Ultrastructural Profile Combined with Immunohistochemistry of a Hepatic Progenitor Cell Line in Pediatric Autoimmune Hepatitis: New Insights into the Morphological Pattern of the Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients’ Profile
2.2. Liver Tissue Processing for Immunohistochemistry for Cytokeratin 7 (CK7)
2.3. Liver Tissue Processing for Transmission Electron Microscopy
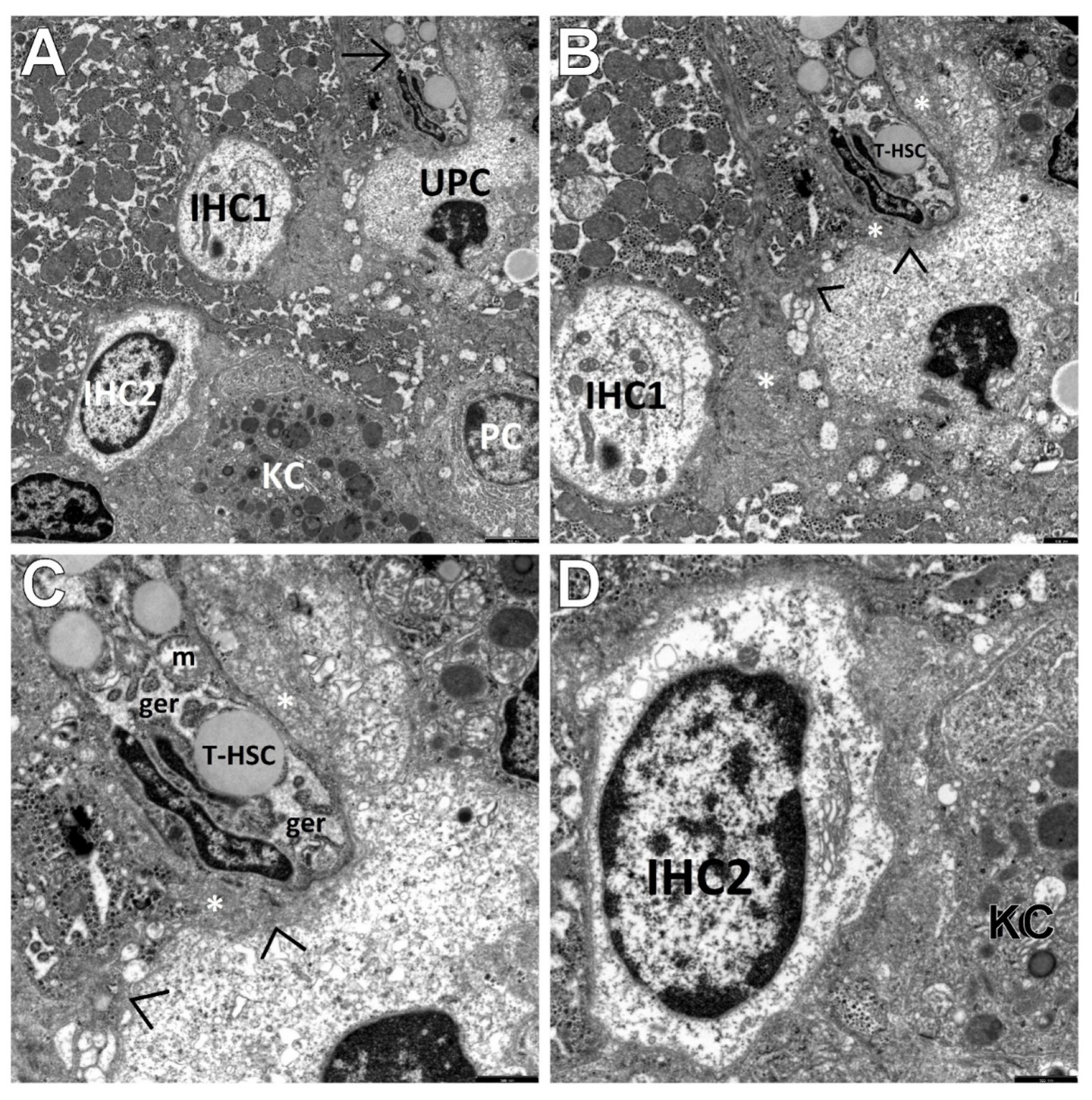
3. Results
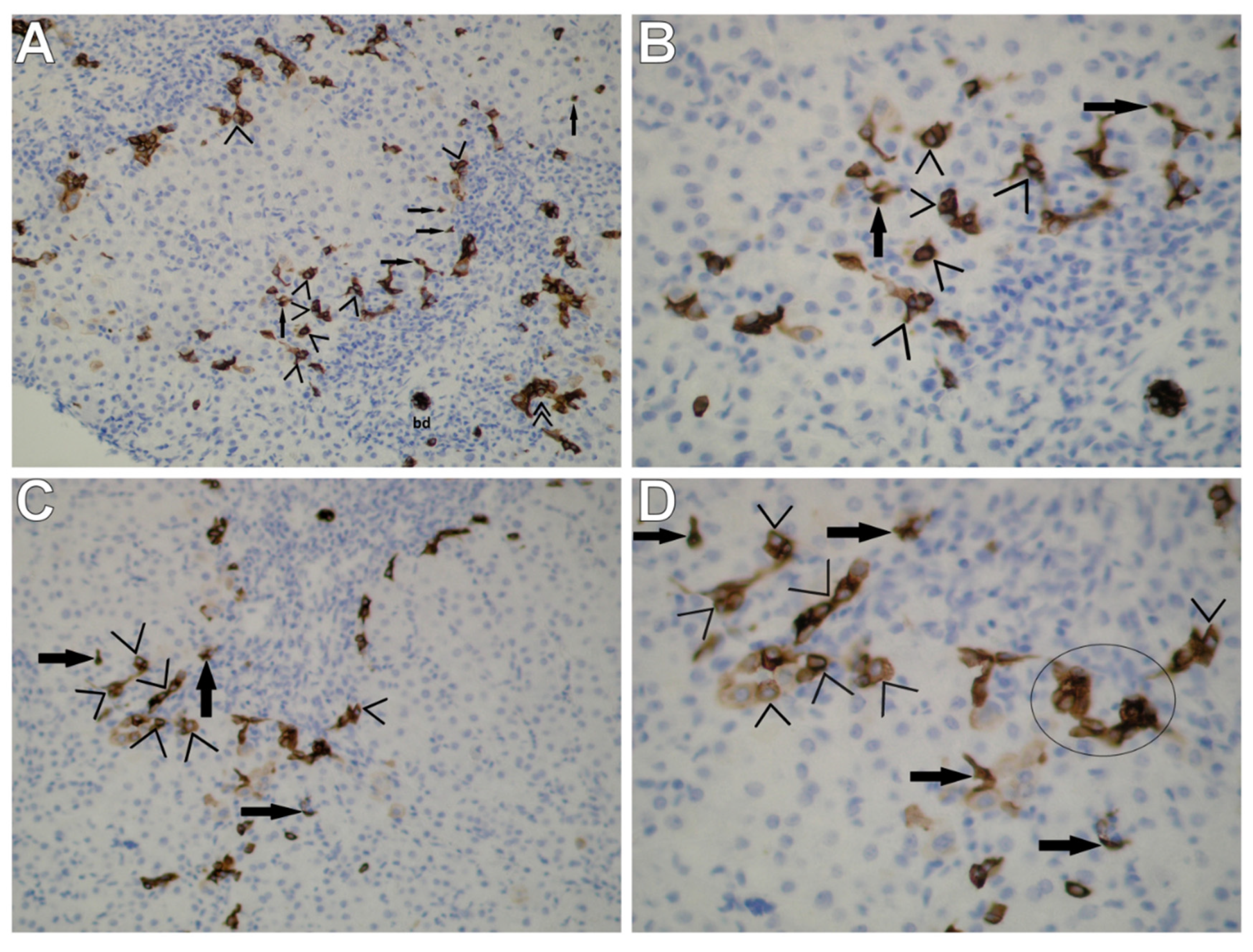
3.1. Immunohistochemical Staining for CK7
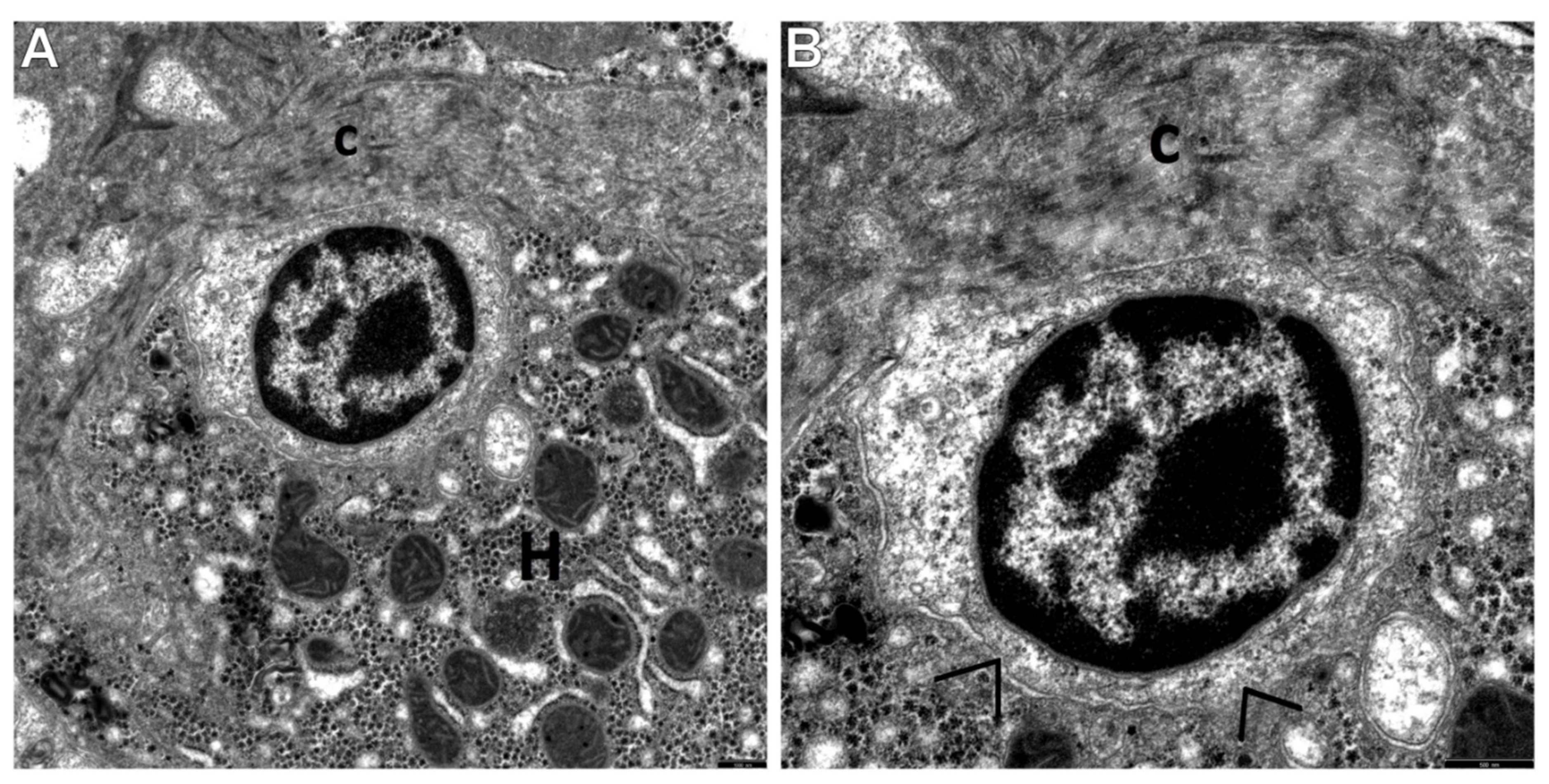
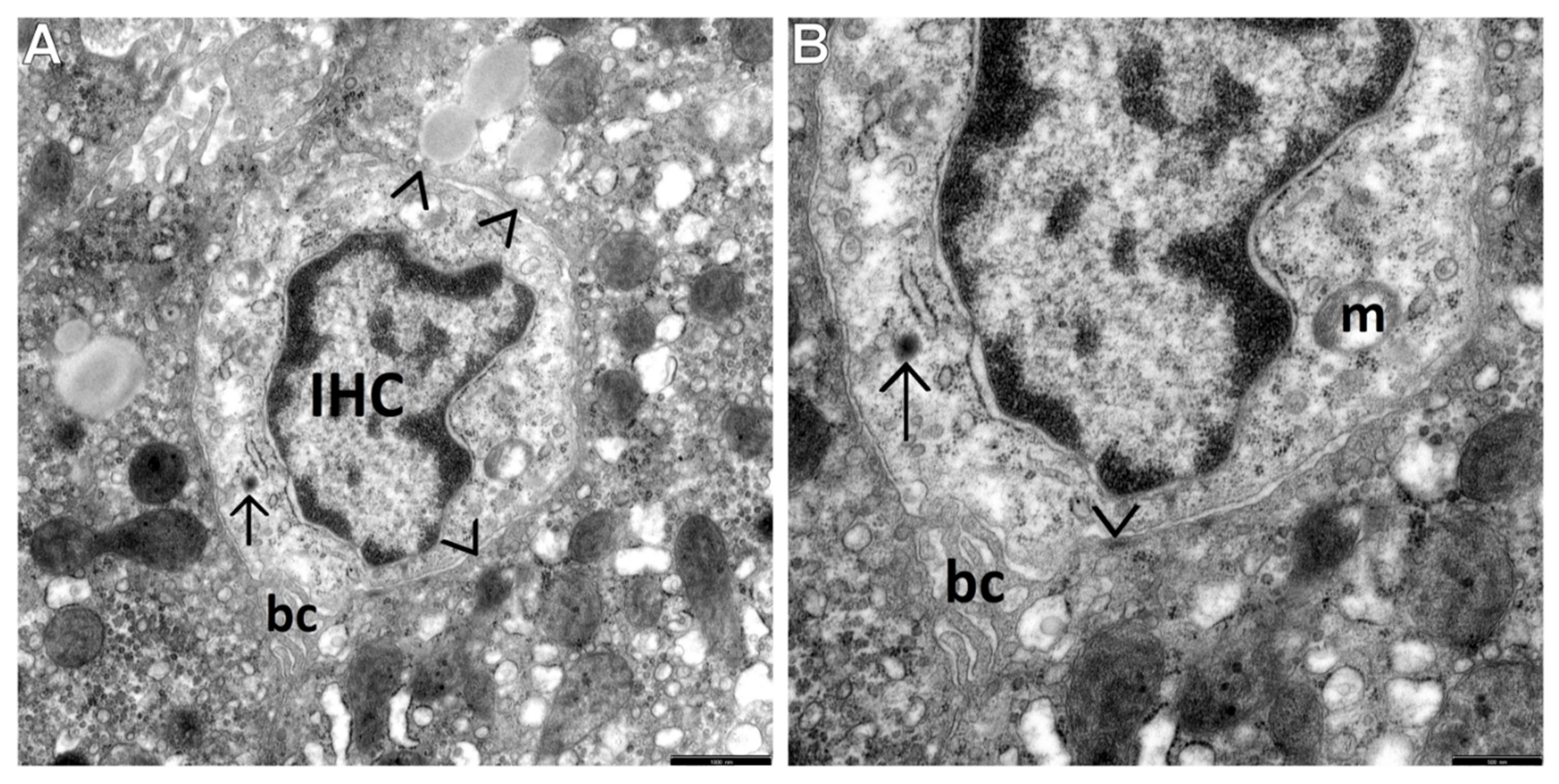
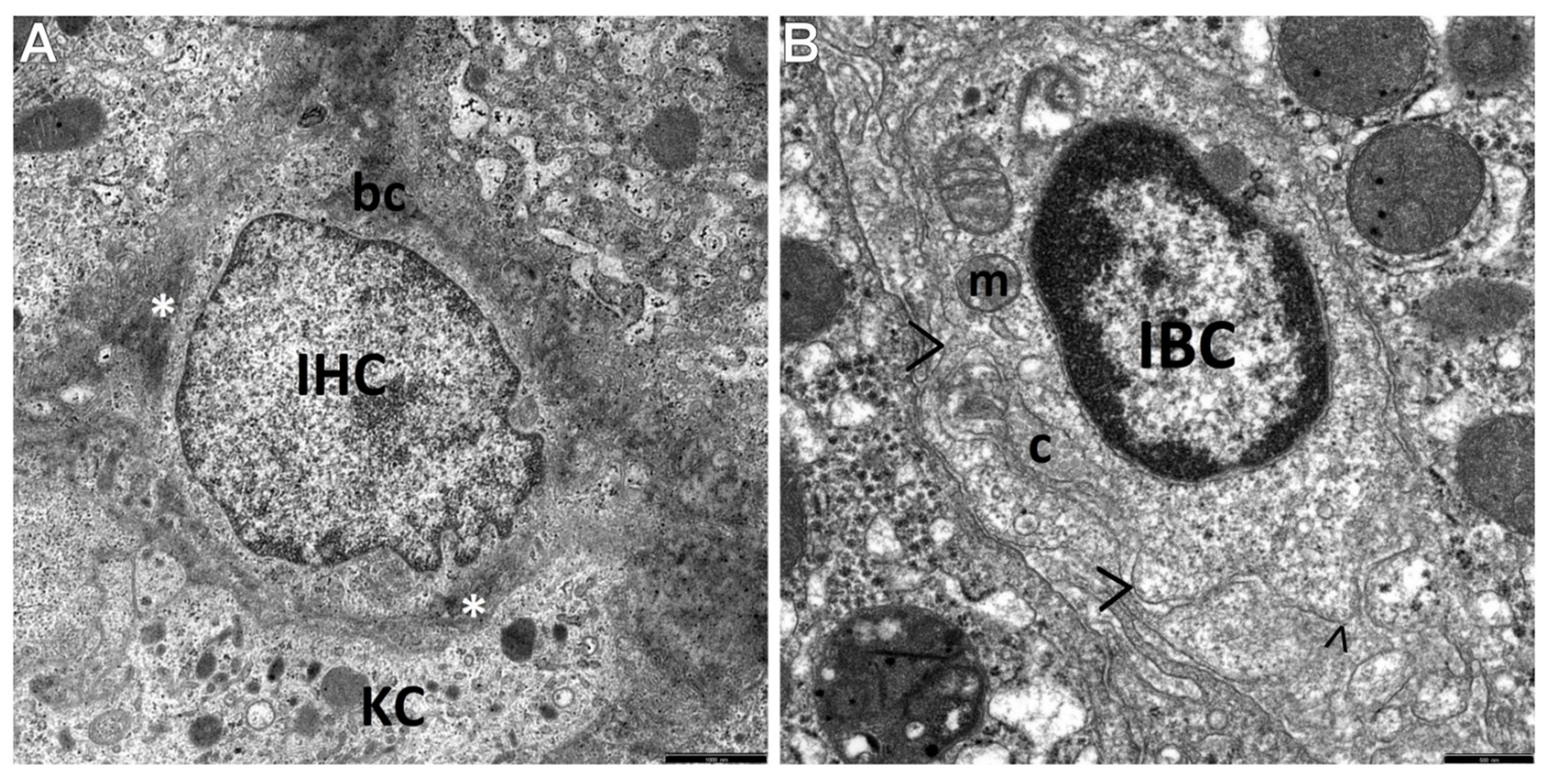
3.2. Transmission Electron Microscopic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heneghan, M.A.; Yeoman, A.D.; Verma, S.; Smith, A.D.; Longhi, M.S. Autoimmune hepatitis. Lancet 2013, 382, 1433–1444. [Google Scholar] [CrossRef]
- Dohmen, K.; Tanaka, H.; Haruno, M.; Aishima, S. Immunoserological and histological differences between autoimmune hepatitis with acute presentation and chronic autoimmune hepatitis. Hepatol. Res. 2017, 47, 1375–1382. [Google Scholar] [CrossRef]
- Mieli-Vergani, G.; Vergani, D.; Baumann, U.; Czubkowski, P.; Debray, D.; Dezsofi, A.; Hadžić, N.; Fischler, B.; Gupte, G.; Hierro, L.; et al. Diagnosis and Management of Paediatric Autoimmune Liver Disease: ESPGHAN Hepatology Committee Position Statement. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Lotowska, J.M.; Sobaniec-Lotowska, M.E.; Daniluk, U.; Lebensztejn, D.M. Glassy droplet inclusions within the cytoplasm of Kupffer cells: A novel ultrastructural feature for the diagnosis of pediatric autoimmune hepatitis. Dig. Liver Dis. 2017, 49, 929–933. [Google Scholar] [CrossRef]
- Fujiwara, K.; Fukuda, Y.; Seza, K.; Saito, M.; Yasui, S.; Nakano, M.; Kato, N. Long-term observation of acute-onset autoimmune hepatitis presenting clinically and radiologically as acute hepatitis. Hepatol. Int. 2018, 12, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Dalekos, G.N.; Koskinas, J.; Papatheodoridis, G.V. Hellenic Association for the Study of the Liver Clinical Practice Guidelines: Autoimmune hepatitis. Ann. Gastroenterol. 2019, 32, 1–23. [Google Scholar] [CrossRef]
- Puustinen, L.; Barner-Rasmussen, N.; Pukkala, E.; Färkkilä, M. Incidence, prevalence, and causes of death of patients with autoimmune hepatitis: A nationwide register-based cohort study in Finland. Dig. Liver Dis. 2019, 51, 1294–1299. [Google Scholar] [CrossRef]
- Tanaka, A.; Mori, M.; Matsumoto, K.; Ohira, H.; Tazuma, S.; Takikawa, H. Increase trend in the prevalence and male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis, and primary sclerosing cholangitis in Japan. Hepatol. Res. 2019, 49, 881–889. [Google Scholar] [CrossRef]
- Tanaka, A. Autoimmune Hepatitis: 2019 Update. Gut Liver 2020, 14, 430–438. [Google Scholar] [CrossRef]
- Kage, M. Pathology of autoimmune liver diseases in children. Hepatol. Res. 2007, 37, S502–S508. [Google Scholar] [CrossRef]
- Lotowska, J.M.; Sobaniec-Lotowska, M.E.; Sobaniec, P.; Lebensztejn, D.M. Liver sinusoidal endothelial cells in morphogenesis of pediatric autoimmune hepatitis. Ultrastructural characteristics—A novel report. Pol. J. Pathol. 2018, 69, 327–334. [Google Scholar] [CrossRef]
- Radhakrishnan, K.R.; Alkhouri, N.; Worley, S.; Arrigain, S.; Hupertz, V.; Kay, M.; Feldstein, A.E. Autoimmune hepatitis in children--impact of cirrhosis at presentation on natural history and long-term outcome. Dig. Liver Dis. 2010, 42, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Borgonovo, A.; Maggi, D.C.; Pasinato, A.P.; Ramos, F.G.; Dantas-Corrêa, E.B.; Narciso-Schiavon, J.L.; Narciso-Schiavon, J.L. Liver dysfunction and fibrosis as predictors of biochemical response to autoimmune hepatitis treatment. Minerva Gastroentero. Dieto. 2016, 62, 138–147. [Google Scholar]
- Nares-Cisneros, J.; Jaramillo-Rodríguez, Y. Autoimmune hepatitis in children: Progression of 20 cases in northern Mexico. Rev. Gastroentero. Mex. 2014, 79, 238–243. [Google Scholar] [CrossRef][Green Version]
- Floreani, A.; Liberal, R.; Vergani, D.; Mieli-Vergani, G. Autoimmune hepatitis: Contrasts and comparisons in children and adults—A comprehensive review. J. Autoimmun. 2013, 46, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.; Berg, P.A.; Bianchi, F.B.; Bianchi, L.; Burroughs, A.K.; Cancado, E.L.; Chapman, R.W.; Cooksley, W.G.; Czaja, A.J.; Desmet, V.J.; et al. International Autoimmune Hepatitis Group Report: Review of criteria for diagnosis of autoimmune hepatitis. J. Hepatol. 1999, 31, 929–938. [Google Scholar] [CrossRef]
- Fujiwara, K.; Nakano, M.; Yasui, S.; Okitsu, K.; Yonemitsu, Y.; Yokosuka, O. Advanced histology and impaired liver regeneration are associated with disease severity in acute-onset autoimmune hepatitis. Histopathology 2011, 58, 693–704. [Google Scholar] [CrossRef]
- Fujiwara, K.; Yasui, S.; Yokosuka, O. Autoimmune acute liver failure: An emerging etiology for intractable acute liver failure. Hepatol. Int. 2013, 7, 335–346. [Google Scholar] [CrossRef]
- Verdonk, R.C.; Lozano, M.F.; van den Berg, A.P.; Gouw, A.S. Bile ductal injury and ductular reaction are frequent phenomena with different significance in autoimmune hepatitis. Liver Int. 2016, 36, 1362–1369. [Google Scholar] [CrossRef]
- Van Haele, M.; Snoeck, J.; Roskams, T. Human Liver Regeneration: An Etiology Dependent Process. Int. J. Mol. Sci. 2019, 20, 2332. [Google Scholar] [CrossRef] [PubMed]
- Paku, S.; Schnur, J.; Nagy, P.; Thorgeirsson, S.S. Origin and structural evolution of the early proliferating ovalcells in rat liver. Am. J. Pathol. 2001, 158, 1313–1323. [Google Scholar] [CrossRef]
- Guettier, C. Which stem cells for adult liver? Ann. Pathol. 2005, 25, 33–44. [Google Scholar] [CrossRef]
- Herrera, M.B.; Fonsato, V.; Gatti, S.; Deregibus, M.C.; Sordi, A.; Cantarella, D.; Calogero, R.; Bussolati, B.; Tetta, C.; Camussi, G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J. Cell. Mol. Med. 2010, 14, 1605–1618. [Google Scholar] [CrossRef]
- He, Z.; Feng, M. Activation, isolation, identification and culture of hepatic stem cells from porcine liver tissues. Cell Prolif. 2011, 44, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Lotowska, J.M.; Sobaniec-Lotowska, M.E.; Lebensztejn, D.M.; Daniluk, U.; Sobaniec, P.; Sendrowski, K.; Daniluk, J.; Reszec, J.; Debek, W. Ultrastructural characteristics of rat hepatic oval cells and their intercellular contacts in the model of biliary fibrosis. New insights into experimental liver fibrogenesis. Gastroenterol. Res. Pract. 2017. [Google Scholar] [CrossRef]
- Yovchev, M.L.; Grozdanov, P.N.; Zhou, H.; Racherla, H.; Guha, C.; Dabeva, M.D. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology 2008, 47, 636–647. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Lu, W.Y. Liver stem cells: Plasticity of the liver epithelium. World J. Gastroenterol. 2019, 2, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Hytiroglou, P.; Wieczorek, R.; Park, Y.N.; Thung, S.N.; Arias, B.; Theise, N.D. Immunohistochemical evidence for hepatic progenitor cells in liver diseases. Liver 2002, 22, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.C.; Ruck, P.; Adam, A.; Wang, T.X.; Kaiserling, E. Small epithelial cells in human liver cirrhosis exhibit features of hepatic stem-like cells: Immunohistochemical, electron microscopic and immunoelectron microscopic findings. Histopathology 2003, 42, 141–149. [Google Scholar] [CrossRef]
- Clouston, A.D.; Powell, E.E.; Walsh, M.J.; Richardson, M.M.; Demetris, A.J.; Jonsson, J.R. Fibrosis correlates with a ductular reaction in hepatitis C: Roles of impaired replication, progenitor cells and steatosis. Hepatology 2005, 41, 809–818. [Google Scholar] [CrossRef]
- El-Araby, H.A.; Ehsan, N.A.; Konsowa, H.A.; Abd-Elaati, B.M.; Sira, A.M. Hepaticprogenitor cells in children with chronic hepatitis C: Correlation with histopathology, viremia, and treatment response. Eur. J. Gastroenterol. Hepatol. 2015, 27, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Rogler, L.E.; Teperman, L.; Morgan, G.; Rogler, C.E. Identification of hepatocytic and bile ductular cell lineages and candidate stem cells in bipolar ductular reactions in cirrhotic human liver. Hepatology 2007, 45, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Vestentoft, P.S.; Jelnes, P.; Andersen, J.B.; Tran, T.A.T.; Jørgensen, T.; Rasmussen, M.; Bornholdt, J.; Melsæther Grøvdal, L.; Jensen, C.H.; Vogel, L.K.; et al. Molecular constituents of the extracellular matrix in rat liver mounting a hepatic progenitor cell response for tissue repair. Fibrogenesis Tissue Repair 2013, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Schotanus, B.A.; Kruitwagen, H.S.; van den Ingh, T.S.; van Wolferen, M.E.; Rothuizen, J.; Penning, L.C.; Spee, B. Enhanced Wnt/β-catenin and Notch signalling in the activated canine hepatic progenitor cell niche. BMC Vet. Res. 2014, 10, 309. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, L.; Fish, M.; Logan, C.; Nusse, R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 2015, 524, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, L.; Zern, M.; Theise, N.; Diehl, A.M.; Ping, L.; Duan, Y. The diversity and plasticity of adult hepatic progenitor cells and their niche. Liver Int. 2017, 37, 1260–1271. [Google Scholar] [CrossRef]
- Sobaniec-Lotowska, M.E.; Lotowska, J.M.; Lebensztejn, D.M. Ultrastructure of oval cells in children with chronic hepatitis B, with special emphasis on the stage of liver fibrosis: The first pediatric study. World J. Gastroenterol. 2007, 13, 2918–2922. [Google Scholar] [CrossRef]
- Lotowska, J.M.; Sobaniec-Lotowska, M.E.; Lebensztejn, D.M. Electron microscopic alterations in intermediate hepatocyte-like cells in children with chronic hepatitis B. The first report in pediatric patients. Eur. J. Gastroenterol. Hepatol. 2010, 22, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Sobaniec-Lotowska, M.E.; Lebensztejn, D.M.; Lotowska, J.M.; Kańczuga-Koda, L.; Sulkowski, S. Ultrastructure of liver progenitor/oval cells in children with nonalcoholic steatohepatitis. Adv. Med. Sci. 2011, 56, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Lotowska, J.M.; Sobaniec-Lotowska, M.E.; Lebensztejn, D.M. The role of Kupffer cells in the morphogenesis of nonalcoholic steatohepatitis—Ultrastructural findings. The first report in pediatric patients. Scand. J. Gastroenterol. 2013, 48, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Batts, K.P.; Ludwig, J. Chronic hepatitis. An update on terminology and reporting. Am. J. Surg. Pathol. 1995, 19, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Roskams, T.A.; Libbrecht, L.; Desmet, V.J. Progenitor cells in diseased human liver. Semin. Liver Dis. 2003, 23, 385–396. [Google Scholar]
- Dezsőfi, A.; Baumann, U.; Dhawan, A.; Durmaz, O.; Fischler, B.; Hadzic, N.; Hierro, L.; Lacaille, F.; McLin, V.A.; Nobili, V.; et al. ESPGHAN Hepatology Committee. Liver biopsy in children: Position paper of the ESPGHAN Hepatology Committee. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 408–420. [Google Scholar] [CrossRef]
- Lotowska, J.M.; Sobaniec-Lotowska, M.E.; Lebensztejn, D.M. Ultrastructural characteristics of the respective forms of hepatic stellate cells in chronic hepatitis B as an example of high fibroblastic cell plasticity. The first assessment in children. Adv. Med. Sci. 2018, 63, 127–133. [Google Scholar] [CrossRef]
- Fiore, E.J.; Mazzolini, G.; Aquino, J.B. Mesenchymal Stem/Stromal Cells in Liver Fibrosis: Recent Findings, Old/New Caveats and Future Perspectives. Stem. Cell Rev. Rep. 2015, 11, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Spirli, C.; Cadamuro, M.; Fiorotto, R.; Strazzabosco, M. Emerging concepts in biliary repair and fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G102–G116. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Tacke, F. Liver Fibrosis: From Pathogenesis to Novel Therapies. Dig. Dis. 2016, 34, 410–422. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotowska, J.M.; Sobaniec-Lotowska, M.E.; Sobaniec, P. Ultrastructural Profile Combined with Immunohistochemistry of a Hepatic Progenitor Cell Line in Pediatric Autoimmune Hepatitis: New Insights into the Morphological Pattern of the Disease. Cells 2021, 10, 1899. https://doi.org/10.3390/cells10081899
Lotowska JM, Sobaniec-Lotowska ME, Sobaniec P. Ultrastructural Profile Combined with Immunohistochemistry of a Hepatic Progenitor Cell Line in Pediatric Autoimmune Hepatitis: New Insights into the Morphological Pattern of the Disease. Cells. 2021; 10(8):1899. https://doi.org/10.3390/cells10081899
Chicago/Turabian StyleLotowska, Joanna Maria, Maria Elzbieta Sobaniec-Lotowska, and Piotr Sobaniec. 2021. "Ultrastructural Profile Combined with Immunohistochemistry of a Hepatic Progenitor Cell Line in Pediatric Autoimmune Hepatitis: New Insights into the Morphological Pattern of the Disease" Cells 10, no. 8: 1899. https://doi.org/10.3390/cells10081899
APA StyleLotowska, J. M., Sobaniec-Lotowska, M. E., & Sobaniec, P. (2021). Ultrastructural Profile Combined with Immunohistochemistry of a Hepatic Progenitor Cell Line in Pediatric Autoimmune Hepatitis: New Insights into the Morphological Pattern of the Disease. Cells, 10(8), 1899. https://doi.org/10.3390/cells10081899





