Fungal Associates of Soft Scale Insects (Coccomorpha: Coccidae)
Abstract
1. Introduction
2. Material and Methods
2.1. Insects
2.2. Light (LM) and Electron Microscopy (TEM)
2.3. Molecular Analyses
2.4. Fluorescence In Situ Hybridization (FISH)
2.5. Phylogenetic and Co-Phylogenetic Analyses
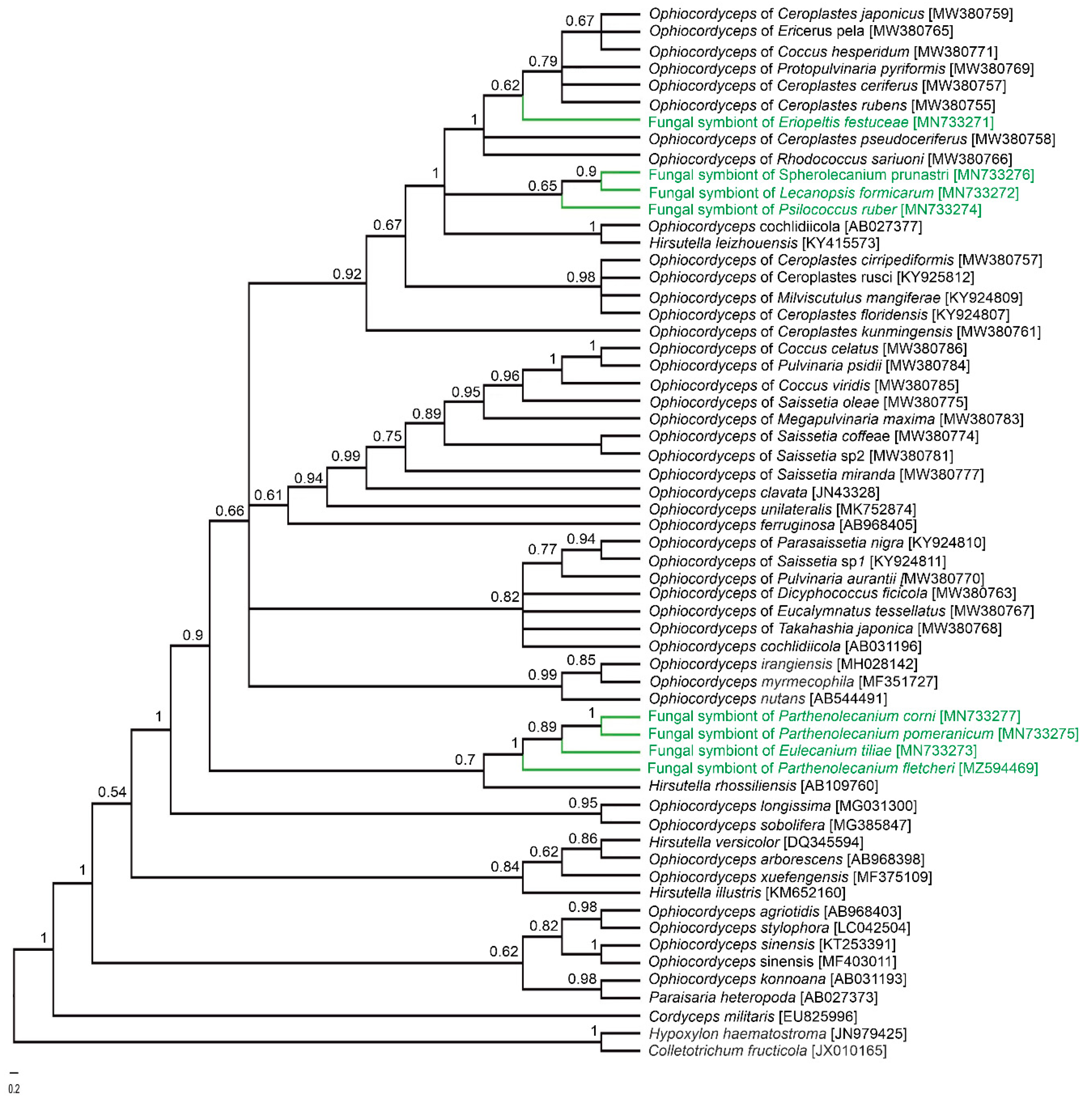
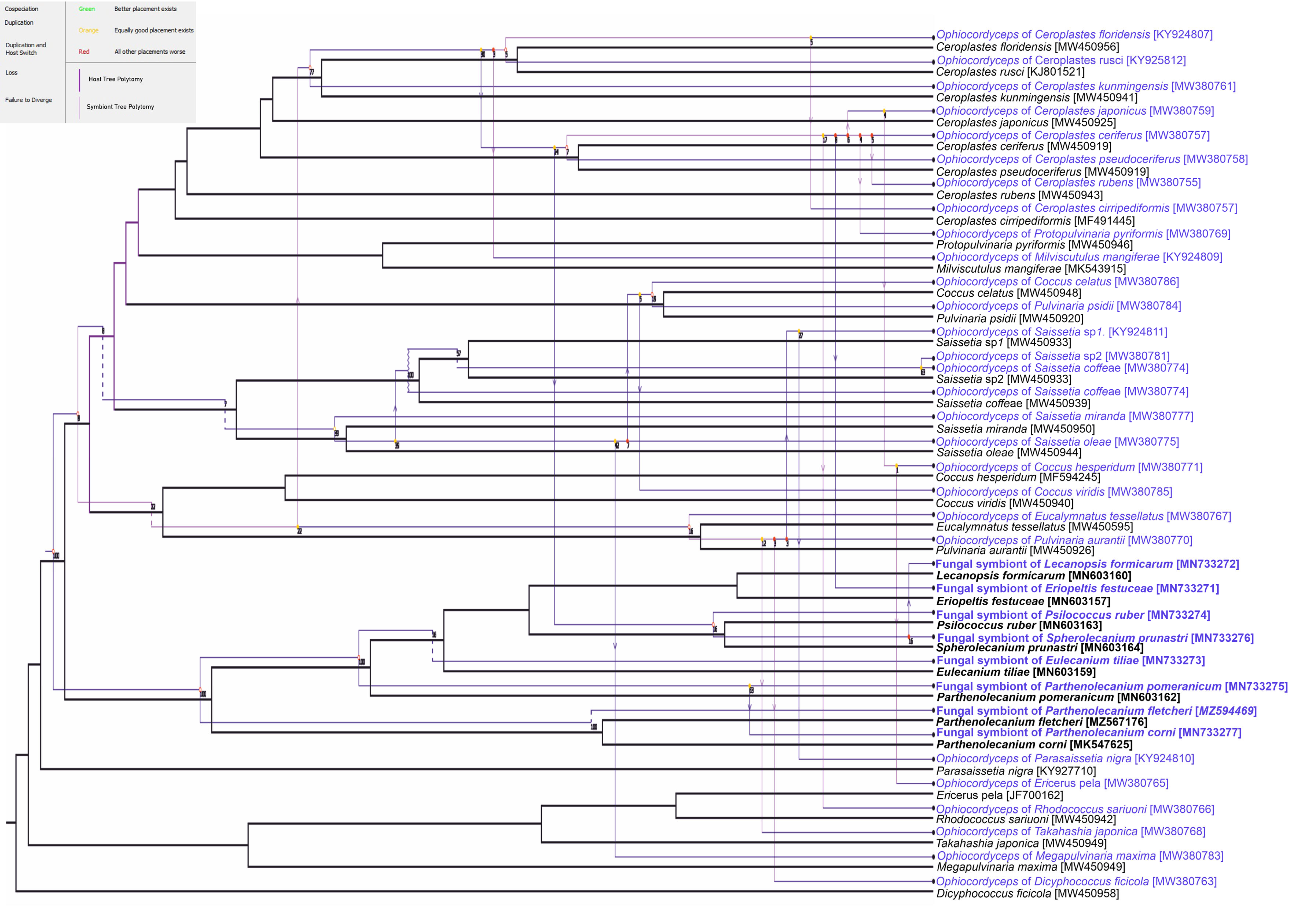
3. Results
3.1. Fungi Belonging to the Ophiocordycypitaceae Family Are Symbionts of the Soft Scale Insects Examined
3.2. Fungal Associates of Soft Scale Insects Are Transovarially Transmitted between Generations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouvrard, D.; Kondo, T.; Gullan, P.J. Scale insects: Major pests and management. In Encyclopedia of Pest Management; Taylor and Francis: New York, NY, USA, 2013; pp. 1–4. [Google Scholar]
- Hodgson, C.J. The Scale Insect Family Coccidae: An Identification Manual to Genera; CAB International: Wallingford, UK, 1994. [Google Scholar]
- Camacho, E.R.; Chong, J.-H. General Biology and Current Management Approaches of Soft Scale Pests (Hemiptera: Coccidae). J. Integr. Pest Manag. 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Gullan, P.J.; Kosztarab, M. Adaptations in Scale Insects. Annu. Rev. Entomol. 1997, 42, 23–50. [Google Scholar] [CrossRef]
- Ben-Dov, Y. A Systematic Catalogue of the Soft Scale Insects of the World (Homoptera: Coccoidea: Coccidae); Sandhill Crane Press: Gainesville, FL, USA, 1993. [Google Scholar]
- Ben-Dov, Y. The soft scale insects. Morphology, Systematics and Phylogeny. In Soft Scale Insects: Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1997; Volume 7A. [Google Scholar]
- Kozár, F.; Ben-Dov, Y. Zoogeographical considerations and status of knowledge of the family. In Soft Scale Insects: Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1997; Volume 7A. [Google Scholar]
- Douglas, A.E. The Molecular Basis of Bacterial-Insect Symbiosis. J. Mol. Biol. 2014, 426, 3830–3837. [Google Scholar] [CrossRef]
- Buchner, P. Endosymbiosis of Animals with Plant Microorganisms; Interscience Publishers: New York, NY, USA, 1965. [Google Scholar]
- Tremblay, E. Advances in Endosymbiont Studies in Coccoidea. Va. Polytech. Inst. State Univ. Res. Div. Bull. 1977, 127, 23–33. [Google Scholar]
- Baumann, P. Biology of Bacteriocyte-Associated Endosymbionts of Plant Sap-Sucking Insects. Annu. Rev. Microbiol. 2005, 59, 155–189. [Google Scholar] [CrossRef]
- Szklarzewicz, T.; Michalik, A.; Michalik, K. The Diversity of Symbiotic Systems in Scale Insects. In Symbiosis: Cellular, Molecular, Medical and Evolutionary Aspects; Kloc, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 469–495. [Google Scholar]
- Tremblay, E. Embryonic development; oviparity and viviparity. In Soft Scale Insects–Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1997; pp. 257–260. [Google Scholar]
- Gomez-Polo, P.; Ballinger, M.J.; Lalzar, M.; Malik, A.; Ben-Dov, Y.; Mozes-Daube, N.; Perlman, S.J.; Iasur-Kruh, L.; Chiel, E. An Exceptional Family: Ophiocordyceps-Allied Fungus Dominates the Microbiome of Soft Scale Insects (Hemiptera: Sternorrhyncha: Coccidae). Mol. Ecol. 2017, 26, 5855–5868. [Google Scholar] [CrossRef]
- Deng, J.; Yu, Y.; Wang, X.; Liu, Q.; Huang, X. The Ubiquity and Development-Related Abundance Dynamics of Ophiocordyceps Fungi in Soft Scale Insects. Microorganisms 2021, 9, 404. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, Y.P.; Xiong, Q.; Liu, W.M.; Xue, J.L. Ultrastructural Exploration on the Histopathological Change in Phenacoccus fraxinus Infected with Lecanicillium lecanii. PLoS ONE 2015, 10, e0117428. [Google Scholar] [CrossRef]
- Sun, X.; Yan, W.; Zhang, J.; Niu, X.; Li, F.; Qin, W.; Ma, G. Frozen Section and Electron Microscopy Studies of the Infection of the Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) by the Entomopathogenic Fungus Metarhizium anisopliae. SpringerPlus 2016, 5, 1748. [Google Scholar] [CrossRef]
- Malsam, O.; Filian, M.; Rudiger, H.; Berg, D. Fungal insecticides. In Fungal Biotechnology; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Assaf, L.H.; Hassan, F.R.; Ahmad, D.S. Pathogenicity Evaluation of Some Local Isolates of Entomopathogenic Fungi against the Nut Scale Insect Eulecanium tiliae L. Int. J. Pure Appl. Sci. Technol. 2013, 19, 37–43. [Google Scholar]
- Suh, S.-O.; Noda, H.; Blackwell, M. Insect Symbiosis: Derivation of Yeast-like Endosymbionts within an Entomopathogenic Filamentous Lineage. Mol. Biol. Evol. 2001, 18, 995–1000. [Google Scholar] [CrossRef]
- Drew, G.C.; Stevens, E.J.; King, K.C. Microbial Evolution and Transitions along the Parasite–Mutualist Continuum. Nat. Rev. Microbiol. 2021. [Google Scholar] [CrossRef]
- Koteja, J. Jak rozpoznawać czerwce (Homoptera, Coccinea). In Diagnostyka Szkodników Roślin i ich Wrogów Naturalnych; Wydawnictwo SGGW: Warszawa, Poland, 1996; Volume 2, pp. 139–231. [Google Scholar]
- Barbosa, P.; Berry, D.L.; Kary, C.S. Insect Histology. Practical Laboratory Techniques; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Hardy, N.B.; Gullan, P.J.; Hodgson, C.J. A Subfamily-Level Classification of Mealybugs (Hemiptera: Pseudococcidae) Based on Integrated Molecular and Morphological Data. Syst. Entomol. 2008, 33, 51–71. [Google Scholar] [CrossRef]
- Matsuura, Y.; Moriyama, M.; Łukasik, P.; Vanderpool, D.; Tanahashi, M.; Meng, X.-Y.; McCutcheon, J.P.; Fukatsu, T. Recurrent Symbiont Recruitment from Fungal Parasites in Cicadas. Proc. Natl. Acad. Sci. USA 2018, 115, E5970. [Google Scholar] [CrossRef]
- Łukasik, P.; Newton, J.A.; Sanders, J.G.; Hu, Y.; Moreau, C.S.; Kronauer, D.J.C.; O’Donnell, S.; Koga, R.; Russell, J.A. The Structured Diversity of Specialized Gut Symbionts of the New World Army Ants. Mol. Ecol. 2017, 26, 3808–3825. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Conow, C.; Fielder, D.; Ovadia, Y.; Libeskind-Hadas, R. Jane: A New Tool for the Cophylogeny Reconstruction Problem. Algorithms Mol. Biol. AMB 2010, 5, 16. [Google Scholar] [CrossRef]
- Szklarzewicz, T. The Ovaries of Scale Insects (Hemiptera, Coccinea). Morphology and Phylogenetic Conclusions. Folia Histochem. Cytobiol. 1998, 36, 157–165. [Google Scholar]
- Araújo, J.P.M.; Hughes, D.P. Chapter One—Diversity of Entomopathogenic Fungi: Which Groups Conquered the Insect Body? In Advances in Genetics; Lovett, B., St. Leger, R.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 94, pp. 1–39. [Google Scholar]
- Podsiadło, E.; Michalik, K.; Michalik, A.; Szklarzewicz, T. Yeast-like Microorganisms in the Scale Insect Kermes quercus (Insecta, Hemiptera, Coccomorpha: Kermesidae). Newly Acquired Symbionts? Arthropod Struct. Dev. 2018, 47, 56–63. [Google Scholar] [CrossRef]
- Vega, F.E.; Dowd, P.F. The role of yeasts as insect endosymbionts. In Insect-Fungal Associations: Ecology and Evolution; Oxford University Press: New York, NY, USA, 2005; pp. 211–243. [Google Scholar]
- Nishino, T.; Tanahashi, M.; Lin, C.-P.; Koga, R.; Fukatsu, T. Fungal and Bacterial Endosymbionts of Eared Leafhoppers of the Subfamily Ledrinae (Hemiptera: Cicadellidae). Appl. Entomol. Zool. 2016, 51, 465–477. [Google Scholar] [CrossRef]
- Kobiałka, M.; Michalik, A.; Walczak, M.; Szklarzewicz, T. Dual “Bacterial-Fungal” Symbiosis in Deltocephalinae Leafhoppers (Insecta, Hemiptera, Cicadomorpha: Cicadellidae). Microb. Ecol. 2018, 75, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Nakashima, N.; Koizumi, M. Phylogenetic Position of Yeast-like Symbiotes of Rice Planthoppers Based on Partial 18S RDNA Sequences. Insect Biochem. Mol. Biol. 1995, 25, 639–646. [Google Scholar] [CrossRef]
- Xet-Mull, A.M.; Quesada, T.; Espinoza, A.M. Phylogenetic Position of the Yeast-like Symbiotes of Tagosodes Orizicolus (Homoptera: Delphacidae) Based on 18S Ribosomal DNA Partial Sequences. Rev. Biol. Trop. 2004, 52, 777–785. [Google Scholar] [CrossRef][Green Version]
- Fan, H.-W.; Noda, H.; Xie, H.-Q.; Suetsugu, Y.; Zhu, Q.-H.; Zhang, C.-X. Genomic Analysis of an Ascomycete Fungus from the Rice Planthopper Reveals How It Adapts to an Endosymbiotic Lifestyle. Genome Biol. Evol. 2015, 7, 2623–2634. [Google Scholar] [CrossRef]
- Fukatsu, T.; Ishikawa, H. Phylogenetic Position of Yeast-like Symbiont of Hamiltonaphis styraci (Homoptera, Aphididae) Based on 18S RDNA Sequence. Insect Biochem. Mol. Biol. 1996, 26, 383–388. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Sayavedra, L.; Sámano-Sánchez, H.; Roth, A.; Martínez-Romero, E. Evolutionary Relationships of Flavobacterial and Enterobacterial Endosymbionts with Their Scale Insect Hosts (Hemiptera: Coccoidea). J. Evol. Biol. 2012, 25, 2357–2368. [Google Scholar] [CrossRef]
- Vera-Ponce de León, A.; Sanchez-Flores, A.; Rosenblueth, M.; Martínez-Romero, E. Fungal Community Associated with Dactylopius (Hemiptera: Coccoidea: Dactylopiidae) and Its Role in Uric Acid Metabolism. Front. Microbiol. 2016, 7, 954. [Google Scholar] [CrossRef]
- Fukatsu, T.; Ishikawa, H. A Novel Eukaryotic Extracellular Symbiont in an Aphid, Astegopteryx styraci (Homoptera, Aphididae, Hormaphidinae). J. Insect Physiol. 1992, 38, 765–773. [Google Scholar] [CrossRef]
- Fukatsu, T.; Aoki, S.; Kurosu, U.; Ishikawa, H. Phylogeny of Cerataphidini Aphids Revealed by Their Symbiotic Microorganisms and Basic Structure of Their Galls -Implications for Host-Symbiont Coevolution and Evolution of Sterile Soldier Castes. Zoolog. Sci. 1994, 11, 613–623. [Google Scholar]
- Hongoh, Y.; Ishikawa, H. Evolutionary Studies on Uricases of Fungal Endosymbionts of Aphids and Planthoppers. J. Mol. Evol. 2000, 51, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Michalik, A.; Jankowska, W.; Szklarzewicz, T. Ultrastructure and Transovarial Transmission of Endosymbiotic Microorganisms in Conomelus anceps and Metcalfa pruinosa (Insecta, Hemiptera, Fulgoromorpha). Folia Biol. Kraków 2009, 57, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kuechler, S.M.; Dettner, K.; Kehl, S. Characterization of an Obligate Intracellular Bacterium in the Midgut Epithelium of the Bulrush Bug Chilacis typhae (Heteroptera, Lygaeidae, Artheneinae). Appl. Environ. Microbiol. 2011, 77, 2869. [Google Scholar] [CrossRef] [PubMed]
- Walczuch, A. Studien an Coccidensymbionten. Z. für Morphol. und Ökol. der Tiere 1932, 25, 623–729. [Google Scholar] [CrossRef]
- von Dohlen, C.D.; Kohler, S.; Alsop, S.T.; McManus, W.R. Mealybug β-Proteobacterial Endosymbionts Contain γ-Proteobacterial Symbionts. Nature 2001, 412, 433–436. [Google Scholar] [CrossRef]
- Szklarzewicz, T.; Kędra, K.; Niżnik, S. Ultrastructure and Transovarial Transmission of Endosymbiotic Microorganisms in Palaeococcus fuscipennis (Burmeister) (Insecta, Hemiptera, Coccinea: Monophlebidae). Folia Biol. Kraków 2006, 54, 69–74. [Google Scholar] [CrossRef]
- Szklarzewicz, T.; Kalandyk-Kolodziejczyk, M.; Kot, M.; Michalik, A. Ovary Structure and Transovarial Transmission of Endosymbiotic Microorganisms in Marchalina hellenica (Insecta, Hemiptera, Coccomorpha: Marchalinidae). Acta Zool. 2013, 94, 184–192. [Google Scholar] [CrossRef]
- Szklarzewicz, T.; Kalandyk-Kołodziejczyk, M.; Michalik, K.; Jankowska, W.; Michalik, A. Symbiotic Microorganisms in Puto superbus (Leonardi, 1907) (Insecta, Hemiptera, Coccomorpha: Putoidae). Protoplasma 2018, 255, 129–138. [Google Scholar] [CrossRef]
- Niżnik, S.; Szklarzewicz, T. Structure and Development of Hermaphroditic Gonad in Icerya purchasi (Insecta, Hemiptera, Coccinea: Monophlebidae). Zool. Pol. 2007, 52, 71–90. [Google Scholar]
- Michalik, K.; Szklarzewicz, T.; Kalandyk-Kołodziejczyk, M.; Jankowska, W.; Michalik, A. Bacteria Belonging to the Genus Burkholderia Are Obligatory Symbionts of the Eriococcids Acanthococcus aceris Signoret, 1875 and Gossyparia spuria (Modeer, 1778) (Insecta, Hemiptera, Coccoidea). Arthropod Struct. Dev. 2016, 45, 265–272. [Google Scholar] [CrossRef]
- Michalik, A.; Schulz, F.; Michalik, K.; Wascher, F.; Horn, M.; Szklarzewicz, T. Coexistence of Novel Gammaproteobacterial and Arsenophonus Symbionts in the Scale Insect Greenisca brachypodii (Hemiptera, Coccomorpha: Eriococcidae). Environ. Microbiol. 2018, 20, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Michalik, A.; Michalik, K.; Grzywacz, B.; Kalandyk-Kołodziejczyk, M.; Szklarzewicz, T. Molecular Characterization, Ultrastructure, and Transovarial Transmission of Tremblaya phenacola in Six Mealybugs of the Phenacoccinae Subfamily (Insecta, Hemiptera, Coccomorpha). Protoplasma 2019, 256, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Szklarzewicz, T.; Michalik, A. Transovarial Transmission of Symbionts in Insects. In Oocytes: Maternal Information and Functions; Kloc, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 43–67. [Google Scholar]
- Moran, N.A. The Coevolution of Bacterial Endosymbionts and Phloem-Feeding Insects. Ann. Mo. Bot. Gard. 2001, 88, 35–44. [Google Scholar] [CrossRef]
- Baumann, L.; Baumann, P. Cospeciation Between the Primary Endosymbionts of Mealybugs and Their Hosts. Curr. Microbiol. 2005, 50, 84–87. [Google Scholar] [CrossRef]
- Downie, D.A.; Gullan, P.J. Phylogenetic Congruence of Mealybugs and Their Primary Endosymbionts. J. Evol. Biol. 2005, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]


| Species | Subfamily | Place of Collection/Locality Coordinates | Date of Collection | Host Plant | No. of Individuals Examined Using Microscopic Techniques | No. of Individuals Examined Using Molecular Techniques | ||
|---|---|---|---|---|---|---|---|---|
| LM | TEM | PCR | FISH | |||||
| Parthenolecanium corni | Coccinae | Katowice 50.245638 19.007992 | V, VI 2017 | Tilia cordata | 10 | 2 | 3 | 1 |
| Parthenolecanium fletcheri | Katowice, 50.245621 19.005127 Olsztyn 50.753157 19.277790 | V 2018; V 2019 | Thuja sp. | 6 | 4 | 3 | 1 | |
| Parthenolecanium pomeranicum | Katowice 50.243978 19.001704 | V, VI 2017; VI 2018 | Taxus baccata | 10 | 2 | 7 | 3 | |
| Eriopeltis festucae | Eriopeltinae | Olsztyn 50.749359 19.272956 | VII 2017; VI 2018 | Calamagrostis epigejos | 5 | 2 | 3 | 1 |
| Lecanopsis formicarum | Mikoszewo 54.341908 18.992342 | VIII 2018 | Festuca ovina | 5 | 2 | 7 | 3 | |
| Psilococcus ruber | Mikoszewo 54.344519 18.978498 | VIII 2018 | Carex sp. | 10 | 4 | 7 | 3 | |
| Sphaerolecanium prunastri | Filippinae | Bukowno 50.274843 19.436405 | VI 2017; IV 2018 | Prunus spinosa | 15 | 3 | 7 | 3 |
| Eulecanium tiliae | Olsztyn 50.749837 19.274678 | V 2018 | Tilia cordata | 6 | 2 | 3 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szklarzewicz, T.; Michalik, K.; Grzywacz, B.; Kalandyk-Kołodziejczyk, M.; Michalik, A. Fungal Associates of Soft Scale Insects (Coccomorpha: Coccidae). Cells 2021, 10, 1922. https://doi.org/10.3390/cells10081922
Szklarzewicz T, Michalik K, Grzywacz B, Kalandyk-Kołodziejczyk M, Michalik A. Fungal Associates of Soft Scale Insects (Coccomorpha: Coccidae). Cells. 2021; 10(8):1922. https://doi.org/10.3390/cells10081922
Chicago/Turabian StyleSzklarzewicz, Teresa, Katarzyna Michalik, Beata Grzywacz, Małgorzata Kalandyk-Kołodziejczyk, and Anna Michalik. 2021. "Fungal Associates of Soft Scale Insects (Coccomorpha: Coccidae)" Cells 10, no. 8: 1922. https://doi.org/10.3390/cells10081922
APA StyleSzklarzewicz, T., Michalik, K., Grzywacz, B., Kalandyk-Kołodziejczyk, M., & Michalik, A. (2021). Fungal Associates of Soft Scale Insects (Coccomorpha: Coccidae). Cells, 10(8), 1922. https://doi.org/10.3390/cells10081922






