint inhibitors’ immunomodulatory effect may enhance the immune reaction to infectious disease, resulting in reactivation and unmasking of latent infections. An immune reconstitution inflammatory syndrome may explain previously reported cases, such as the progression of chronic progressive pulmonary aspergillosis in a patient affected by lung adenocarcinoma treated with nivolumab in Japan [71].
As for previously reported infections, ICIs are not directly associated with an increased risk of PJP. A recent meta-analysis [72] investigating the incidence and relative risk (RR) of lung infections observed in phase II/III studies including patients with solid tumors undergoing treatment with ICIs did not show an increased RR compared to the standard chemotherapy. However, as previously described, treatment of irAEs may lead to an increased risk of PJP. Del Castillo et al. [66] described 3 cases of PJP in patients treated with corticosteroids and infliximab. Seven additional clinical cases described the development of PJP as a consequence of the use of immunosuppressants for the management of irAEs. Four patients were affected by melanoma [65,73,74], four by lung cancer [75], and one by Hodgkin’s lymphoma [73]. In a retrospective study of 515 patients treated with ICIs for malignancy, 23 required treatment with high doses of corticosteroids secondary to the development of immune-mediated pneumonia, six (26%) of whom developed PJP (all in the absence of specific prophylaxis for PJP); in this study the presence of lymphopenia (lymphocytes < 755 cells/µL) was a predisposing factor for the development of PJP during steroid treatment [76]. Few cases of PJP associated with treatment with ICIs in the absence of previous or concomitant immunosuppressive treatment for irAEs have been described in the literature; the majority of patients presented significant comorbidities or anamnestic history of chemotherapy or radiotherapy treatments with a possible etiological role in the development of opportunistic infections [77].
The burden of opportunistic infections in PLWHIV depends on their viro-immunological status. Recovery of immunological competence has been made possible with the advent of cART. As is widely known, today the commonest opportunistic infections of the pre-cART era can be avoided in PLWHIV with suppressed viral load and CD4 cell counts > 200/mmc.
Currently, no cases of opportunistic infections have been reported in patients with HIV and cancer treated with ICIs. This is probably related to the non-extensive use of these drugs in this population. Further studies on the use of ICIs as immunological enhancers are needed. In fact, their use has been hypothesized in the treatment of opportunistic or latent infections, reversing T cell immune exhaustion [78].
6. Conclusions
ICIs are currently reshaping the landscape of oncologic therapy, opening opportunities for patients previously considered untreatable. PLWHIV have only partially benefited from this revolution, having been excluded from trials investigating the efficacy of these drugs. Nonetheless, due to the growing volume of reassuring data on ICI safety in PLWHIV, currently in those patients with well-controlled infections, it is suggested to employ ICIs as in patients without HIV. Several RCTs are ongoing, enrolling PLWHIV specifically, and more solid data are expected in the near future. The presence of immune exhaustion is a well-described feature of HIV infection, with several pieces of evidence highlighting the role of the immune checkpoints CTLA-4, PD-1, TIM-3, LAG-3, and TIGIT. Nonetheless, the use of ICIs as a therapeutic instrument to eradicate the viral reservoir, despite having been proposed by several authors, is still limited to the in vitro experimental setting and far from use in clinical practice. Finally, due to the lack of ICI use among PLWHIV, it is not possible to draw definitive conclusions regarding the risk of opportunistic infections in this specific population.
Author Contributions
Conceptualization, A.L., A.B. and A.G.; writing—original draft preparation, A.L., V.C. and G.B.; writing—review and editing, A.L., V.C., G.B., E.P., A.M., D.M., R.U., L.A., A.B. and A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Drug Administration (FDA). Drug Approval Package: YERVOY (ipilimumab) Injection. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/125377Orig1s000TOC.cfm (accessed on 23 April 2021).
- FDA Approves New, Targeted Treatment for Bladder Cancer | FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-targeted-treatment-bladder-cancer (accessed on 24 April 2021).
- FDA Approves First Treatment for Rare Form of Skin Cancer | FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-rare-form-skin-cancer (accessed on 24 April 2021).
- Food and Drug Administration. Drug Approval Package: LIBTAYO (cemiplimab-rwlc) Injection. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761097s007lbl.pdf (accessed on 24 April 2021).
- FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Endometrial Cancer|FDA. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-endometrial-cancer (accessed on 24 April 2021).
- FDA Approves Durvalumab after Chemoradiation for Unresectable Stage III NSCLC | FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-durvalumab-after-chemoradiation-unresectable-stage-iii-nsclc (accessed on 24 April 2021).
- Center for Drug Evaluation and Research Approval Package for: Nivolumab. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125527Orig1s000Approv.pdf (accessed on 24 April 2021).
- FDA Pembrolizumab Label. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf (accessed on 24 April 2021).
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; Borghaei, H.; Ramalingam, S.S.; Horn, L.; De Castro Carpeño, J.; Pluzanski, A.; Burgio, M.A.; Garassino, M.; Chow, L.Q.M.; Gettinger, S.; et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: A pooled analysis. Lancet Oncol. 2019, 20, 1395–1408. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase i KEYNOTE-001 study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Vora, K.B.; Awad, M.M. Exclusion rates of patients living with HIV from cancer immunotherapy clinical trials. J. Clin. Oncol. 2020, 38, e19035. [Google Scholar] [CrossRef]
- Moskophidis, D.; Lechner, F.; Pircher, H.; Zinkernagel, R.M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 1993, 362, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Gallimore, A.; Glithero, A.; Godkin, A.; Tissot, A.C.; Plückthun, A.; Elliott, T.; Hengartner, H.; Zinkernagel, R. Induction and Exhaustion of Lymphocytic Choriomeningitis Virus–specific Cytotoxic T Lymphocytes Visualized Using Soluble Tetrameric Major Histocompatibility Complex Class I–Peptide Complexes. J. Exp. Med. 1998, 187, 1383–1393. [Google Scholar] [CrossRef]
- Zajac, A.J.; Blattman, J.N.; Murali-Krishna, K.; Sourdive, D.J.D.; Suresh, M.; Altman, J.D.; Ahmed, R. Viral Immune Evasion Due to Persistence of Activated T Cells Without Effector Function. J. Exp. Med. 1998, 188, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Klenerman, P.; Hill, A. T cells and viral persistence: Lessons from diverse infections. Nat. Immunol. 2005, 6, 873–879. [Google Scholar] [CrossRef]
- Blank, C.; Mackensen, A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: An update on implications for chronic infections and tumor evasion. Cancer Immunol. Immunother. 2007, 56, 739–745. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Kahan, S.M.; Wherry, E.J.; Zajac, A.J. T cell exhaustion during persistent viral infections. Virology 2015, 479–480, 180–193. [Google Scholar] [CrossRef]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Douek, D.C.; Brenchley, J.M.; Betts, M.R.; Ambrozak, D.R.; Hill, B.J.; Okamoto, Y.; Casazza, J.P.; Kuruppu, J.; Kunstman, K.; Wolinsky, S.; et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature 2002, 417, 95–98. [Google Scholar] [CrossRef]
- Papagno, L.; Spina, C.A.; Marchant, A.; Salio, M.; Rufer, N.; Little, S.; Dong, T.; Chesney, G.; Waters, A.; Easterbrook, P.; et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004, 2, e120. [Google Scholar] [CrossRef] [PubMed]
- The HIV-CAUSAL Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. Aids 2010, 24, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.M.P.; Sereti, I. Immune restoration after antiretroviral therapy: The pitfalls of hasty or incomplete repairs. Immunol. Rev. 2013, 254, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Pantazis, N.; Martin, G.E.; Hickling, S.; Hurst, J.; Meyerowitz, J.; Willberg, C.B.; Robinson, N.; Brown, H.; Fisher, M.; et al. Exhaustion of Activated CD8 T Cells Predicts Disease Progression in Primary HIV-1 Infection. PLoS Pathog. 2016, 12, 1–19. [Google Scholar] [CrossRef]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.-R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef]
- Steiner, K.; Waase, I.; Rau, T.; Dietrich, M.; Fleischer, B.; Bröker, B.M. Enhanced expression of CTLA-4 (CD152) on CD4+ T cells in HIV infection. Clin. Exp. Immunol. 1999, 115, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, D.E.; Kavanagh, D.G.; Pereyra, F.; Zaunders, J.J.; Mackey, E.W.; Miura, T.; Palmer, S.; Brockman, M.; Rathod, A.; Piechocka-Trocha, A.; et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007, 8, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Ndhlovu, L.C.; Barbour, J.D.; Sheth, P.M.; Jha, A.R.; Long, B.R.; Wong, J.C.; Satkunarajah, M.; Schweneker, M.; Chapman, J.M.; et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008, 205, 2763–2779. [Google Scholar] [CrossRef] [PubMed]
- Rallón, N.; García, M.; García-Samaniego, J.; Cabello, A.; Álvarez, B.; Restrepo, C.; Nistal, S.; Górgolas, M.; Benito, J.M. Expression of PD-1 and tim-3 markers of T-cell exhaustion is associated with CD4 dynamics during the course of untreated and treated HIV infection. PLoS ONE 2018, 13, e0193829. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, A.; Qiu, C.; Wang, W.; Yang, Y.; Qiu, C.; Liu, A.; Zhu, L.; Yuan, S.; Hu, H.; et al. The Upregulation of LAG-3 on T Cells Defines a Subpopulation with Functional Exhaustion and Correlates with Disease Progression in HIV-Infected Subjects. J. Immunol. 2015, 194, 3873–3882. [Google Scholar] [CrossRef] [PubMed]
- Chew, G.M.; Fujita, T.; Webb, G.M.; Burwitz, B.J.; Wu, H.L.; Reed, J.S.; Hammond, K.B.; Clayton, K.L.; Ishii, N.; Abdel-Mohsen, M.; et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog. 2016, 12, e1005349. [Google Scholar] [CrossRef] [PubMed]
- Brockman, M.A.; Kwon, D.S.; Tighe, D.P.; Pavlik, D.F.; Rosato, P.C.; Sela, J.; Porichis, F.; Le Gall, S.; Waring, M.T.; Moss, K.; et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 2009, 114, 346–356. [Google Scholar] [CrossRef]
- Burke, M.M.; Kluger, H.M.; Golden, M.; Heller, K.N.; Hoos, A.; Sznol, M. Case Report: Response to Ipilimumab in a Patient with HIV with Metastatic Melanoma. J. Clin. Oncol. 2011, 29, e792–e794. [Google Scholar] [CrossRef]
- Morris, V.K.; Salem, M.E.; Nimeiri, H.; Iqbal, S.; Singh, P.; Ciombor, K.; Polite, B.; Deming, D.; Chan, E.; Wade, J.L.; et al. Nivolumab for previously treated unresectable metastatic anal cancer (NCI9673): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 446–453. [Google Scholar] [CrossRef]
- Kim, C.; Cook, M.R. Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients with HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol. 2019, 5, 1049–1053. [Google Scholar]
- Spano, J.P.; Veyri, M.; Gobert, A.; Guihot, A.; Perré, P.; Kerjouan, M.; Brosseau, S.; Cloarec, N.; Montaudié, H.; Helissey, C.; et al. Immunotherapy for cancer in people living with HIV: Safety with an efficacy signal from the series in real life experience. Aids 2019, 33, F13–F19. [Google Scholar] [CrossRef]
- Rico, G.T.; Chan, M.M.; Loo, K.F. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): A review of the available evidence. Cancer Treat. Rev. 2020, 86, 102011. [Google Scholar] [CrossRef]
- Letizia, A.G.; Ge, Y.; Vangeti, S.; Goforth, C.; Weir, D.L.; Kuzmina, N.A.; Balinsky, C.A.; Chen, H.W.; Ewing, D.; Soares-Schanoski, A.; et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: A prospective cohort study. Lancet Respir. Med. 2021, 2600, 1–9. [Google Scholar] [CrossRef]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Banga, R.; Rebecchini, C.; Procopio, F.A.; Noto, A.; Munoz, O.; Ioannidou, K.; Fenwick, C.; Ohmiti, K.; Cavassini, M.; Corpataux, J.M.; et al. Lymph node migratory dendritic cells modulate HIV-1 transcription through PD-1 engagement. PLoS Pathog. 2019, 15, e1007918. [Google Scholar] [CrossRef]
- Troeger, C.E.; Blacker, B.F.; Khalil, I.A.; Zimsen, S.R.M.; Albertson, S.B.; Abate, D.; Abdela, J.; Adhikari, T.B.; Aghayan, S.A.; Agrawal, S.; et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019, 7, 69–89. [Google Scholar] [CrossRef]
- Wightman, F.; Solomon, A.; Kumar, S.S.; Urriola, N.; Gallagher, K.; Hiener, B.; Palmer, S.; McNeil, C.; Garsia, R.; Lewin, S.R. Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. Aids 2015, 29, 504–506. [Google Scholar] [CrossRef]
- Guihot, A.; Marcelin, A.G.; Massiani, M.A.; Samri, A.; Soulié, C.; Autran, B.; Spano, J.P. Drastic decrease of the HIV reservoir in a patient treated with nivolumab for lung cancer. Ann. Oncol. 2018, 29, 517–518. [Google Scholar] [CrossRef]
- Abbar, B.; Baron, M.; Katlama, C.; Marcelin, A.G.; Veyri, M.; Autran, B.; Guihot, A.; Spano, J.P. Immune checkpoint inhibitors in people living with HIV: What about anti-HIV effects? Aids 2020, 34, 167–175. [Google Scholar] [CrossRef]
- Fromentin, R.; DaFonseca, S.; Costiniuk, C.T.; El-Far, M.; Procopio, F.A.; Hecht, F.M.; Hoh, R.; Deeks, S.G.; Hazuda, D.J.; Lewin, S.R.; et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4 + T cells from ART-suppressed individuals. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Reuss, J.E.; Stern, D.; Foster, J.C.; Ramaswami, R.; Lurain, K.; Chen, H.X.; Streicher, H.; Kem, R.; Little, R.F.; Sharon, E. Assessment of Cancer Therapy Evaluation Program Advocacy and Inclusion Rates of People Living With HIV in Anti-PD1/PDL1 Clinical Trials. JAMA Netw. Open 2020, 3, e2027110. [Google Scholar] [CrossRef]
- Park, S.; Sacco, A.G.; Cohen, E.E.W.; Daniels, G.A. Safety and efficacy of checkpoint inhibition (CI) in cancer patients (pts) with concurrent human immunodeficiency virus (HIV) infection. J. Clin. Oncol. 2018, 36, 136. [Google Scholar] [CrossRef]
- Uldrick, T.S.; Gonçalves, P.H.; Abdul-Hay, M.; Claeys, A.J.; Emu, B.; Ernstoff, M.S.; Fling, S.P.; Fong, L.; Kaiser, J.C.; Lacroix, A.M.; et al. Assessment of the Safety of Pembrolizumab in Patients with HIV and Advanced Cancer—A Phase 1 Study. JAMA Oncol. 2019, 5, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Church, C.; Alexander, N.A.; Shinohara, M.M.; Paulson, K.G.; Lewis, K.D.; Lee, N.S.; Nghiem, P. Immune checkpoint inhibitor therapy in HIV-associated Merkel cell carcinoma: A case series of 3 patients. JAAD Case Rep. 2021, 8, 28–33. [Google Scholar] [CrossRef]
- Galanina, N.; Goodman, A.M.; Cohen, P.R.; Frampton, G.M.; Kurzrock, R. Successful Treatment of HIV-associated Kaposi’s Sarcoma with Immune Checkpoint Blockade. Cancer Immunol Res. 2018, 176, 139–148. [Google Scholar] [CrossRef]
- Bari, S.; Chan, A.; Jain, S.R.; Hostler, C.J. Outcomes of programmed cell death protein 1 (PD-1) and programmed death-ligand 1(PD-L1) inhibitor therapy in HIV patients with advanced cancer. J. Oncol. 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Identifier: NCT02595866. Available online: https://clinicaltrials.gov/ct2/show/NCT02595866 (accessed on 24 April 2021).
- ClinicalTrials.gov Identifier: NCT03354936. Available online: https://clinicaltrials.gov/ct2/show/NCT03354936 (accessed on 24 April 2021).
- ClinicalTrials.gov Identifier: NCT02408861. Available online: https://clinicaltrials.gov/ct2/show/NCT02408861 (accessed on 24 April 2021).
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv264–iv266. [Google Scholar] [CrossRef]
- Ford, A.C.; Peyrin-Biroulet, L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: Meta-analysis of randomized controlled trials. Am. J. Gastroenterol. 2013, 108, 1268–1276. [Google Scholar] [CrossRef]
- Klein, N.C.; Go, C.H.U.; Cunha, B.A. Infections associated with steroid use. Infect. Dis. Clin. N. Am. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Kyi, C.; Hellmann, M.D.; Wolchok, J.D.; Chapman, P.B.; Postow, M.A. Opportunistic infections in patients treated with immunotherapy for cancer. J. Immunother. Cancer 2014, 2, 2–4. [Google Scholar] [CrossRef]
- Uslu, U.; Agaimy, A.; Hundorfean, G.; Harrer, T.; Schuler, G.; Heinzerling, L. Autoimmune Colitis and Subsequent CMV-induced Hepatitis After Treatment With Ipilimumab. J. Immunother. 2015, 38, 212–215. [Google Scholar] [CrossRef]
- Arriola, E.; Wheater, M.; Krishnan, R.; Smart, J.; Foria, V.; Ottensmeier, C. Immunosuppression for ipilimumab-related toxicity can cause pneumocystis pneumonia but spare antitumor immune control. Oncoimmunology 2015, 4, 1–4. [Google Scholar] [CrossRef]
- Del Castillo, M.; Romero, F.A.; Argüello, E.; Kyi, C.; Postow, M.A.; Redelman-Sidi, G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin. Infect. Dis. 2016, 63, 1490–1493. [Google Scholar] [CrossRef]
- Fujita, K.; Kim, Y.H.; Kanai, O.; Yoshida, H.; Mio, T.; Hirai, T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir. Med. 2019, 146, 66–70. [Google Scholar] [CrossRef]
- Lee, J.J.X.; Chan, A.; Tang, T. Tuberculosis reactivation in a patient receiving anti-programmed death-1 (PD-1) inhibitor for relapsed Hodgkin’s lymphoma. Acta Oncol. 2016, 55, 519–520. [Google Scholar] [CrossRef]
- Langan, E.A.; Graetz, V.; Allerheiligen, J.; Zillikens, D.; Rupp, J.; Terheyden, P. Immune checkpoint inhibitors and tuberculosis: An old disease in a new context. Lancet Oncol. 2020, 21, e55–e65. [Google Scholar] [CrossRef]
- Zaemes, J.; Kim, C. Immune checkpoint inhibitor use and tuberculosis: A systematic review of the literature. Eur. J. Cancer 2020, 132, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Fujita, K.; Nakatani, K.; Mio, T. Acute progression of aspergillosis in a patient with lung cancer receiving nivolumab. Respirol. Case Rep. 2018, 6, 3–5. [Google Scholar] [CrossRef]
- Su, Q.; Zhu, E.C.; Wu, J.B.; Li, T.; Hou, Y.L.; Wang, D.Y.; Gao, Z.H. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: A systematic review and meta-analysis. Front. Immunol. 2019, 10, 108. [Google Scholar] [CrossRef]
- Sadek, M.; Loizidou, A.; Drowart, A.; Van den Wijngaert, S.; Gomez-Galdon, M.; Aspeslagh, S. Pneumocystis infection in two patients treated with both immune checkpoint inhibitor and corticoids. J. Immunother. Precis. Oncol. 2020, 3, 27–30. [Google Scholar] [CrossRef]
- Slevin, F.; Mulatero, C.; Marples, M. Pneumocystis jirovecii pneumonia in a patient with melanoma treated with infliximab and corticosteroids for ipilimumab-associated colitis. Glob. Dermatol. 2016, 3, 381–384. [Google Scholar] [CrossRef][Green Version]
- Schwarz, M.; Kocher, F.; Niedersuess-Beke, D.; Rudzki, J.; Hochmair, M.; Widmann, G.; Hilbe, W.; Pircher, A. Immunosuppression for Immune Checkpoint-related Toxicity Can Cause Pneumocystis jirovecii Pneumonia (PJP) in Non-small-cell Lung Cancer (NSCLC): A Report of 2 Cases. Clin. Lung Cancer 2019, 20, E247–E250. [Google Scholar] [CrossRef] [PubMed]
- Omene, A.A.; Ferguson, R.P. Absolute lymphocyte count as a predictor of Pneumocystis pneumonia in patients previously unknown to have HIV. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 15696. [Google Scholar] [CrossRef] [PubMed]
- Cadranel, J.; Canellas, A.; Matton, L.; Darrason, M.; Parrot, A.; Naccache, J.-M.; Lavole, A.; Ruppert, A.-M.; Fallet, V. Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur. Respir. Rev. 2019, 28, 190058. [Google Scholar] [CrossRef]
- Better, J.; Matt, U. Pneumocystis pneumonia: Checkpoint inhibition to the rescue? Am. J. Respir. Cell Mol. Biol. 2020, 62, 674–675. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).