How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation
Abstract
1. Introduction
2. Mechanical Properties of the Endometrium Change during the Menstrual Cycle
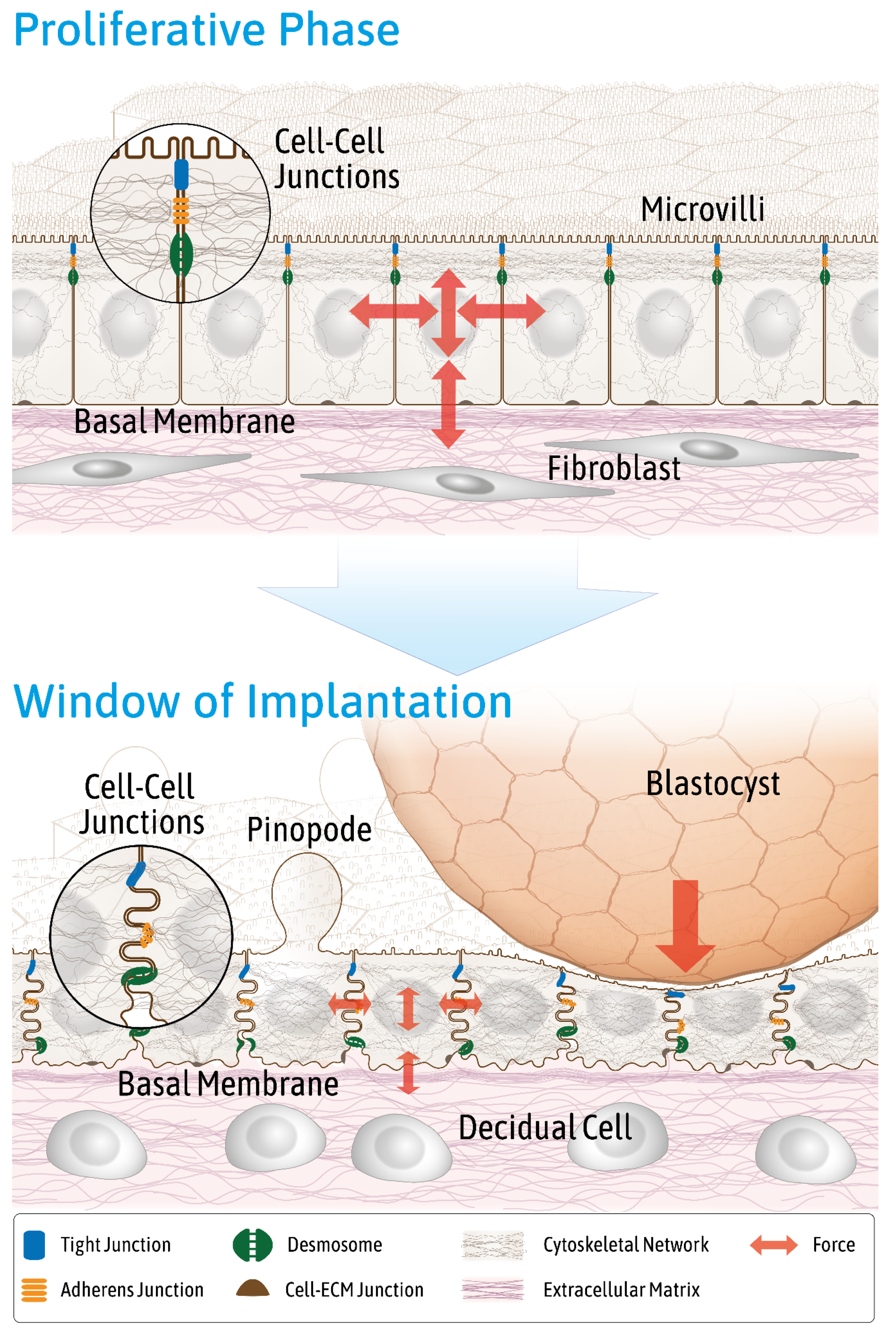
3. Endometrial Epithelial Cells Change Junctional Arrangement to Become Receptive
4. Hormone-Dependent Cytoskeletal Rearrangements Regulate Trophoblast Adhesion
5. Steroid Hormones Regulate Apicobasal Organization in Endometrial Epithelial Cells
6. Mechanical Stimuli Enhance Decidualization of Endometrial Stromal Cells
7. Mechanical Cues Modulate Embryo–Endometrial Interaction
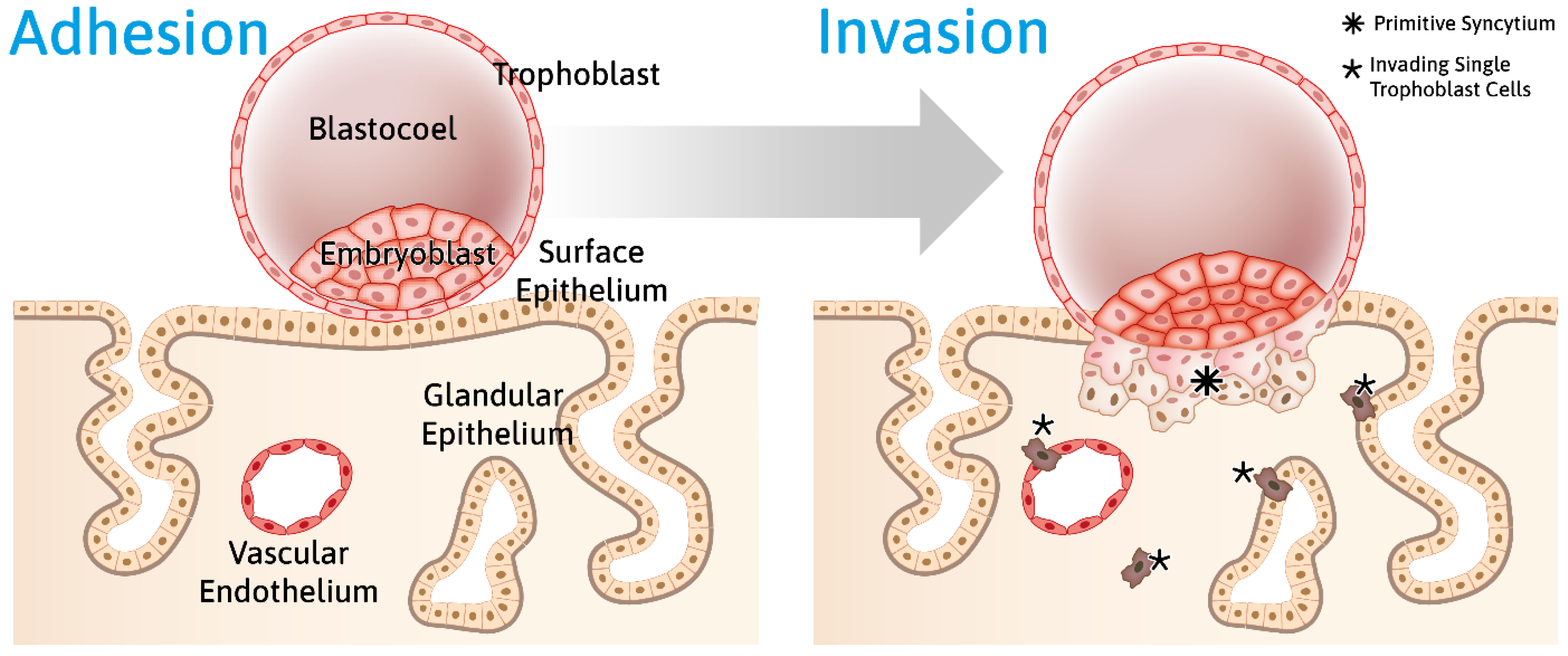
8. Trophoblast Penetration of the Epithelial Barrier Is a Multimodal Process
9. Invading Extravillous Trophoblast Cells Increase Decidual Stiffness by Remodeling Vasculature and Extracellular Matrix
10. Superficial Endometrial Injuries May Improve Embryo Implantation Rates
11. ENaC, Piezo1 and TRP Are Functionally Expressed in Endometrial Epithelial Cells and May Contribute to Embryo–Endometrial Epithelial Cell Crosstalk
12. TAZ Protein Is Downregulated in Endometrial Stromal Cells during Decidualization
13. The Endometrium Stiffens during Pregnancy
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Atomic force microscopy (AFM) | Method used in mechanobiology to quantify and apply mechanical forces in biological systems and biomaterials. The tip of a cantilever is used to probe and indent the underlying surface. |
| Biomechanics | Approach to describe the structural properties of cells, tissues, organs and organisms by mechanical concepts of engineering sciences. |
| Complex shear modulus |G *| | Response of viscoelastic material to vibration, expressed in Pa (=N/m²). |
| Compression | Application of a mechanical force that results in a contraction of the material. |
| Elasticity | Ability of a material to resume its normal shape after being stretched or compressed. It can be quantified, for example, by Young’s modulus “E”. |
| Mechanical force | A force that involves contact with another object and that produces a change in state of rest or motion. Mechanical forces are expressed in Newton (N = kg m/sec²). |
| Mechanobiology | Interdisciplinary field that studies the effects of mechanical forces on the physiology and pathology of living systems. |
| Mechanosensing | Ability of cells to sense and respond to mechanical stimuli. |
| Mechanosensitivity | Quantifiable ability of a cell to sense and respond to a specific mechanical stimulus. |
| Mechanotransduction | The molecular conversion of a mechanical signal into a biological cell response. |
| Mechanotransmission | The transmission of an applied mechanical load to specialized structures. |
| Nanoindentation | Technique to measure the hardness of a material by pressing a probe against a sample. |
| Shear stress “τ” | Stress that is applied parallel or tangential to the surface of a material. |
| Stiffness | Technical term to describe the elastic properties of a material. It can be quantified by Young’s modulus “E”. |
| Strain “ε” | Measure of material deformation as a result of tension or compression expressed in percentage. |
| Stress | Force per unit area within materials that arises from externally applied forces. It is measured in Pascal (Pa = N/m²). |
| Tension | The application of a pulling force that results in an elongation of the material. |
| Young’s modulus “E” | Stiffness value expressed in Pa (=N/m²). It describes the elastic properties of a solid undergoing tension or compression in only one direction (E = σ/ε). |
| Viscoelasticity | Property of materials that display a mixture of viscous and elastic behavior when stressed. |
Appendix A
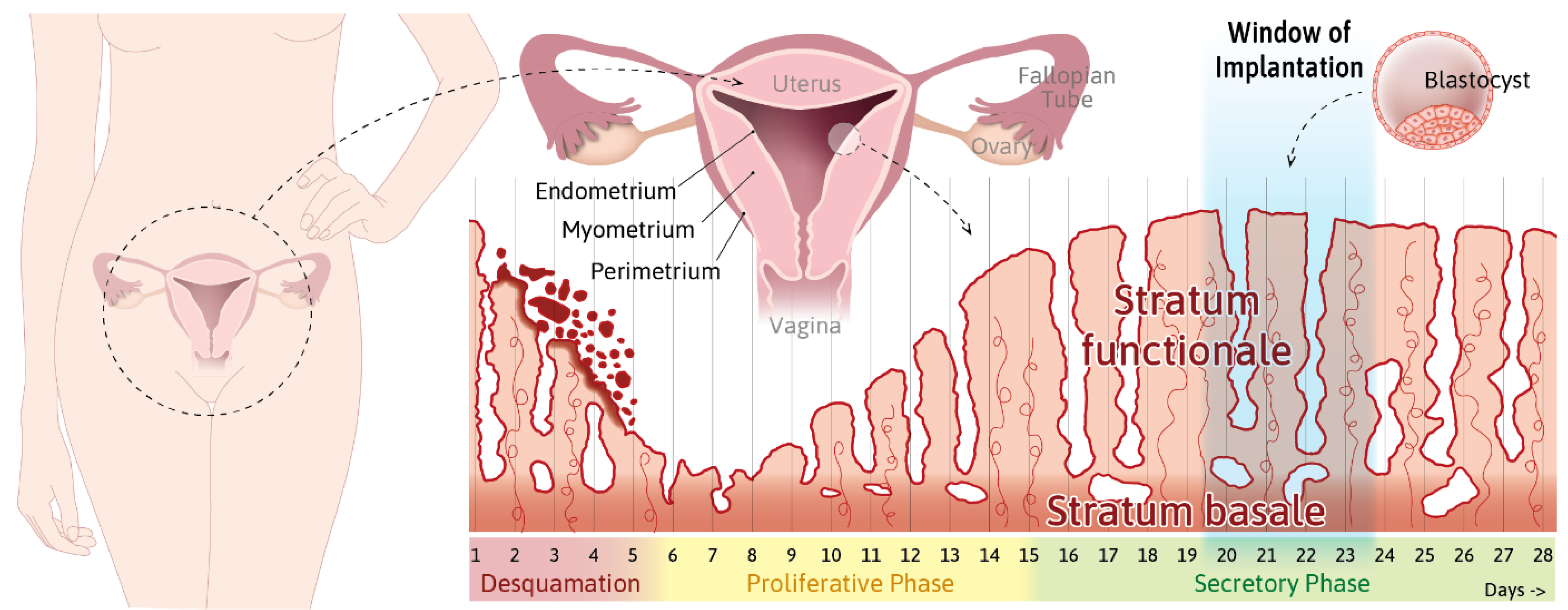
- Info Box 1—The Uterine Wall (See Also Figure 1)
- Info Box 2—The Endometrial Epithelium and Underlying Connective Tissue (See Also Figure 3)
- Info Box 3—Human Embryo Implantation (See Also Figure 2)
- Info Box 4—Mechanobiology in Health and Disease
References
- Cui, W. Mother or nothing: The agony of infertility. World Health Organ. Bull. World Health Organ. 2010, 88, 881. [Google Scholar] [CrossRef]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef]
- Barbieri, R.L. Female infertility. In Yen and Jaffe’s Reproductive Endocrinology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 556–581.e557. [Google Scholar]
- De Kretser, D.M. Male infertility. Lancet 1997, 349, 787–790. [Google Scholar] [CrossRef]
- Evans, J.P.; Leppert, P.C. “Feeling the force” in reproduction: Mechanotransduction in reproductive processes. Connect. Tissue Res. 2016, 57, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Pan-Castillo, B.; Gazze, S.A.; Thomas, S.; Lucas, C.; Margarit, L.; Gonzalez, D.; Francis, L.W.; Conlan, R.S. Morphophysical dynamics of human endometrial cells during decidualization. Nanomedicine 2018, 14, 2235–2245. [Google Scholar] [CrossRef]
- Muthupillai, R.; Lomas, D.; Rossman, P.; Greenleaf, J.F.; Manduca, A.; Ehman, R.L. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 1995, 269, 1854–1857. [Google Scholar] [CrossRef]
- Jiang, X.; Asbach, P.; Streitberger, K.-J.; Thomas, A.; Hamm, B.; Braun, J.; Sack, I.; Guo, J. In vivo high-resolution magnetic resonance elastography of the uterine corpus and cervix. Eur. Radiol. 2014, 24, 3025–3033. [Google Scholar] [CrossRef]
- Noyes, R.W.; Hertig, A.T.; Rock, J. Dating the endometrial biopsy. Obstet. Gynecol. Surv. 1950, 5, 561–564. [Google Scholar] [CrossRef]
- Aplin, J.D.; Charlton, A.K.; Ayad, S. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res. 1988, 253, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, D.; Klentzeris, L.; Fleming, S. Uterus and endometrium: Immunohistochemical localization of extracellular matrix proteins in luteal phase endometrium of fertile and infertile patients. Hum. Reprod. 1996, 11, 2713–2718. [Google Scholar] [CrossRef]
- Tanaka, T.; Wang, C.; Umesaki, N. Remodeling of the human endometrial epithelium is regulated by laminin and type iv collagen. Int. J. Mol. Med. 2009, 23, 173–180. [Google Scholar] [CrossRef]
- Fidler, A.L.; Vanacore, R.M.; Chetyrkin, S.V.; Pedchenko, V.K.; Bhave, G.; Yin, V.P.; Stothers, C.L.; Rose, K.L.; McDonald, W.H.; Clark, T.A.; et al. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. USA 2014, 111, 331–336. [Google Scholar] [CrossRef]
- Vanacore, R.; Ham, A.J.; Voehler, M.; Sanders, C.R.; Conrads, T.P.; Veenstra, T.D.; Sharpless, K.B.; Dawson, P.E.; Hudson, B.G. A sulfilimine bond identified in collagen iv. Science 2009, 325, 1230–1234. [Google Scholar] [CrossRef]
- Uchida, H.; Maruyama, T.; Masuda, H.; Uchida, S.; Miki, F.; Hihara, H.; Katakura, S.; Yoshimasa, Y.; Tanaka, M. How to create an embryo penetration route. Am. J. Reprod. Immunol. 2016, 75, 326–332. [Google Scholar] [CrossRef]
- Whitby, S.; Zhou, W.; Dimitriadis, E. Alterations in epithelial cell polarity during endometrial receptivity: A systematic review. Front. Endocrinol. 2020, 11, 596324. [Google Scholar] [CrossRef]
- Murphy, C.R. Uterine receptivity and the plasma membrane transformation. Cell Res. 2004, 14, 259–267. [Google Scholar] [CrossRef]
- Tsuchiya, B.; Sato, Y.; Kameya, T.; Okayasu, I.; Mukai, K. Differential expression of n-cadherin and e-cadherin in normal human tissues. Arch. Histol. Cytol. 2006, 69, 135–145. [Google Scholar] [CrossRef]
- Imai, K.; Maeda, M.; Fujiwara, H.; Kariya, M.; Takakura, K.; Kanzaki, H.; Mori, T. Dipeptidyl peptidase iv as a differentiation marker of the human endometrial glandular cells. Hum. Reprod. 1992, 7, 1189–1194. [Google Scholar] [CrossRef]
- Suzuki, M.; Kuramoto, H.; Izumi, S.; Shirane, H.; Watanabe, K. Cyclic changes of alkaline phosphatase in the human endometrium: Histochemical and biochemical analyses. Acta Histochem. Cytochem. 1981, 14, 524–533. [Google Scholar] [CrossRef]
- Martel, D.; Frydman, R.; Glissant, M.; Maggioni, C.; Roche, D.; Psychoyos, A. Scanning electron microscopy of postovulatory human endometrium in spontaneous cycles and cycles stimulated by hormone treatment. J. Endocrinol. 1987, 114, 319–324. [Google Scholar] [CrossRef]
- Nikas, G. Pinopodes as markers of endometrial receptivity in clinical practice. Hum. Reprod. 1999, 14, 99–106. [Google Scholar] [CrossRef]
- Gompel, C. The ultrastructure of the human endometrial cell studied by electron microscopy. Am. J. Obs. Gynecol. 1962, 84, 1000–1009. [Google Scholar] [CrossRef]
- Thie, M.; Herter, P.; Pommerenke, H.; Dürr, F.; Sieckmann, F.; Nebe, B.; Rychly, J.; Denker, H.W. Adhesiveness of the free surface of a human endometrial monolayer for trophoblast as related to actin cytoskeleton. Mol. Hum. Reprod. 1997, 3, 275–283. [Google Scholar] [CrossRef]
- Olson, G.E.; Winfrey, V.P.; Blaeuer, G.L.; Palisano, J.R.; NagDas, S.K. Stage-specific expression of the intermediate filament protein cytokeratin 13 in luminal epithelial cells of secretory phase human endometrium and peri-implantation stage rabbit endometrium. Biol. Reprod. 2002, 66, 1006–1015. [Google Scholar] [CrossRef][Green Version]
- Buck, V.; Kohlen, M.; Sternberg, A.; Rösing, B.; Neulen, J.; Leube, R.; Classen-Linke, I. Steroid hormones and human choriogonadotropin influence the distribution of alpha6-integrin and desmoplakin 1 in gland-like endometrial epithelial spheroids. Histochem. Cell Biol. 2021, 155, 581–591. [Google Scholar] [CrossRef]
- Buck, V.U.; Windoffer, R.; Leube, R.E.; Classen-Linke, I. Redistribution of adhering junctions in human endometrial epithelial cells during the implantation window of the menstrual cycle. Histochem. Cell Biol. 2012, 137, 777–790. [Google Scholar] [CrossRef]
- Murphy, C.R. Junctional barrier complexes undergo major alterations during the plasma membrane transformation of uterine epithelial cells. Hum. Reprod. 2000, 15 (Suppl. S3), 182–188. [Google Scholar] [CrossRef]
- Murphy, C.; Swift, J.; Need, J.; Mukherjee, T.; Rogers, A. A freeze-fracture electron microscopic study of tight junctions of epithelial cells in the human uterus. Anat. Embryol. 1982, 163, 367–370. [Google Scholar] [CrossRef]
- Lindsay, L.A.; Nasir, R.F.; Dowland, S.N.; Madawala, R.J.; Murphy, C.R. Rab13 and desmosome redistribution in uterine epithelial cells during early pregnancy. Reprod. Sci. 2021, 28, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Buck, V.U.; Gellersen, B.; Leube, R.E.; Classen-Linke, I. Interaction of human trophoblast cells with gland-like endometrial spheroids: A model system for trophoblast invasion. Hum. Reprod. 2015, 30, 906–916. [Google Scholar] [CrossRef]
- Albers, A.; Thie, M.; Hohn, H.-P.; Denker, H.-W. Differential expression and localization of integrins and cd44 in the membrane domains of human uterine epithelial cells during the menstrual cycle. Cells Tissues Organs 1995, 153, 12–19. [Google Scholar] [CrossRef]
- Lessey, B.A.; Castelbaum, A.J.; Sawin, S.W.; Buck, C.A.; Schinnar, R.; Bilker, W.; Strom, B. Aberrant integrin expression in the endometrium of women with endometriosis. J. Clin. Endocrinol. Metab. 1994, 79, 643–649. [Google Scholar]
- Bi, D.; Yang, X.; Marchetti, M.C.; Manning, M.L. Motility-driven glass and jamming transitions in biological tissues. Phys. Rev. X 2016, 6, 021011. [Google Scholar] [CrossRef]
- Park, J.A.; Atia, L.; Mitchel, J.A.; Fredberg, J.J.; Butler, J.P. Collective migration and cell jamming in asthma, cancer and development. J. Cell Sci. 2016, 129, 3375–3383. [Google Scholar] [CrossRef]
- Palamidessi, A.; Malinverno, C.; Frittoli, E.; Corallino, S.; Barbieri, E.; Sigismund, S.; Beznoussenko, G.V.; Martini, E.; Garre, M.; Ferrara, I.; et al. Unjamming overcomes kinetic and proliferation arrest in terminally differentiated cells and promotes collective motility of carcinoma. Nat. Mater. 2019, 18, 1252–1263. [Google Scholar] [CrossRef]
- Ketene, A.N.; Roberts, P.C.; Shea, A.A.; Schmelz, E.M.; Agah, M. Actin filaments play a primary role for structural integrity and viscoelastic response in cells. Integr. Biol. 2012, 4, 540–549. [Google Scholar] [CrossRef]
- Lim, C.T.; Zhou, E.H.; Quek, S.T. Mechanical models for living cells—A review. J. Biomech. 2006, 39, 195–216. [Google Scholar] [CrossRef]
- Quan, F.-S.; Kim, K.S. Medical applications of the intrinsic mechanical properties of single cells. Acta Biochim. Biophys. Sin. 2016, 48, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef]
- Wang, N.; Stamenovic, D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am. J. Physiol. Cell Physiol. 2000, 279, C188–C194. [Google Scholar] [CrossRef]
- Raboudi, N.; Julian, J.; Rohde, L.H.; Carson, D.D. Identification of cell-surface heparin/heparan sulfate-binding proteins of a human uterine epithelial cell line (rl95). J. Biol. Chem. 1992, 267, 11930–11939. [Google Scholar] [CrossRef]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for study of human embryo implantation: Choice of cell lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef]
- Coch, R.A.; Leube, R.E. Intermediate filaments and polarization in the intestinal epithelium. Cells 2016, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Leube, R.E.; Moch, M.; Windoffer, R. Intracellular motility of intermediate filaments. Cold Spring Harb. Perspect. Biol. 2017, 9, a021980. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, H.; Suda, M. Seven kinds of intermediate filament networks in the cytoplasm of polarized cells: Structure and function. Acta Histochem. Cytochem. 2010, 43, 19–31. [Google Scholar] [CrossRef]
- Tateishi, K.; Nishida, T.; Inoue, K.; Tsukita, S. Three-dimensional organization of layered apical cytoskeletal networks associated with mouse airway tissue development. Sci. Rep. 2017, 7, 43783. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Wang, X.; Cui, X.; Jin, L. Keratin 86 is up-regulated in the uterus during implantation, induced by oestradiol. BMC Dev. Biol. 2020, 20, 3. [Google Scholar] [CrossRef]
- Margolis, B.; Borg, J.-P. Apicobasal polarity complexes. J. Cell Sci. 2005, 118, 5157–5159. [Google Scholar] [CrossRef]
- Whitby, S.; Salamonsen, L.A.; Evans, J. The endometrial polarity paradox: Differential regulation of polarity within secretory-phase human endometrium. Endocrinology 2018, 159, 506–518. [Google Scholar] [CrossRef]
- Korch, C.; Spillman, M.A.; Jackson, T.A.; Jacobsen, B.M.; Murphy, S.K.; Lessey, B.A.; Jordan, V.C.; Bradford, A.P. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol. Oncol. 2012, 127, 241–248. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liang, X.; Liang, X.H.; Wang, T.S.; Qi, Q.R.; Deng, W.B.; Sha, A.G.; Yang, Z.M. The mesenchymal-epithelial transition during in vitro decidualization. Reprod. Sci. 2013, 20, 354–360. [Google Scholar] [CrossRef]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Gnecco, J.S.; Ding, T.; Smith, C.; Lu, J.; Bruner-Tran, K.L.; Osteen, K.G. Hemodynamic forces enhance decidualization via endothelial-derived prostaglandin e2 and prostacyclin in a microfluidic model of the human endometrium. Hum. Reprod. 2019, 34, 702–714. [Google Scholar] [CrossRef]
- Ivaska, J.; Pallari, H.-M.; Nevo, J.; Eriksson, J.E. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Canis, M.; Pouly, J.L.; Darcha, C. Soft matrices inhibit cell proliferation and inactivate the fibrotic phenotype of deep endometriotic stromal cells in vitro. Hum. Reprod. 2016, 31, 541–553. [Google Scholar] [CrossRef]
- John, N.J.; Linke, M.; Denker, H.-W. Quantitation of human choriocarcinoma spheroid attachment to uterine epithelial cell monolayers. Vitr. Cell. Dev. Biol. Anim. 1993, 29, 461–468. [Google Scholar] [CrossRef]
- Thie, M.; Fuchs, P.; Butz, S.; Sieckmann, F.; Hoschützky, H.; Kemler, R.; Denker, H. Adhesiveness of the apical surface of uterine epithelial cells: The role of junctional complex integrity. Eur. J. Cell Biol. 1996, 70, 221–232. [Google Scholar]
- Thie, M.; Fuchs, P.; Denker, H.W. Epithelial cell polarity and embryo implantation in mammals. Int. J. Dev. Biol. 1996, 40, 389–393. [Google Scholar]
- Thie, M.; Denker, H. Endometrial receptivity for trophoblast attachment: Model studies using cell lines. In Microscopy of Reproduction and Development. A Dynamic Approach; Antonio Delfino Editore Srl: Roma, Italy, 1997; pp. 241–249. [Google Scholar]
- Pattillo, R.; Hussa, R.; Bernstein, R.; Delfs, E. The jar cell line. Continuous lines of human choriocarcinoma. In Vitro 1971, 6, 398–399. [Google Scholar]
- Thie, M.; Röspel, R.; Dettmann, W.; Benoit, M.; Ludwig, M.; Gaub, H.E.; Denker, H.-W. Interactions between trophoblast and uterine epithelium: Monitoring of adhesive forces. Hum. Reprod. 1998, 13, 3211–3219. [Google Scholar] [CrossRef] [PubMed]
- Enders, A. Anatomical aspects of implantation. J. Reprod. Fertil. Suppl. 1976, 25, 1–15. [Google Scholar]
- Enders, A.C. Embryo implantation, with emphasis on the rhesus monkey and the human. Reproduccion 1981, 5, 163–167. [Google Scholar] [PubMed]
- Hertig, A.T.; Rock, J.; Adams, E.C. A description of 34 human ova within the first 17 days of development. Am. J. Anat. 1956, 98, 435–493. [Google Scholar] [CrossRef]
- Hustin, J.; Schaaps, J.-P. Echocardiograhic and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am. J. Obstet. Gynecol. 1987, 157, 162–168. [Google Scholar] [CrossRef]
- Moser, G.; Gauster, M.; Orendi, K.; Glasner, A.; Theuerkauf, R.; Huppertz, B. Endoglandular trophoblast, an alternative route of trophoblast invasion? Analysis with novel confrontation co-culture models. Hum. Reprod. 2010, 25, 1127–1136. [Google Scholar] [CrossRef]
- Moser, G.; Weiss, G.; Gauster, M.; Sundl, M.; Huppertz, B. Evidence from the very beginning: Endoglandular trophoblasts penetrate and replace uterine glands in situ and in vitro. Hum. Reprod. 2015, 30, 2747–2757. [Google Scholar] [CrossRef]
- Assali, N.; Douglass, R.A., Jr.; Baird, W.W.; Nicholson, D.; Suyemoto, R. Measurement of uterine blood flow and uterine metabolism: Iv. Results in normal pregnancy. Am. J. Obstet. Gynecol. 1953, 66, 248–253. [Google Scholar] [CrossRef]
- Ziegler, W.F.; Bernstein, I.; Badger, G.; Leavitt, T.; Cerrero, M.L. Regional hemodynamic adaptation during the menstrual cycle. Obstet. Gynecol. 1999, 94, 695–699. [Google Scholar]
- Abbas, Y.; Carnicer-Lombarte, A.; Gardner, L.; Thomas, J.; Brosens, J.J.; Moffett, A.; Sharkey, A.M.; Franze, K.; Burton, G.J.; Oyen, M.L. Tissue stiffness at the human maternal-fetal interface. Hum. Reprod. 2019, 34, 1999–2008. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Dixon, G.; Robertson, W.B.; Brosens, I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980, 1, 3–19. [Google Scholar] [CrossRef]
- Burrows, T.D.; King, A.; Loke, Y. Expression of integrins by human trophoblast and differential adhesion to laminin or fibronectin. Hum. Reprod. 1993, 8, 475–484. [Google Scholar] [CrossRef]
- Oefner, C.M.; Sharkey, A.; Gardner, L.; Critchley, H.; Oyen, M.; Moffett, A. Collagen type iv at the fetal-maternal interface. Placenta 2015, 36, 59–68. [Google Scholar] [CrossRef]
- Iwahashi, M.; Muragaki, Y.; Ooshima, A.; Yamoto, M.; Nakano, R. Alterations in distribution and composition of the extracellular matrix during decidualization of the human endometrium. J. Reprod. Fertil. 1996, 108, 147–155. [Google Scholar] [CrossRef]
- Klotzsch, E.; Smith, M.L.; Kubow, K.E.; Muntwyler, S.; Little, W.C.; Beyeler, F.; Gourdon, D.; Nelson, B.J.; Vogel, V. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc. Natl. Acad. Sci. USA 2009, 106, 18267. [Google Scholar] [CrossRef]
- Miyazaki, K.; Oyanagi, J.; Hoshino, D.; Togo, S.; Kumagai, H.; Miyagi, Y. Cancer cell migration on elongate protrusions of fibroblasts in collagen matrix. Sci. Rep. 2019, 9, 292. [Google Scholar] [CrossRef]
- Sherratt, M.J.; Baldock, C.; Louise Haston, J.; Holmes, D.F.; Jones, C.J.P.; Adrian Shuttleworth, C.; Wess, T.J.; Kielty, C.M. Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. J. Mol. Biol. 2003, 332, 183–193. [Google Scholar] [CrossRef]
- Burrows, T.D.; King, A.; Loke, Y. Trophoblast migration during human placental implantation. Hum. Reprod. Update 1996, 2, 307–321. [Google Scholar] [CrossRef]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.-E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Sagrillo-Fagundes, L.; Mok, S.; Vaillancourt, C.; Moraes, C. Mechanobiological regulation of placental trophoblast fusion and function through extracellular matrix rigidity. Sci. Rep. 2020, 10, 5837. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Bland, J.; Robertson, W.; Dixon, G.; Brosens, I. The pattern of interstitial trophoblasticinvasion of the myometrium in early human pregnancy. Placenta 1981, 2, 303–315. [Google Scholar] [CrossRef]
- Boyd, J.D.; Hamilton, W.J. The giant cells of the pregnant human uterus. BJOG Int. J. Obstet. Gynaecol. 1960, 67, 208–218. [Google Scholar] [CrossRef]
- Barash, A.; Dekel, N.; Fieldust, S.; Segal, I.; Schechtman, E.; Granot, I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil. Steril. 2003, 79, 1317–1322. [Google Scholar] [CrossRef]
- Raziel, A.; Schachter, M.; Strassburger, D.; Bern, O.; Ron-El, R.; Friedler, S. Favorable influence of local injury to the endometrium in intracytoplasmic sperm injection patients with high-order implantation failure. Fertil. Steril. 2007, 87, 198–201. [Google Scholar] [CrossRef]
- Eskew, A.M.; Reschke, L.D.; Woolfolk, C.; Schulte, M.B.; Boots, C.E.; Broughton, D.E.; Jimenez, P.T.; Omurtag, K.R.; Keller, S.L.; Ratts, V.S.; et al. Effect of endometrial mechanical stimulation in an unselected population undergoing in vitro fertilization: Futility analysis of a double-blind randomized controlled trial. J. Assist. Reprod. Genet. 2019, 36, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tal, R.; Chao, H.; Liu, M.; Liu, Y. Effect of local endometrial injury in proliferative vs. Luteal phase on ivf outcomes in unselected subfertile women undergoing in vitro fertilization. Reprod. Biol. Endocrinol. RBE 2017, 15, 75. [Google Scholar] [CrossRef][Green Version]
- Sar-Shalom Nahshon, C.; Sagi-Dain, L.; Wiener-Megnazi, Z.; Dirnfeld, M. The impact of intentional endometrial injury on reproductive outcomes: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Finn, C.; Keen, P. The induction of deciduomata in the rat. Development 1963, 11, 673–682. [Google Scholar] [CrossRef]
- Kalma, Y.; Granot, I.; Gnainsky, Y.; Or, Y.; Czernobilsky, B.; Dekel, N.; Barash, A. Endometrial biopsy-induced gene modulation: First evidence for the expression of bladder-transmembranal uroplakin ib in human endometrium. Fertil. Steril. 2009, 91, 1042–1049.e9. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.R.; Brar, A.K.; Cedars, M.I.; Handwerger, S. Prostaglandin e2 enhances human endometrial stromal cell differentiation. Endocrinology 1994, 134, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsohn, P.A.; Zorn, T.M. Implantation and decidualization in rodents. J. Exp. Zool. 1993, 266, 603–628. [Google Scholar] [CrossRef] [PubMed]
- Fronius, M.; Clauss, W.G. Mechano-sensitivity of enac: May the (shear) force be with you. Pflüg. Arch. Eur. J. Physiol. 2008, 455, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Shenton, F.; Hunter, I.; Banks, R.W.; Bewick, G.S. Amiloride-sensitive channels are a major contributor to mechanotransduction in mammalian muscle spindles. J. Physiol. 2010, 588, 171–185. [Google Scholar] [CrossRef]
- Enuka, Y.; Hanukoglu, I.; Edelheit, O.; Vaknine, H.; Hanukoglu, A. Epithelial sodium channels (enac) are uniformly distributed on motile cilia in the oviduct and the respiratory airways. Histochem. Cell Biol. 2012, 137, 339–353. [Google Scholar] [CrossRef]
- Ruan, Y.C.; Guo, J.H.; Liu, X.; Zhang, R.; Tsang, L.L.; Da Dong, J.; Chen, H.; Yu, M.K.; Jiang, X.; Zhang, X.H.; et al. Activation of the epithelial Na+ channel triggers prostaglandin E2 release and production required for embryo implantation. Nat. Med. 2012, 18, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Chang, S.; Barzilai, J.J.; Leppert, P.; Segars, J.H. Mechanical signaling in reproductive tissues: Mechanisms and importance. Reprod. Sci. 2014, 21, 1093–1107. [Google Scholar] [CrossRef]
- Bae, C.; Sachs, F.; Gottlieb, P.A. The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMT×4. Biochemistry 2011, 50, 6295–6300. [Google Scholar] [CrossRef]
- Hennes, A.; Held, K.; Boretto, M.; De Clercq, K.; Van den Eynde, C.; Vanhie, A.; Van Ranst, N.; Benoit, M.; Luyten, C.; Peeraer, K.; et al. Functional expression of the mechanosensitive piezo1 channel in primary endometrial epithelial cells and endometrial organoids. Sci. Rep. 2019, 9, 1779. [Google Scholar] [CrossRef]
- De Clercq, K.; Held, K.; Van Bree, R.; Meuleman, C.; Peeraer, K.; Tomassetti, C.; Voets, T.; D’Hooghe, T.; Vriens, J. Functional expression of transient receptor potential channels in human endometrial stromal cells during the luteal phase of the menstrual cycle. Hum. Reprod. 2015, 30, 1421–1436. [Google Scholar] [CrossRef]
- Vallet, V.; Chraibi, A.; Gaeggeler, H.-P.; Horisberger, J.-D.; Rossier, B.C. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 1997, 389, 607–610. [Google Scholar] [CrossRef]
- Nourse, J.L.; Pathak, M.M. How cells channel their stress: Interplay between piezo1 and the cytoskeleton. Semin. Cell Dev. Biol. 2017, 71, 3–12. [Google Scholar] [CrossRef]
- Syeda, R.; Xu, J.; Dubin, A.E.; Coste, B.; Mathur, J.; Huynh, T.; Matzen, J.; Lao, J.; Tully, D.C.; Engels, I.H. Chemical activation of the mechanotransduction channel Piezo1. eLife 2015, 4, e07369. [Google Scholar] [CrossRef]
- Morachevskaya, E.A.; Sudarikova, A.V. Actin dynamics as critical ion channel regulator: ENaC and Piezo in focus. Am. J. Physiol. Cell Physiol. 2021, 320, C696–C702. [Google Scholar] [CrossRef]
- Montazeri, M.; Sanchez-Lopez, J.A.; Caballero, I.; Maslehat Lay, N.; Elliott, S.; Lopez-Martin, S.; Yanez-Mo, M.; Fazeli, A. Activation of toll-like receptor 3 reduces actin polymerization and adhesion molecule expression in endometrial cells, a potential mechanism for viral-induced implantation failure. Hum. Reprod. 2015, 30, 893–905. [Google Scholar] [CrossRef]
- Barritt, G.; Rychkov, G. TRPs as mechanosensitive channels. Nat. Cell Biol. 2005, 7, 105–107. [Google Scholar] [CrossRef]
- O’Neil, R.G.; Heller, S. The mechanosensitive nature of TRPV channels. Pflüg. Arch. 2005, 451, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Majeed, Y.; Amer, M.S.; Agarwal, A.K.; McKeown, L.; Porter, K.E.; O’Regan, D.J.; Naylor, J.; Fishwick, C.W.; Muraki, K.; Beech, D.J. Stereo-selective inhibition of transient receptor potential trpc5 cation channels by neuroactive steroids. Br. J. Pharmacol. 2011, 162, 1509–1520. [Google Scholar] [CrossRef]
- Yang, H.; Choi, K.C.; Hyun, S.H.; Jeung, E.B. Coexpression and estrogen-mediated regulation of TRPV6 and PMCA1 in the human endometrium during the menstrual cycle. Mol. Reprod. Dev. 2011, 78, 274–282. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.-L. The hippo pathway: Biology and pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L. Inactivation of yap oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Fu, J.; Zhou, M.; Xiao, L.; Feng, X.; Chen, H.; Huang, W. Activated hippo/yes-associated protein pathway promotes cell proliferation and anti-apoptosis in endometrial stromal cells of endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 1552–1561. [Google Scholar] [CrossRef]
- Strakova, Z.; Kruss, S.; Morris, K.; Reed, J. Members of the Hippo Pathway are Regulated in the Uterus during the Menstrual Cycle; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Strakova, Z.; Reed, J.; Ihnatovych, I. Human transcriptional coactivator with PDZ-binding motif (TAZ) is downregulated during decidualization. Biol. Reprod. 2010, 82, 1112–1118. [Google Scholar] [CrossRef]
- Chen, H.; Song, Y.; Yang, S.; Fu, J.; Feng, X.; Huang, W. Yap mediates human decidualization of the uterine endometrial stromal cells. Placenta 2017, 53, 30–35. [Google Scholar] [CrossRef]
- Baah-Dwomoh, A.; McGuire, J.; Tan, T.; De Vita, R. Mechanical properties of female reproductive organs and supporting connective tissues: A review of the current state of knowledge. Appl. Mech. Rev. 2016, 68, 060801. [Google Scholar] [CrossRef]
- Conrad, J.T.; Johnson, W.L.; Kuhn, W.K.; Hunter, C.A., Jr. Passive stretch relationships in human uterine muscle. Am. J. Obstet. Gynecol. 1966, 96, 1055–1059. [Google Scholar] [CrossRef]
- Pearsall, G.; Roberts, V. Passive mechanical properties of uterine muscle (myometrium) tested in vitro. J. Biomech. 1978, 11, 167–176. [Google Scholar] [CrossRef]
- Kauer, M.; Vuskovic, V.; Dual, J.; Szekely, G.; Bajka, M. Inverse finite element characterization of soft tissues. Med. Image Anal. 2002, 6, 275–287. [Google Scholar] [CrossRef]
- Kiss, M.Z.; Hobson, M.A.; Varghese, T.; Harter, J.; Kliewer, M.A.; Hartenbach, E.M.; Zagzebski, J.A. Frequency-dependent complex modulus of the uterus: Preliminary results. Phys. Med. Biol. 2006, 51, 3683–3695. [Google Scholar] [CrossRef] [PubMed]
- Omari, E.A.; Varghese, T.; Kliewer, M.A.; Harter, J.; Hartenbach, E.M. Dynamic and quasi-static mechanical testing for characterization of the viscoelastic properties of human uterine tissue. J. Biomech. 2015, 48, 1730–1736. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Altayyeb, A.; Othman, E.; Khashbah, M.; Esmaeel, A.; El-Mokhtar, M.; Lambalk, C.; Mijatovic, V.; Abdelgawad, M. Characterization of mechanical signature of eutopic endometrial stromal cells of endometriosis patients. Reprod. Sci. 2020, 27, 364–374. [Google Scholar] [CrossRef]
- Izmailova, L.S.; Vorotelyak, E.A.; Vasiliev, A.V. In vitro modeling of the early development of mouse and human embryos. Russ. J. Dev. Biol. 2020, 51, 271–282. [Google Scholar] [CrossRef]
- You, Y.; Stelzl, P.; Zhang, Y.; Porter, J.; Liu, H.; Liao, A.H.; Aldo, P.B.; Mor, G. Novel 3D in vitro models to evaluate trophoblast migration and invasion. Am. J. Reprod. Immunol. 2019, 81, e13076. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef]
- Luddi, A.; Pavone, V.; Semplici, B.; Governini, L.; Criscuoli, M.; Paccagnini, E.; Gentile, M.; Morgante, G.; Leo, V.; Belmonte, G.; et al. Organoids of human endometrium: A powerful in vitro model for the endometrium-embryo cross-talk at the implantation site. Cells 2020, 9, 1121. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef]
- Alzamil, L.; Nikolakopoulou, K.; Turco, M.Y. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2021, 28, 35–51. [Google Scholar] [CrossRef]
- Fitzgerald, H.C.; Schust, D.J.; Spencer, T.E. In vitro models of the human endometrium: Evolution and application for women’s health. Biol. Reprod. 2021, 104, 282–293. [Google Scholar] [CrossRef]
- Psychoyos, A. Uterine receptivity for nidation a. Ann. N. Y. Acad. Sci. 1986, 476, 36–42. [Google Scholar] [CrossRef]
- Sharkey, A.M.; Macklon, N.S. The science of implantation emerges blinking into the light. Reprod. Biomed. Online 2013, 27, 453–460. [Google Scholar] [CrossRef][Green Version]
- Singh, H.; Aplin, J.D. Adhesion molecules in endometrial epithelium: Tissue integrity and embryo implantation. J. Anat. 2009, 215, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, J.S. A review of mechanisms of implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.W.; Roca-Cusachs, P.; Sheetz, M.P. Stretchy proteins on stretchy substrates: The important elements of integrin-mediated rigidity sensing. Dev. Cell 2010, 19, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Helmke, B.P.; Blackman, B.R.; Schwartz, M.A. Mechanisms of mechanotransduction. Dev. Cell 2006, 10, 11–20. [Google Scholar] [CrossRef]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Unal, M.; Alapan, Y.; Jia, H.; Varga, A.G.; Angelino, K.; Aslan, M.; Sayin, I.; Han, C.; Jiang, Y.; Zhang, Z.; et al. Micro and nano-scale technologies for cell mechanics. Nanobiomedicine 2014, 1, 5. [Google Scholar] [CrossRef]
- Eyckmans, J.; Boudou, T.; Yu, X.; Chen, C.S. A hitchhiker’s guide to mechanobiology. Dev. Cell 2011, 21, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Thorne, J.T.; Segal, T.R.; Chang, S.; Jorge, S.; Segars, J.H.; Leppert, P.C. Dynamic reciprocity between cells and their microenvironment in reproduction. Biol. Reprod. 2015, 92, 25. [Google Scholar] [CrossRef] [PubMed]
- Molnar, K.; Labouesse, M. The plastic cell: Mechanical deformation of cells and tissues. Open Biol. 2021, 11, 210006. [Google Scholar] [CrossRef]
- Xie, Y.; Cui, D.; Sui, L.; Xu, Y.; Zhang, N.; Ma, Y.; Li, Y.; Kong, Y. Induction of forkhead box M1 (FoxM1) by EGF through ERK signaling pathway promotes trophoblast cell invasion. Cell Tissue Res. 2015, 362, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Li, Q.; Melnichouk, O.; Huszti, E.; Martin, L.J.; Gunasekara, A.; Mawdsley, G.; Yaffe, M.J.; Minkin, S. Evidence that breast tissue stiffness is associated with risk of breast cancer. PLoS ONE 2014, 9, e100937. [Google Scholar] [CrossRef] [PubMed]
- Upagupta, C.; Shimbori, C.; Alsilmi, R.; Kolb, M. Matrix abnormalities in pulmonary fibrosis. Eur. Respir. Rev. 2018, 27, 180033. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sternberg, A.K.; Buck, V.U.; Classen-Linke, I.; Leube, R.E. How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells 2021, 10, 2008. https://doi.org/10.3390/cells10082008
Sternberg AK, Buck VU, Classen-Linke I, Leube RE. How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells. 2021; 10(8):2008. https://doi.org/10.3390/cells10082008
Chicago/Turabian StyleSternberg, Anna K., Volker U. Buck, Irmgard Classen-Linke, and Rudolf E. Leube. 2021. "How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation" Cells 10, no. 8: 2008. https://doi.org/10.3390/cells10082008
APA StyleSternberg, A. K., Buck, V. U., Classen-Linke, I., & Leube, R. E. (2021). How Mechanical Forces Change the Human Endometrium during the Menstrual Cycle in Preparation for Embryo Implantation. Cells, 10(8), 2008. https://doi.org/10.3390/cells10082008






