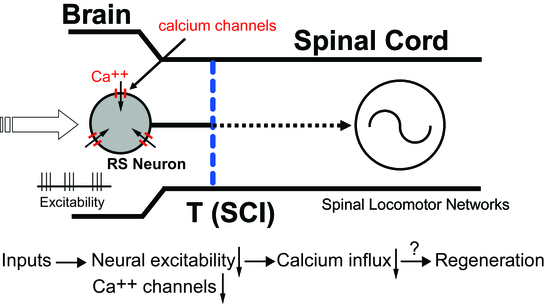
Spinal Cord Injury Significantly Alters the Properties of Reticulospinal Neurons: I. Biophysical Properties, Firing Patterns, Excitability, and Synaptic Inputs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Neurophysiological Properties of Uninjured and Injured RS Neurons
2.2.1. Animal Groups
2.2.2. Isolated Brain-Spinal Cord Preparation
2.2.3. Passive Electrical Properties
2.2.4. Action Potential Properties
2.2.5. Afterpotentials
2.2.6. Repetitive Firing Patterns
2.3. Manipulations of the sAHP
2.4. Data Acquisition/Storage and Statistics
2.5. Sensory-Evoked Synaptic Responses of Uninjured and Injured RS Neurons
3. Results
3.1. Time Course of Altered Firing Patterns for Injured RS Neurons Following SCI
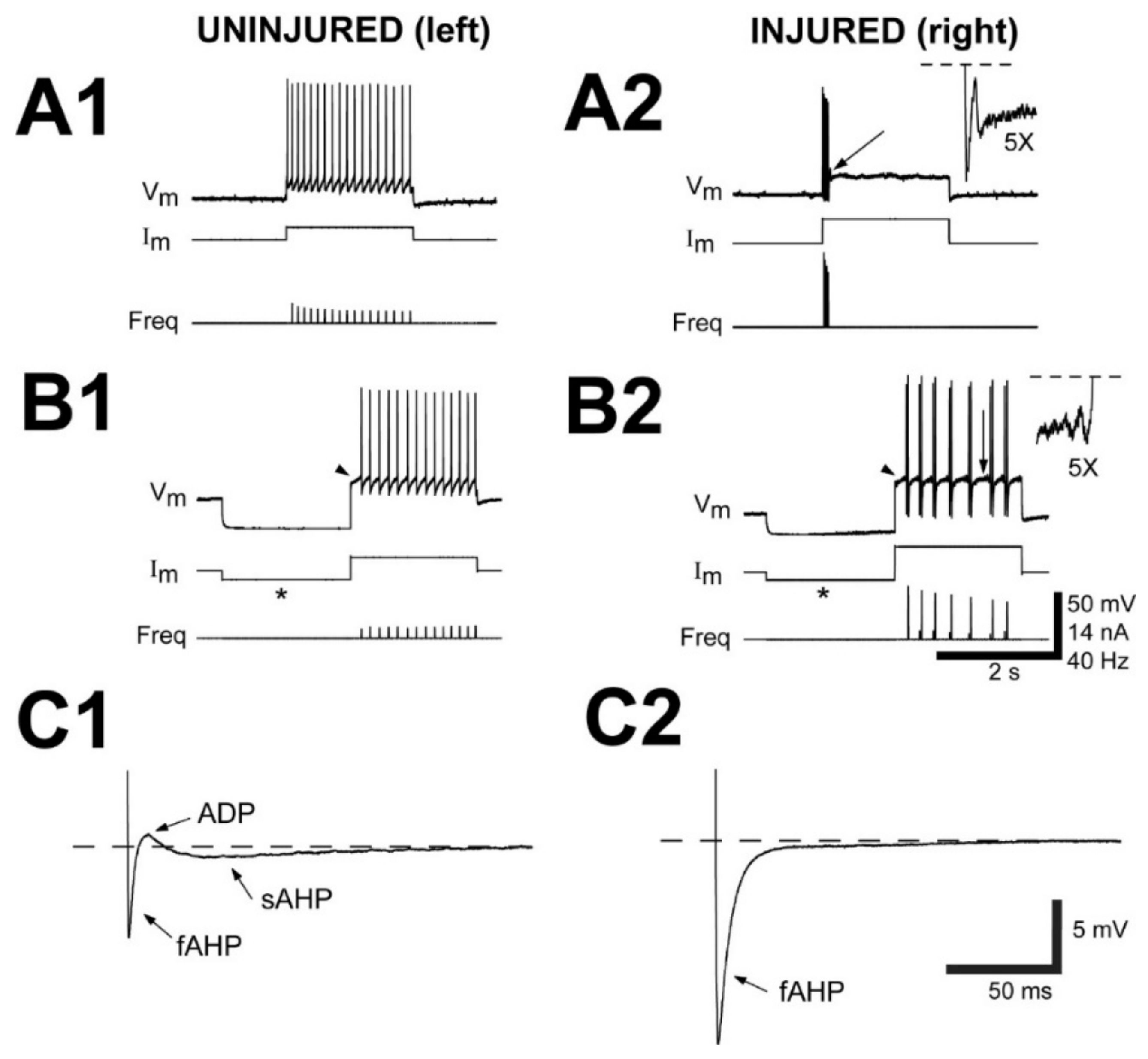
3.2. Neurophysiological Properties of Uninjured (Left)-Injured (Right) Pairs of RS Neurons
3.2.1. Passive Electrical Properties
3.2.2. Action Potential Properties
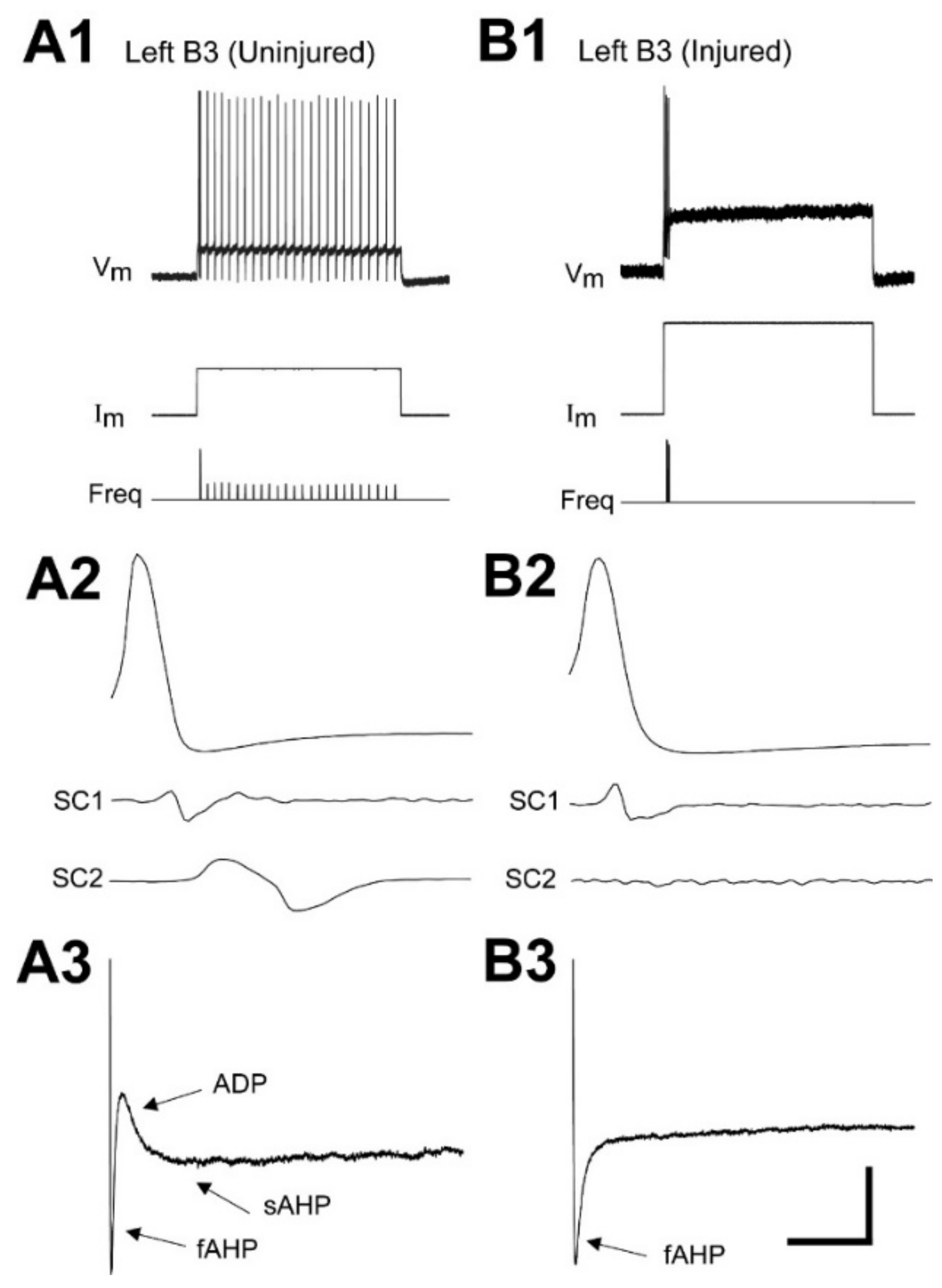
3.2.3. Properties of Afterpotentials
3.2.4. Repetitive Firing
3.3. Dependency of Firing Patterns of Injured RS Neurons on Depolarizing Current Levels
3.4. Do Injured Ascending Pathways Contribute to the Injury Phenotype of RS Neurons?
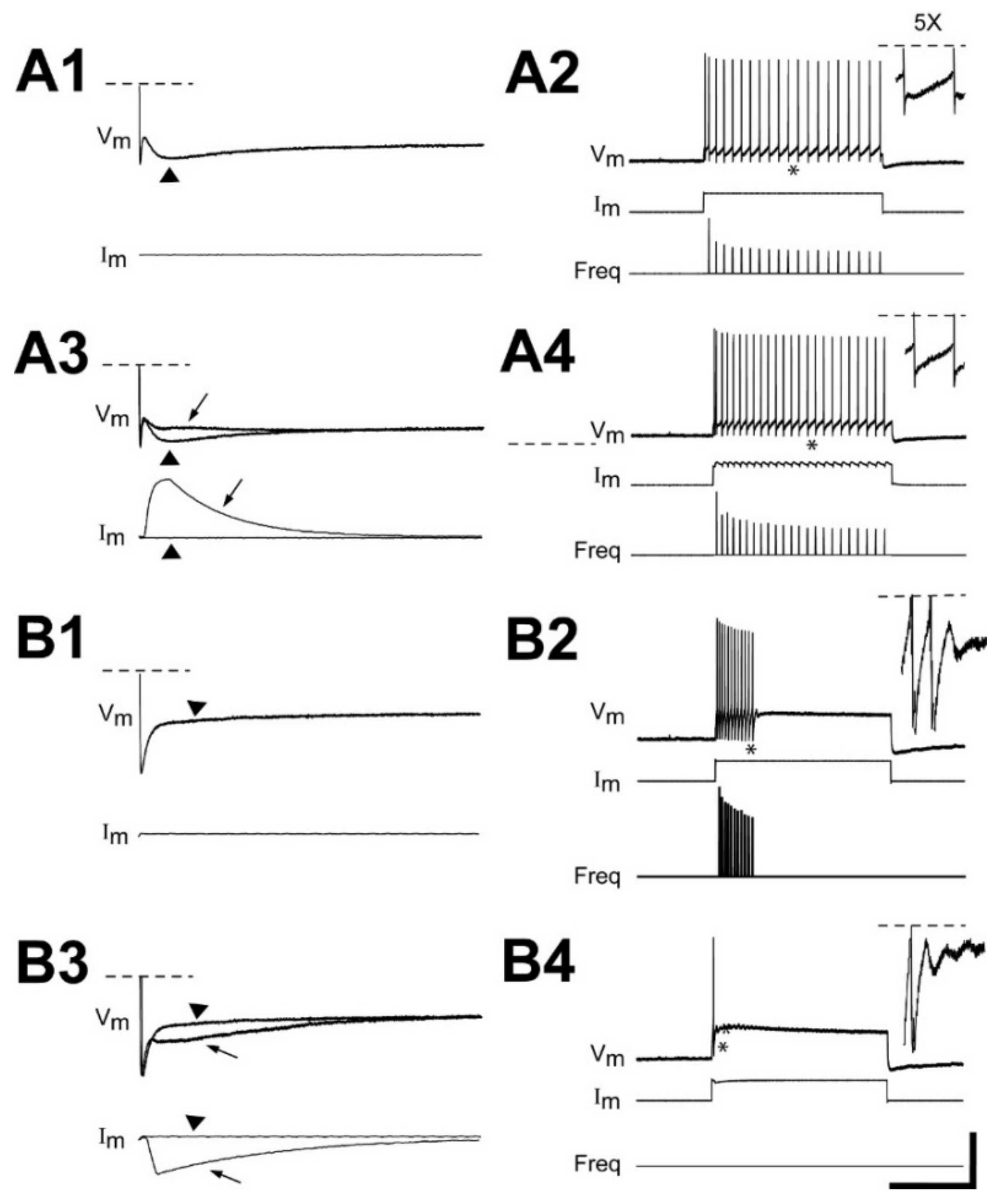
3.5. Contributions of the sAHP to Firing Patterns of Uninjured and Injured RS Neurons
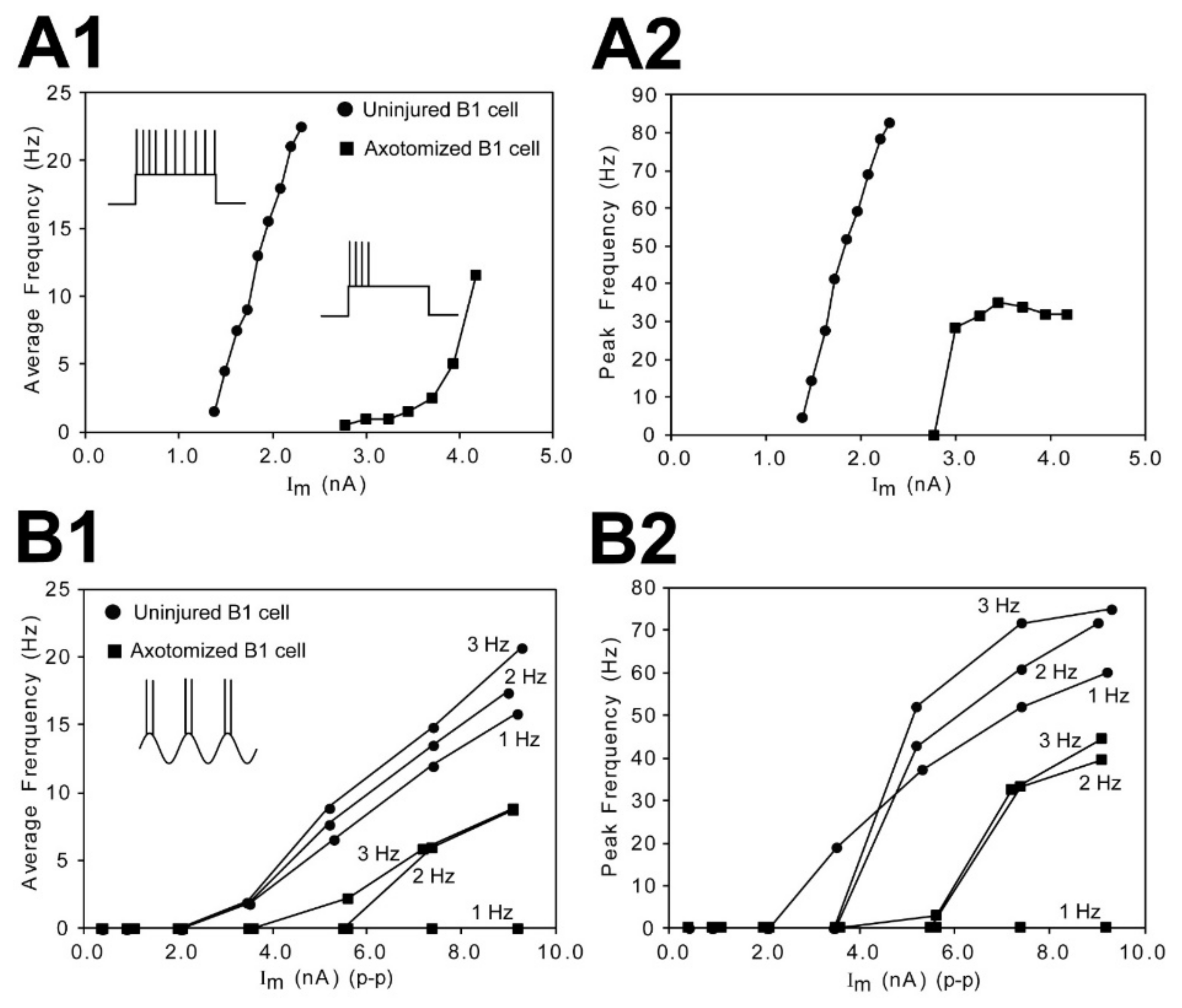
3.6. Excitability of Uninjured vs. Injured RS Neurons
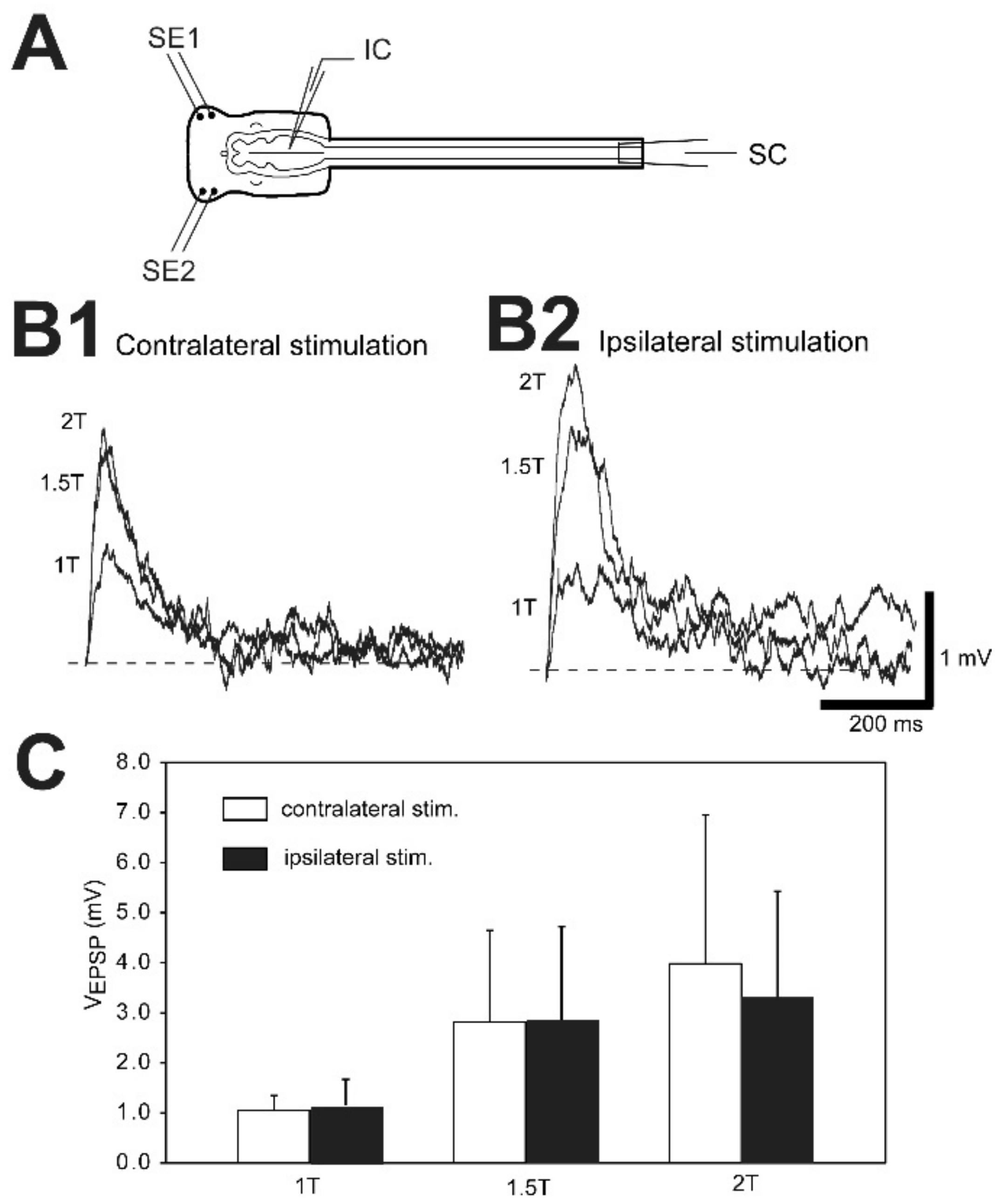
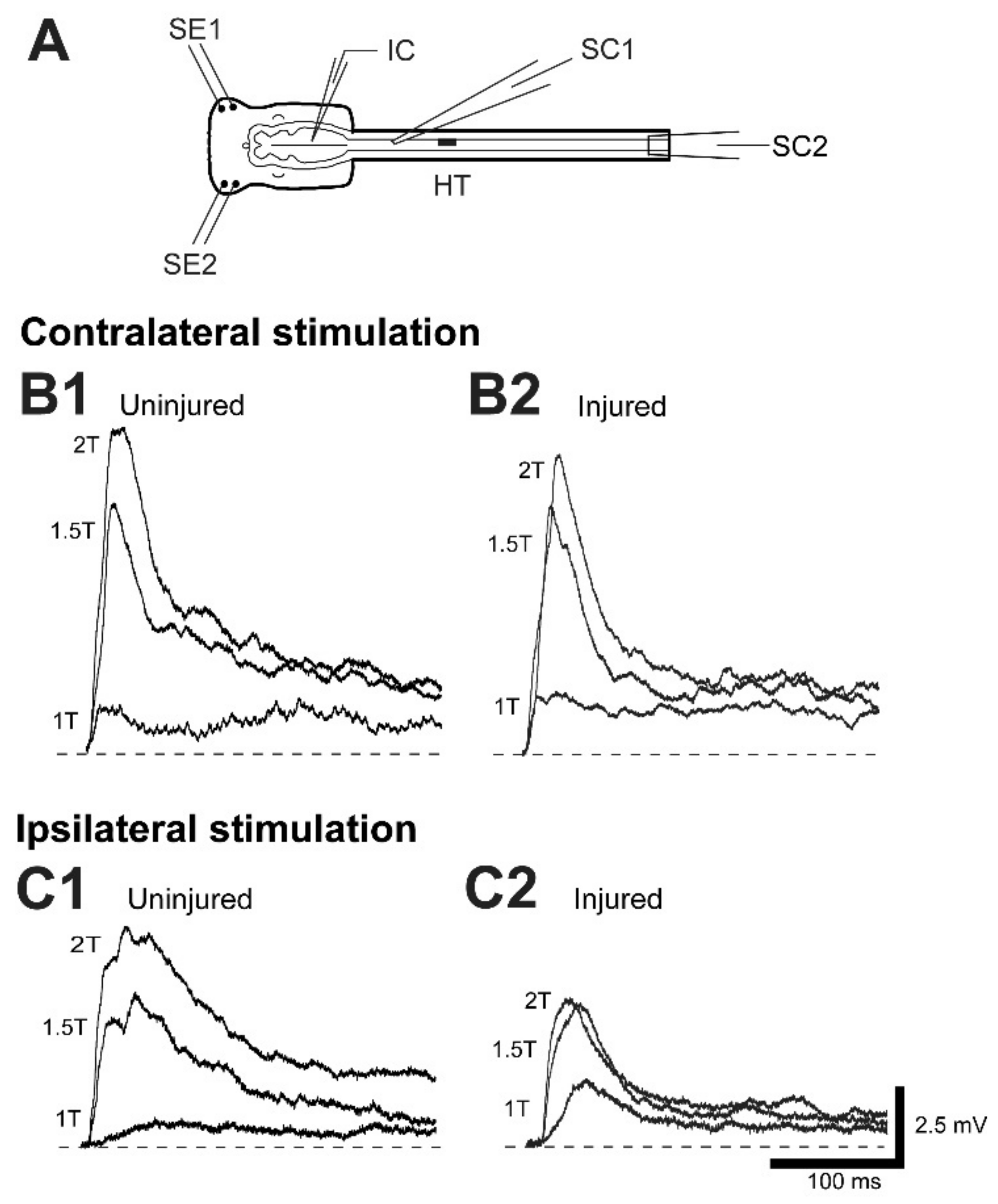
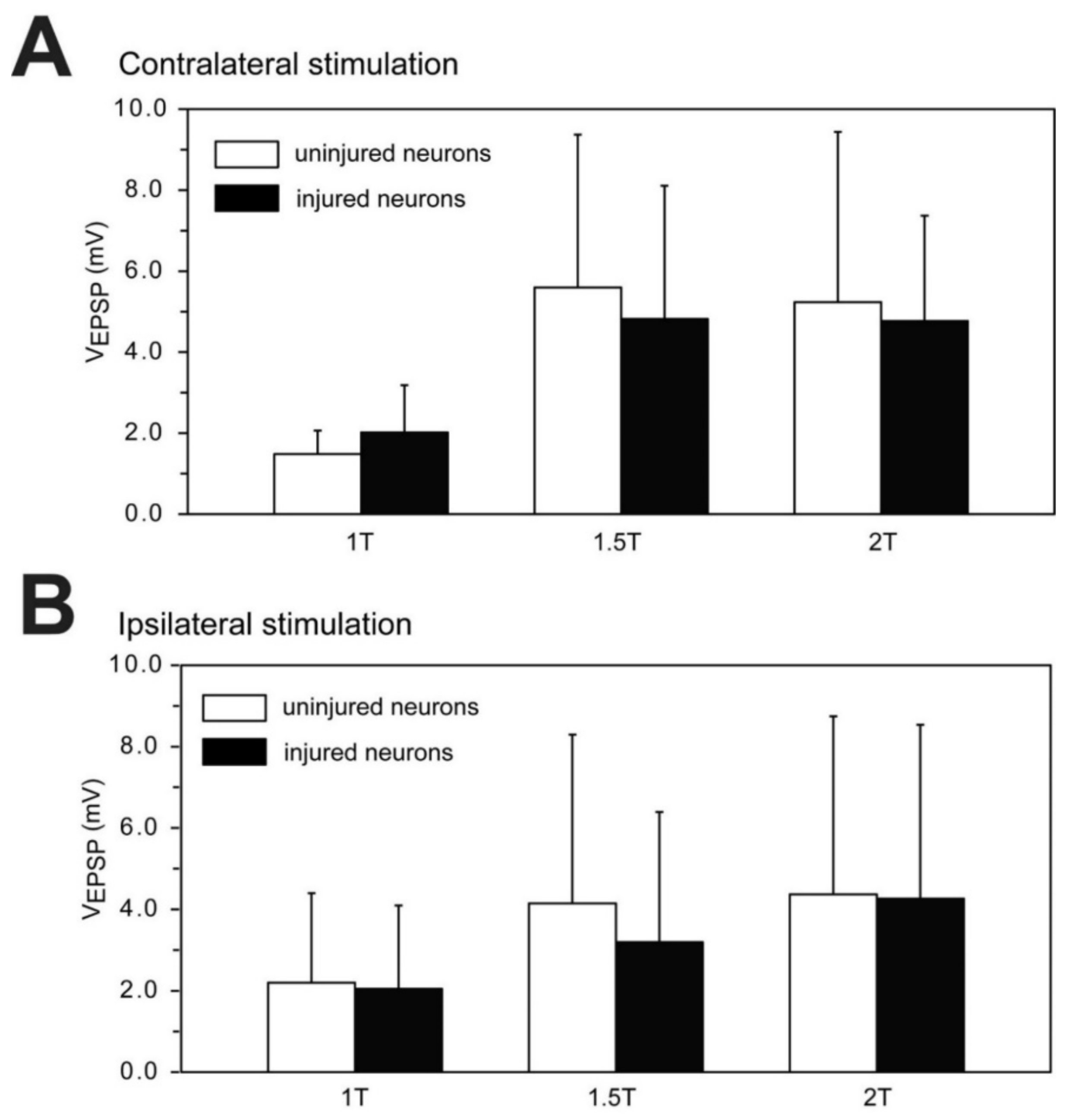
3.7. Sensory-Evoked Synaptic Responses for Uninjured and Injured RS Neurons
3.7.1. Synaptic Potential Types
3.7.2. Contralaterally-Evoked vs. Ipsilaterally-Evoked EPSPs for RS Neurons
3.7.3. Sensory-Evoked EPSPs for Injured and Uninjured Neurons
4. Discussion
4.1. Injury Phenotype for Lamprey RS Neurons Following SCI
4.2. Contribution of Injury Phenotype to Axonal Regeneration of Lamprey RS Neurons
4.3. Comparisons to Other Studies of Lamprey Neurons Following SCI
4.4. Biophysical and Morphological Properties of Injured Neurons in Other Animals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Recovery Time | M2 | M3 | I1 | B1 | B3 | B4 |
| 2–3 days (n = 13 neurons) | 0% b | 7.7% | 15.4% | 23.1% | 7.7% | 15.4% |
| 1 wk (n = 15) | 20.0% | 20.0% | 13.8% | 13.3% | 6.7% | 13.3% |
| 2–3 wks (n = 145) | 16.6% | 13.1% | 11.7% | 22.1% | 17.2% | 17.2% |
| 4 wks (n = 25) | 12.0% | 24.0% | 16.0% | 24.0% | 8.0% | 16.0% |
| 6 wks (n = 33) | 10.7% | 32.1% | 14.3% | 7.1% | 10.7% | 25.0% |
| 8 wks (n = 9) | 11.1% | 11.1% | 22.2% | 11.1% | 11.1% | 22.2% |
| 12–16 wks (n = 19) | 10.5% | 5.3% | 10.5% | 31.6% | 21.1% | 10.5% |
References
- Orlovsky, G.N.; Deliagina, T.G.; Grillner, S. Neuronal Control of Locomotion; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Grillner, S. Control of locomotion in bipeds, tetrapods and fish. In Handbook of Physiology, Motor Control; Brooks, V., Ed.; American Physiological Society: Rockville, MD, USA, 1981; pp. 1179–1236. [Google Scholar]
- Brownstone, R.M.; Chopek, J.W. Reticulospinal systems for tuning motor commands. Front. Neural Circuits 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Orlovsky, G.N. Activity of reticulo-spinal neurones during locomotion. Biophysics 1970, 15, 761–771. [Google Scholar]
- Steeves, J.D.; Sholomenko, G.N.; Webster, D.M.S. Stimulation of the pontomedullary reticular formation initiates locomotion in decerebrate birds. Brain Res. 1987, 401, 205–212. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Skinner, R.D. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 1987, 411, 1–12. [Google Scholar] [CrossRef]
- Valenzuela, J.I.; Hasan, S.J.; Steeves, J.D. Stimulation of brainstem reticular formation evokes locomotor activity in embryonic chicken (in ovo). Dev. Brain. Res. 1990, 56, 13–18. [Google Scholar] [CrossRef]
- Rossignol, S.; Frigon, A. Recovery of locomotion after spinal cord injury: Some facts and mechanisms. Annu. Rev. Neurosci. 2011, 34, 413–440. [Google Scholar] [CrossRef]
- Schwab, M.E.; Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996, 76, 319–370. [Google Scholar] [CrossRef]
- Bradbury, E.J.; McMahon, S.B. Spinal cord repair strategies: Why do they work? Nat. Rev. Neurosci. 2006, 7, 644–653. [Google Scholar] [CrossRef]
- David, S.; Lacroix, S. Molecular approaches to spinal cord repair. Annu. Rev. Neurosci. 2003, 26, 411–440. [Google Scholar] [CrossRef]
- Rossignol, S.; Schwab, M.; Schwartz, M.; Fehlings, M.G. Spinal cord injury: Time to move. J. Neurosci. 2007, 27, 11782–11792. [Google Scholar] [CrossRef]
- McClellan, A.D. Functional regeneration and restoration of locomotor activity following spinal cord transection in the lamprey. In Progress in Brain Research; Seil, F.J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 1994; Volume 103, pp. 203–217. [Google Scholar]
- McClellan, A.D. Spinal cord injury: Lessons from locomotor recovery and axonal regeneration in lower vertebrates. Neuroscientist 1998, 4, 250–263. [Google Scholar] [CrossRef]
- McClellan, A.D. Spinal cord injury—The lamprey model. In Neuromethods Animal Models of Spinal Cord Repair; Aldskogius, H., Ed.; Humana Press: New York, NY, USA, 2013; Volume 76, Chap. 4; pp. 63–108. [Google Scholar]
- Davis, G.R.; Troxel, M.T.; Kohler, V.J.; Grossmann, E.M.; McClellan, A.D. Time course of locomotor recovery and functional regeneration in spinal-transected lamprey: Kinematics and electromyography. Exp. Brain Res. 1993, 97, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.R.; McClellan, A.D. Extent and time course of restoration of descending brainstem projections in spinal cord-transected lamprey. J. Comp. Neurol. 1994, 344, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.R.; McClellan, A.D. Long distance axonal regeneration of identified lamprey reticulospinal neurons. Exp. Neurol. 1994, 127, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; McClellan, A.D. Axonal regeneration of descending brain neurons in larval lamprey demonstrated by retrograde double labeling. J. Comp. Neurol. 1999, 410, 612–626. [Google Scholar] [CrossRef]
- McClellan, A.D. Time course of locomotor recovery and functional regeneration in spinal cord-transected lamprey: In vitro preparations. J. Neurophysiol. 1994, 72, 847–860. [Google Scholar] [CrossRef]
- Paggett, K.C.; Jackson, A.W.; McClellan, A.D. Organization of higher order brain areas that initiate locomotor activity in larval lamprey. Neuroscience 2004, 125, 25–33. [Google Scholar] [CrossRef][Green Version]
- Jackson, A.W.; Pino, F.A.; Wiebe, E.D.; McClellan, A.D. Movements and muscle activity initiated by brain locomotor areas in semi-intact preparations from larval lamprey. J. Neurophysiol. 2007, 97, 3229–3241. [Google Scholar] [CrossRef]
- Shaw, A.C.; Jackson, A.W.; Holmes, T.; Thurman, S.; Davis, G.R.; McClellan, A.D. Descending brain neurons in larval lamprey: Spinal projection patterns and initiation of locomotion. Exp. Neurol. 2010, 224, 527–541. [Google Scholar] [CrossRef][Green Version]
- Kasicki, S.; Grillner, S.; Ohta, Y.; Dubuc, R.; Brodin, L. Phasic modulation of reticulospinal neurones during fictive locomotion and other types of spinal motor activity in lamprey. Brain Res. 1989, 484, 203–216. [Google Scholar] [CrossRef]
- Deliagina, T.G.; Zelenin, P.V.; Fagerstedt, P.; Grillner, S.; Orlovsky, G.N. Activity of reticulopsinal neurons during locomotion in the freely behaving lamprey. J. Neurophysiol. 2000, 83, 853–863. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zelenin, P.V. Activity of individual reticulospinal neurons during different forms of locomotion in the lamprey. Eur. J. Neurosci. 2005, 22, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Sirota, M.G.; Viana Di Prisco, G.; Dubuc, R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur. J. Neurosci. 2000, 12, 4081–4092. [Google Scholar] [CrossRef]
- McClellan, A.D.; Kovalenko, M.; Benes, J.A.; Schulz, D.J. Spinal cord injury induces changes in electrophysiological properties and ion channel expression of reticulospinal neurons in larval lamprey. J. Neurosci. 2008, 28, 650–659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rovainen, C.M. Müller cells, Mauthner cells, and other reticulospinal neurons in the lamprey. In Neurobiology of the Mauthner Cell; Faber, D., Korn, H., Eds.; Raven Press: New York, NY, USA, 1978; pp. 245–269. [Google Scholar]
- Benes, J.A.; House, K.N.; Burks, F.N.; Conaway, K.P.; Julien, D.P.; Donley, J.P.; Iayamu, M.A.; McClellan, A.D. Regulation of axonal regeneration following spinal cord injury in the lamprey. J. Neurophysiol. 2017, 118, 1439–1456. [Google Scholar] [CrossRef]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Transm. 2021, 119, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Rouse, D.T.; Quan, X.; McClellan, A.D. Biophysical properties of reticulospinal neurons in larval lamprey. Brain Res. 1998, 779, 301–308. [Google Scholar] [CrossRef]
- Meer, D.P.; Buchanan, J.T. Apamin reduces the late afterhyperpolarization of lamprey spinal neurons, with little effect on fictive swimming. Neurosci. Lett. 1992, 143, 1–4. [Google Scholar] [CrossRef]
- El Manira, A.; Tegner, J.; Grillner, S. Calcium-dependent potassium channels play a critical role for burst termination in the locomotor network in lamprey. J. Neurophysiol. 1994, 72, 1852–1861. [Google Scholar] [CrossRef]
- Sharp, A.A.; O’Neil, M.B.; Abbot, L.F.; Marder, E. The dynamic clamp: Artificial conductances in biological neurons. Trends Neurosci. 1993, 16, 389–394. [Google Scholar] [CrossRef]
- Martin, R.J. A study of the morphology of the large reticulospinal neurons of the lamprey ammocoete by intracellular injection of procion yellow. Brain Behav. Evol. 1979, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Calton, J.; Philbrick, K.; McClellan, A.D. Anatomical regeneration and behavioral recovery following crush injury of the trigeminal nerve in lamprey. J. Comp. Neurol. 1998, 396, 322–337. [Google Scholar] [CrossRef]
- Ly, L.T.; Benthall, K.N.; Pale, T.; McClellan, A.D. Restoration of the topological organization of the trigeminal system following trigeminal nerve root injury in the lamprey. Neuroscience 2019, 423, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, R.; Bongianni, F.; Ohta, Y.; Grillner, S. Dorsal root and dorsal column mediated synaptic inputs to reticulospinal neurons in lampreys: Involvement of glutamatergic, glycinergic, and GABAergic transmission. J. Comp. Neurol. 1993, 327, 251–259. [Google Scholar] [CrossRef]
- Vinay, L.; Bongianni, F.; Ohta, Y.; Grillner, S.; Dubuc, R. Spinal inputs from lateral columns to reticulospinal neurons in lampreys. Brain Res. 1998, 808, 279–293. [Google Scholar] [CrossRef]
- Einum, J.F.; Buchanan, J.T. Membrane potential oscillations in reticulospinal and spinobulbar neurons during locomotor activity. J. Neurophysiol. 2005, 94, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wickelgren, W.O. Physiological and anatomical characteristics of reticulospinal neurons in lamprey. J. Physiol. 1977, 270, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Viana Di Prisco, G.; Boutin, T.; Petropoulos, D.; Brocard, F.; Dubuc, R. The trigeminal sensory relay to reticulospinal neurones in lampreys. Neuroscience 2005, 131, 535–546. [Google Scholar] [CrossRef]
- Shifman, M.I.; Zhang, G.; Selzer, M.E. Delayed death of identified reticulospinal neurons after spinal cord injury in lampreys. J. Comp. Neurol. 2008, 510, 269–282. [Google Scholar] [CrossRef]
- Ryan, S.K.; Shotts, L.R.; Hong, S.-K.; Nehra, D.; Groat, C.R.; Armstrong, J.R.; McClellan, A.D. Glutamate regulates neurite outgrowth of cultured descending brain neurons from larval lamprey. Dev. Neurobiol. 2007, 67, 173–188. [Google Scholar] [CrossRef]
- Kater, S.B.; Mattson, M.P.; Cohan, C.S.; Connor, J. Calcium regulation of the neuronal growth cone. Trends Neurosci. 1988, 11, 315–321. [Google Scholar] [CrossRef]
- Lipton, S.A.; Kater, S.B. Neurotransmitter regulation of neuronal outgrowth, plasticity, and survival. Trends Neurosci. 1989, 12, 265–270. [Google Scholar] [CrossRef]
- Henley, J.; Poo, M.M. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004, 14, 320–330. [Google Scholar] [CrossRef]
- Bandtlow, C.E.; Schmidt, M.F.; Hassinger, T.D.; Schwab, M.E.; Kater, S.B. Role of intracellular calcium in NI-35 evoked collapse of neuronal growth cones. Science 1993, 259, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Moorman, S.J.; Hume, R.I. ω-Conotoxin prevents myelin-evoked growth cone collapse in neonatal rat locus coeruleus neurons in vitro. J. Neurosci. 1993, 13, 4727–4736. [Google Scholar] [CrossRef]
- Enes, J.; Langwieser, N.; Ruschel, J.; Carballosa-Gonzalez, M.M.; Klug, A.; Traut, M.H.; Ylera, B.; Tahirovic, S.; Hofmann, F.; Stein, V.; et al. Electrical activity suppresses axon growth through Cav1.2 channels in adult primary sensory neurons. Curr. Biol. 2010, 20, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, F.A.; Smith, P.A. Axotomy- and autotomy-induced changes in excitability of rat dorsal root ganglion neurons. J. Neurophysiol. 2001, 85, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Jassar, B.S.; Pennefather, P.S.; Smith, P.A. Changes in sodium and calcium channel activity following axotomy of B-cells in bullfrog sympathetic ganglion. J. Physiol. 1993, 472, 203–231. [Google Scholar] [CrossRef]
- Hall, G.F. Cellular responses of identified lamprey central neurons to axonal and dendritic injury. Ann. N. Y. Acad. Sci. 1993, 679, 43–64. [Google Scholar] [CrossRef]
- Benes, J.A. The Effects of Axotomy on the Biophysical Properties of Reticulospinal Neurons in Larval Lamprey. Master’s Thesis, University of Missouri, Columbia, MO, USA, 2006. [Google Scholar]
- Hall, G.F.; Cohen, M. Extensive dendritic sprouting induced by close axotomy of central neurons in the lamprey. Science 1983, 222, 518–521. [Google Scholar] [CrossRef]
- Hall, G.F.; Cohen, M. The pattern of dendritic sprouting and retraction induced by axotomy of lamprey central neurons. J. Neurosci. 1988, 8, 3584–3597. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.S.; Wellerstein, K.K.; Selzer, M.E. Effects of axotomy on lamprey spinal neurons. Exp. Neurol. 1981, 73, 750–761. [Google Scholar] [CrossRef]
- Armstrong, J.R.; Zhang, L.; McClellan, A.D. Axonal regeneration of descending and ascending spinal projection neurons in spinal cord-transected larval lamprey. Exp. Neurol. 2003, 180, 156–166. [Google Scholar] [CrossRef]
- Buchanan, J.T. Electrophysiological properties of identified classes of lamprey spinal neurons. J. Neurophysiol. 1993, 70, 2313–2325. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.S.; Mackler, S.A.; Selzer, M.E. Axon reaction of lamprey spinal interneurons. Brain Res. 1987, 421, 48–56. [Google Scholar] [CrossRef]
- Pinter, M.J.; Vanden Noven, S. Effects of preventing reinnervation on axotomized spinal motoneurons in the cat. I. Motoneuron electrical properties. J. Neurophysiol. 1989, 62, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.E.; Gordon, T.; Shapiro, J.; Smith, P.A. Axotomy affects calcium-sensitive potassium conductance in sympathetic neurones. Neurosci. Lett. 1986, 67, 163–168. [Google Scholar] [CrossRef]
- Kelly, M.E.; Bisby, M.A.; Lukowiak, K. Regeneration restores some of the altered electrical properties of axotomized bullfrog B-cells. J. Neurobiol. 1988, 19, 357–372. [Google Scholar] [CrossRef]
- Sanchez-Vives, M.V.; Gallego, R. Effects of axotomy or target atrophy on membrane properties of rat sympathetic ganglion cells. J. Physiol. 1993, 471, 801–815. [Google Scholar] [CrossRef]
- Gallego, R.; Ivorra, I.; Morales, A. Effects of central or peripheral axotomy on membrane properties of sensory neurones in the petrosal ganglion of the cat. J. Physiol. 1987, 391, 39–56. [Google Scholar] [CrossRef]
- Sapunar, D.; Ljubkovic, M.; Lirk, P.; McCallum, J.B.; Hogan, Q.H. Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats. Anesthesiology 2005, 103, 360–376. [Google Scholar] [CrossRef]
- Gurta, S.; Smith, P.A. Electrophysiological characteristics of hamster dorsal root ganglion cells and their response to axotomy. J. Neurophysiol. 1988, 59, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Devor, M.; Waxman, S.G.; Kocsis, J.D. Subthreshold oscillations induced by spinal nerve injury in dissociated muscle and cutaneous afferents of mouse DRG. J. Neurophysiol. 2002, 87, 2009–2017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flake, N.M.; Lancaster, E.; Weinreich, D.; Gold, M.S. Absence of an association between axotomy-induced changes in sodium currents and excitability in DRG neurons from adult rat. Pain 2004, 109, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.N.; Michaelis, M.; Amir, R.; Devor, M. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: Relation to neuropathic pain. J. Neurophysiol. 2000, 84, 205–215. [Google Scholar] [CrossRef]
- Stebbing, M.J.; Eschenfelder, S.; Häbler, H.J.; Acosta, M.C.; Jänig, W.; McLachlan, E.M. Changes in the action potential in sensory neurones after peripheral axotomy in vivo. NeuroReport 1999, 10, 201–206. [Google Scholar] [CrossRef]
- Kim, Y.I.; Na, S.; Kim, S.H.; Han, H.C.; Yoon, Y.W.; Sung, B.; Nam, H.J.; Shin, S.I.; Hong, S.K. Cell type-specific changes of the membrane properties of peripherally-axotomized dorsal root ganglion neurons in a rat model of neurophathic pain. Neuroscience 1998, 86, 301–309. [Google Scholar] [CrossRef]
- Tseng, G.F.; Prince, D.A. Structural and functional alterations in rat corticospinal neurons after axotomy. J. Neurophysiol. 1996, 75, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Tseng, G.F. Membrane properties and inhibitory connections of normal and upper cervically axotomized rubrospinal neurons in the rat. Neuroscience 1997, 79, 449–462. [Google Scholar] [CrossRef]
- Titmus, M.J.; Faber, D.S.; Zottoli, S.J. Altered excitability of goldfish Mauthner cell following axotomy. I. Characterization and correlations with somatic and axonal morphological reactions. J. Neurophysiol. 1986, 55, 1424–1439. [Google Scholar] [CrossRef]
- Faber, D.S.; Zottoli, S.J. Axotomy-induced changes in cell structure and membrane excitability are sustained in a vertebrate central neuron. Brain Res. 1981, 223, 436–443. [Google Scholar] [CrossRef]
- Kuno, M.; Llinas, R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J. Physiol. 1970, 210, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Mendell, L.; Munson, J.B.; Scott, J.G. Alterations of synapses of axotomized motoneurones. J. Physiol. 1976, 255, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Rotterman, T.M.; Akhter, E.T.; Lane, A.R.; English, A.W.; Cope, T.C. Synaptic plasticity on motoneurons after axotomy: A necessary change in paradigm. Front. Mol. Neurosci. 2020, 13, 1–23. [Google Scholar] [CrossRef]
- Tseng, G.F.; Hu, M.E. Axotomy induces retraction of the dendritic arbor of adult rat rubrospinal neurons. Acta Anat. 1996, 155, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yawo, H. Changes in the dendritic geometry of mouse superior cervical ganglion cells following postganglionic axotomy. J. Neurosci. 1987, 7, 3703–3711. [Google Scholar] [CrossRef]
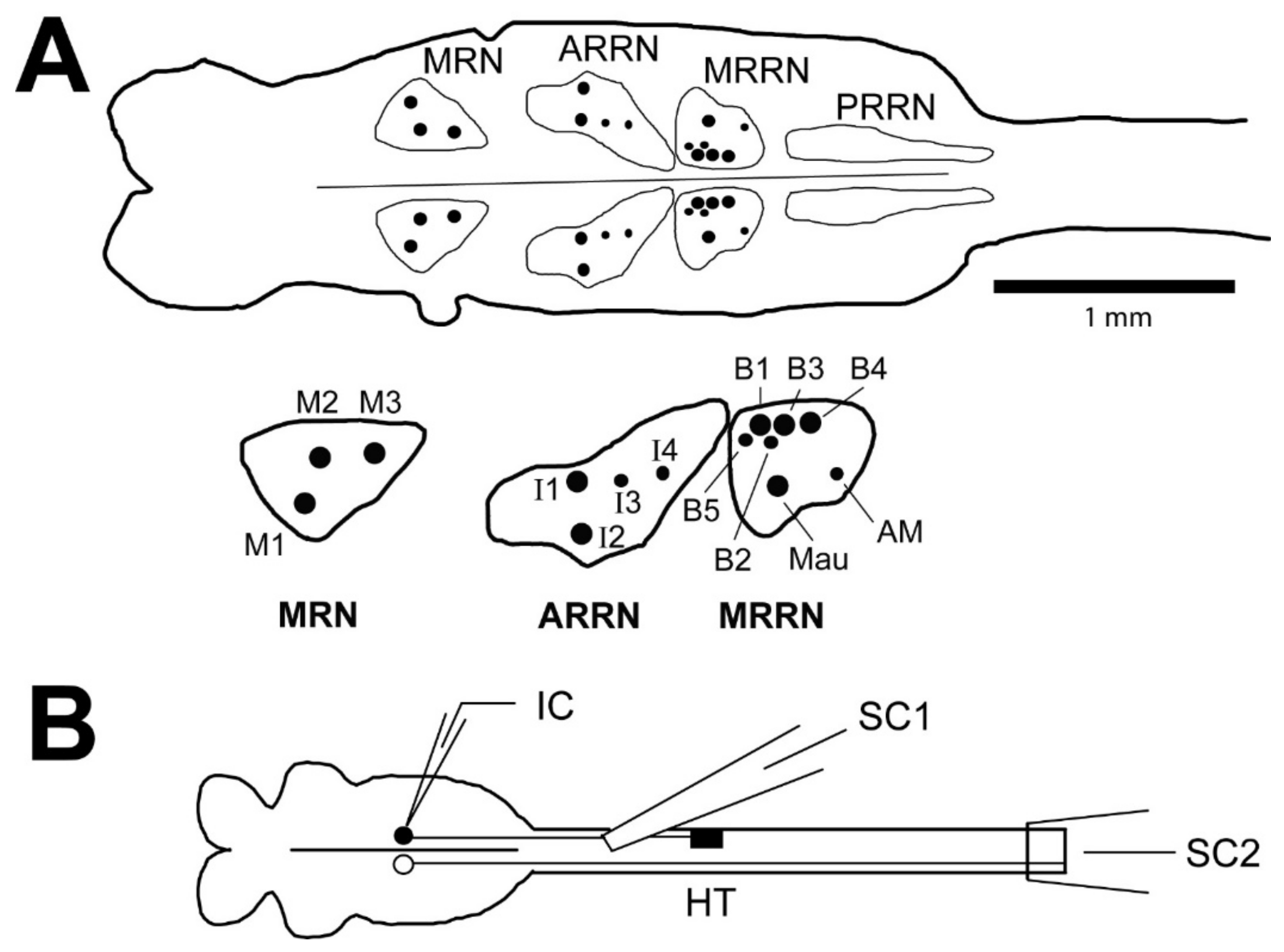
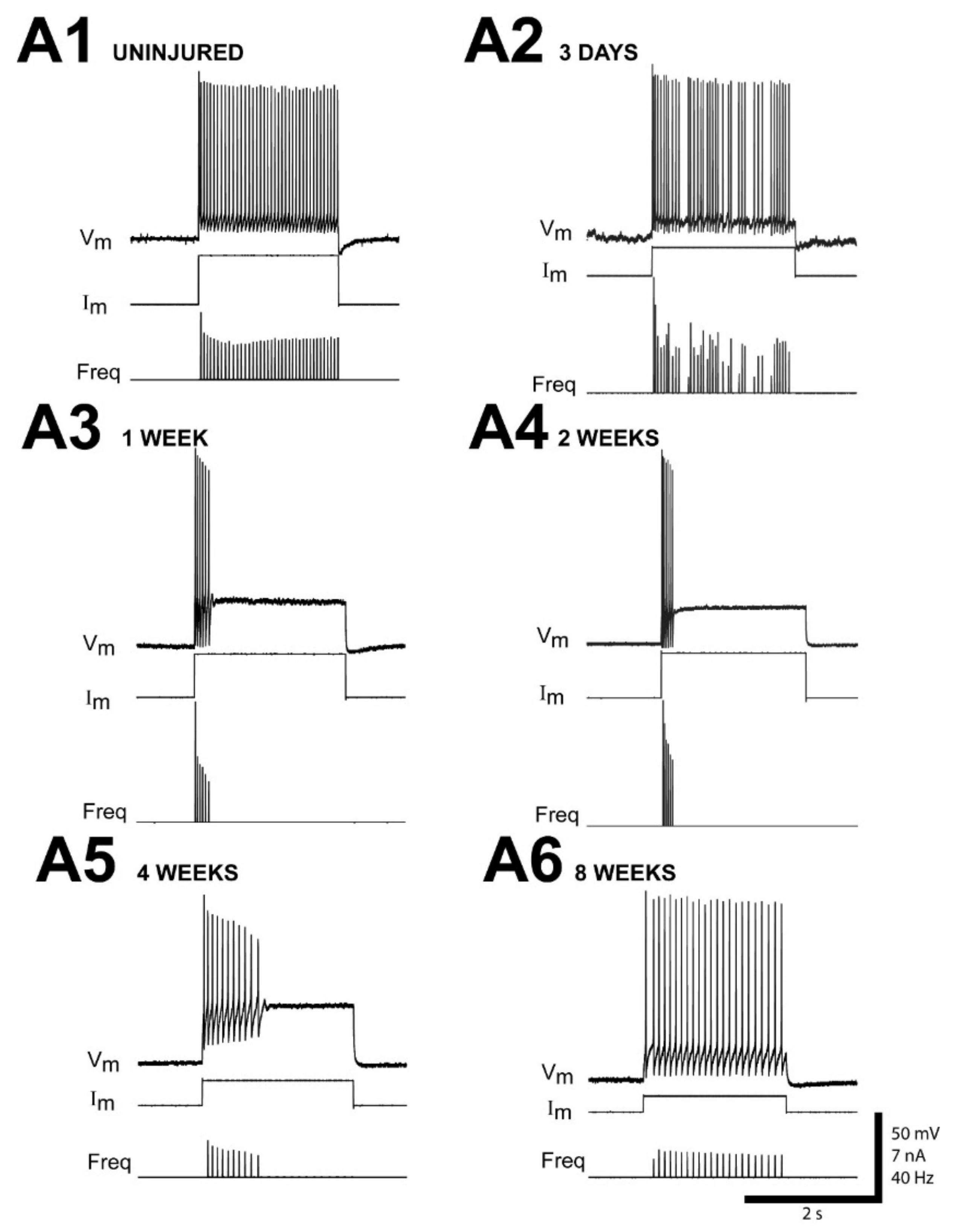
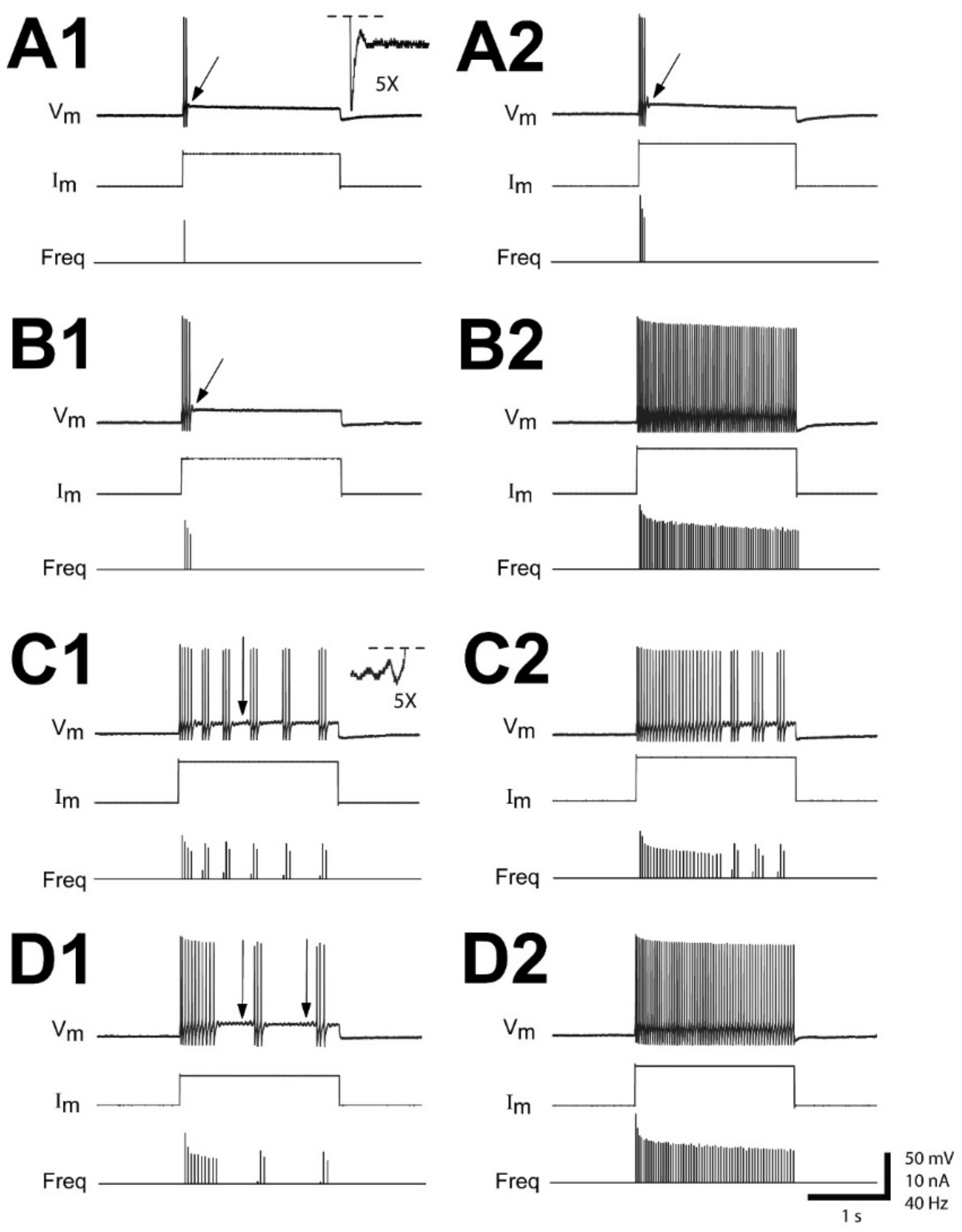
| Recovery Time | Single Short Burst or Single AP | Multiple Short Bursts | Irregular | Smooth |
| 2–3 days (n = 13 neurons) (N = 4 animals) | 8% b (0/1) c | 0% | 69% (0/9) | 23% (0/3) |
| 1 wk (n = 15) (N = 7) | 27% (0/4) | 46% (0/7) | 27% (1/4) | 0% |
| 2–3 wks (n = 145) (N = 53) | 46% (0/67) | 30% (2/43) | 13% (0/19) | 11% (3/16) |
| 4 wks (n = 25) (N = 9) | 24% (0/6) | 44% (0/11) | 16% (1/4) | 16% (0/4) |
| 6 wks (n = 33) (N = 16) | 49% (1/16) | 15% (0/5) | 9% (0/3) | 27% (4/9) |
| 8 wks (n = 9) (N = 5) | 33% (0/3) | 11% (0/1) | 0% | 56% (4/5) |
| 12–16 wks (n = 19) (N = 8) | 26% (3/5) | 0% | 5% (1/1) | 69% (12/13) |
| RS Neuron | Single Short Burst or Single AP | Multiple Short Bursts | Irregular | Smooth |
| M2 (n = 24 neurons) | 42% b | 25% | 17% | 16% |
| M3 (n = 19) | 58% | 26% | 0% | 16% |
| I1 (n = 17) | 47% | 29% | 12% | 12% |
| B1 (n = 32) | 47% | 34% | 13% | 6% |
| B3 (n = 25) | 52% | 28% | 16% | 4% |
| B4 (n = 25) | 36% | 32% | 20% | 12% |
| Passive Properties | ||||||
| Vrest (mV) | Rin (MΩ) | τin (ms) | Cin (nF) | |||
| Uninjured | −72.20 ± 5.24 b (n = 38) c | 5.21 ± 2.82 (n = 36) | 7.24 ± 3.35 (n = 35) | 1.71 ± 1.00 (n = 35) | ||
| Injured | −74.72 ± 4.32 * (n = 38) | 5.38 ± 2.84 (n = 36) | 9.50 ± 4.49 *** (n = 35) | 2.02 ± 1.08 * (n = 35) | ||
| Action Potential Features | ||||||
| ΔVTH (mV) | ITH (nA) | VAP (mV) | DAP (ms) | dVm/dtrise (mV/ms) | dVm/dtfall (mV/ms) | |
| Uninjured | 11.48 ± 4.89 (n = 36) | 3.28 ± 1.62 (n = 37) | 103.21 ± 10.18 (n = 37) | 0.98 ± 0.12 (n = 36) | 249.67 ± 54.47 (n = 36) | −135.08 ± 24.33 (n = 36) |
| Injured | 18.07 + 5.73 *** (n = 36) | 4.68 ± 2.46 ** (n = 37) | 109.31 ± 8.45 ** (n = 37) | 1.04 ± 0.17 * (n = 36) | 254.48 ± 55.80 (n = 36) | −147.10 ± 28.33 * (n = 36) |
| Afterpotential Properties | ||||||
| VfAHP (mV) | DfAHP (ms) | dfAHP (ms) | VADP (mV) | DADP (ms) | dADP (ms) | |
| Uninjured | −5.50 ± 3.37 (n = 28) | 3.67 ± 4.68 (n = 27) | 2.14 ± 0.51 (n = 28) | 2.41 ± 1.81 (n = 26) | 9.94 ± 3.69 (n = 19) | 6.75 ± 2.20 (n = 24) |
| Injured | −11.36 ± 3.04 *** (n = 37) | 8.21 ± 4.15 *** (n = 37) | 2.65 ± 0.43 *** (n = 37) | 0.06 ± 0.21 *** (n = 33) | 18.43 ± 10.03 (n = 4) | 22.58 ± 2.98 *** (n = 4) |
| VsAHP (mV) | DsAHP (ms) | dsAHP (ms) | ||||
| Uninjured | −1.19 ± 0.98 (n = 35) | 96.62 ± 20.37 (n = 24) | 49.91 ± 28.68 (n = 32) | |||
| Injured | −0.10 ± 0.28 *** (n = 34) | 77.29 ± 55.24 (n = 3) | 60.61 ± 14.32 (n = 6) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hough, R.A.; Pale, T.; Benes, J.A.; McClellan, A.D. Spinal Cord Injury Significantly Alters the Properties of Reticulospinal Neurons: I. Biophysical Properties, Firing Patterns, Excitability, and Synaptic Inputs. Cells 2021, 10, 1921. https://doi.org/10.3390/cells10081921
Hough RA, Pale T, Benes JA, McClellan AD. Spinal Cord Injury Significantly Alters the Properties of Reticulospinal Neurons: I. Biophysical Properties, Firing Patterns, Excitability, and Synaptic Inputs. Cells. 2021; 10(8):1921. https://doi.org/10.3390/cells10081921
Chicago/Turabian StyleHough, Ryan A., Timothee Pale, Jessica A. Benes, and Andrew D. McClellan. 2021. "Spinal Cord Injury Significantly Alters the Properties of Reticulospinal Neurons: I. Biophysical Properties, Firing Patterns, Excitability, and Synaptic Inputs" Cells 10, no. 8: 1921. https://doi.org/10.3390/cells10081921
APA StyleHough, R. A., Pale, T., Benes, J. A., & McClellan, A. D. (2021). Spinal Cord Injury Significantly Alters the Properties of Reticulospinal Neurons: I. Biophysical Properties, Firing Patterns, Excitability, and Synaptic Inputs. Cells, 10(8), 1921. https://doi.org/10.3390/cells10081921