Ciliary Dyneins and Dynein Related Ciliopathies
Abstract
1. Enigmatic Cilia
2. Primary Cilia
2.1. Primary (Non-Motile) Cilia Function
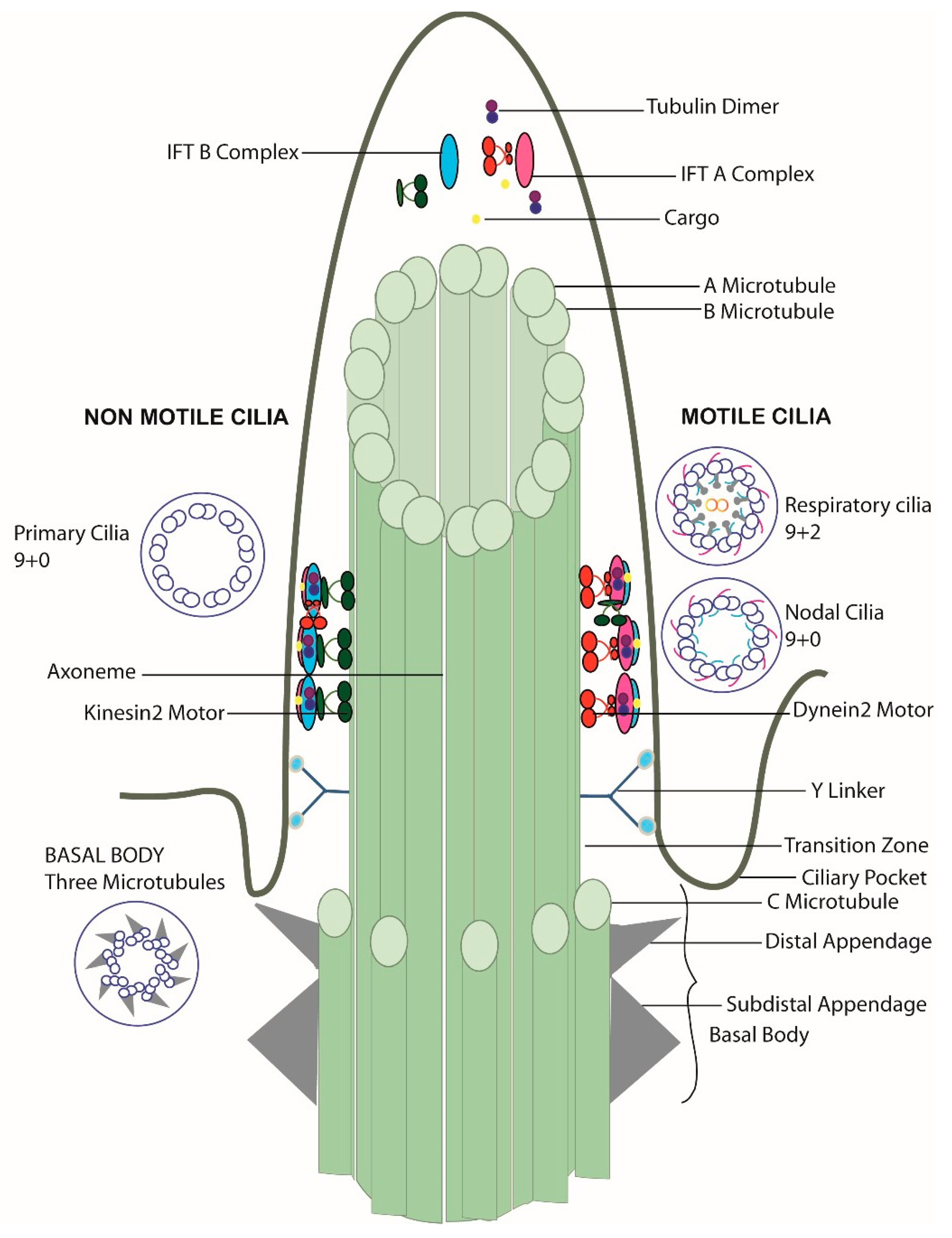
2.2. Primary Cilia Structure
2.3. Primary Ciliogenesis
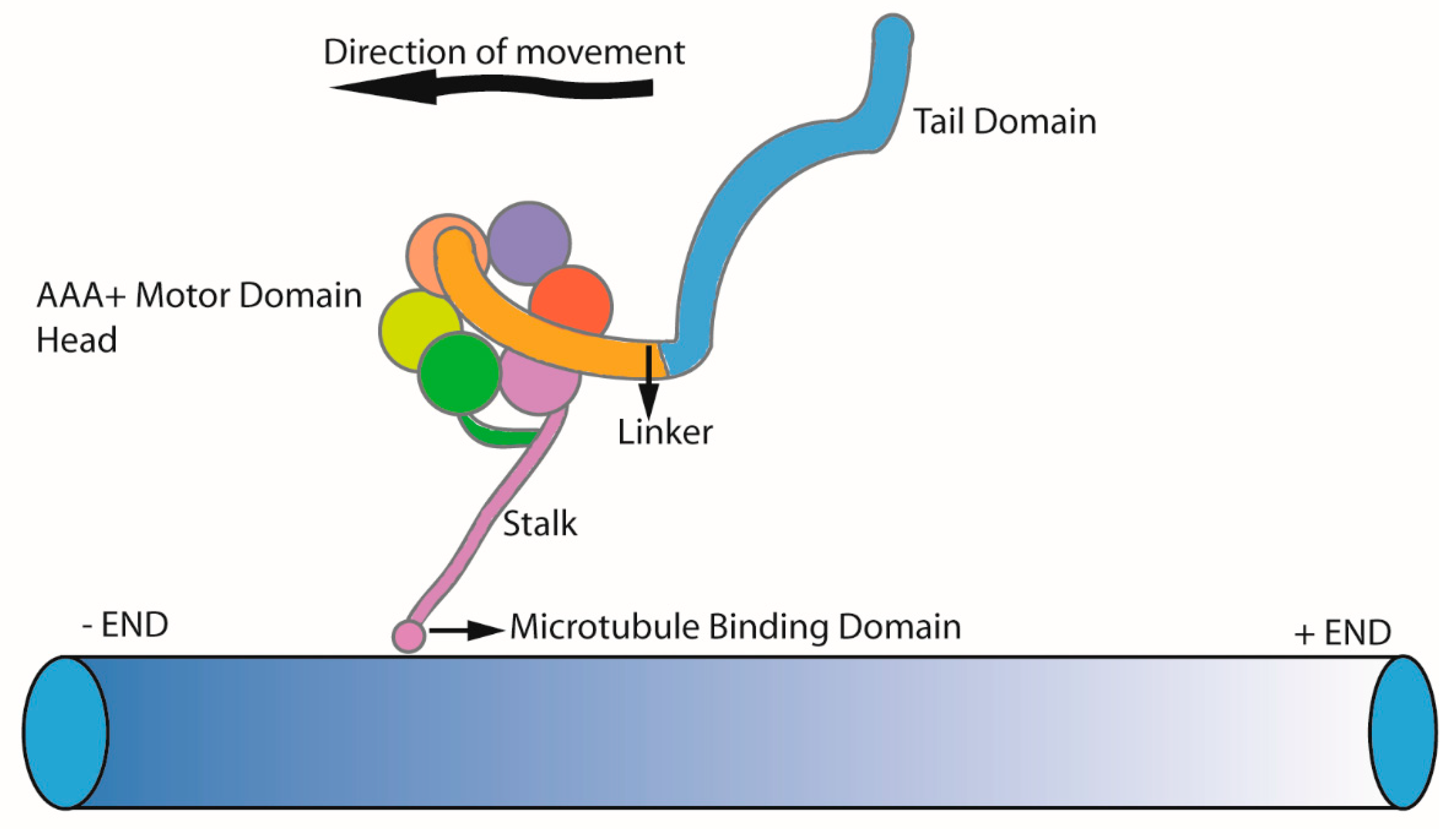
3. Dyneins
4. Intraflagellar Transport Dynein (IFT Dynein or Dynein-2)
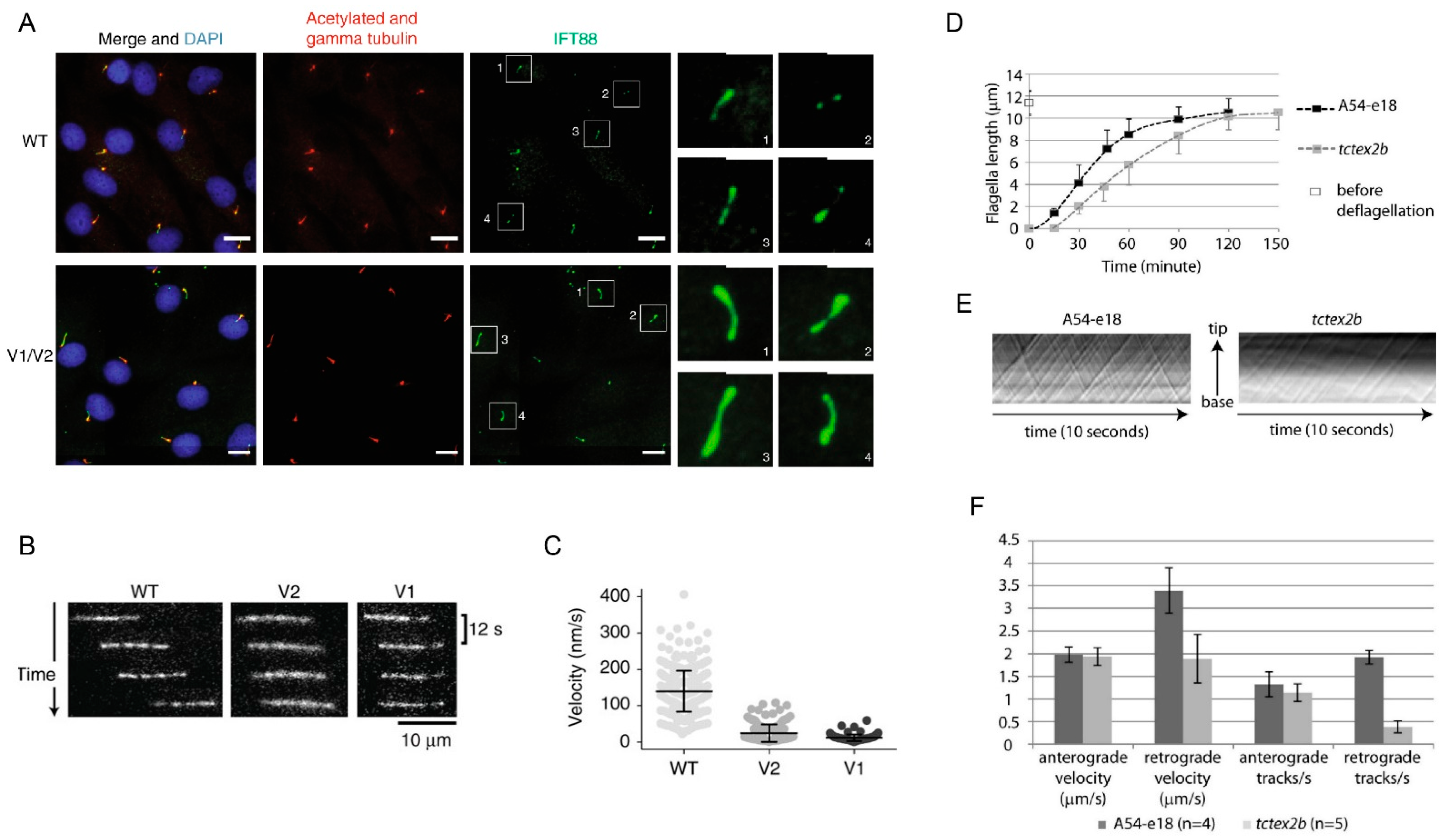
4.1. IFT Dynein Function
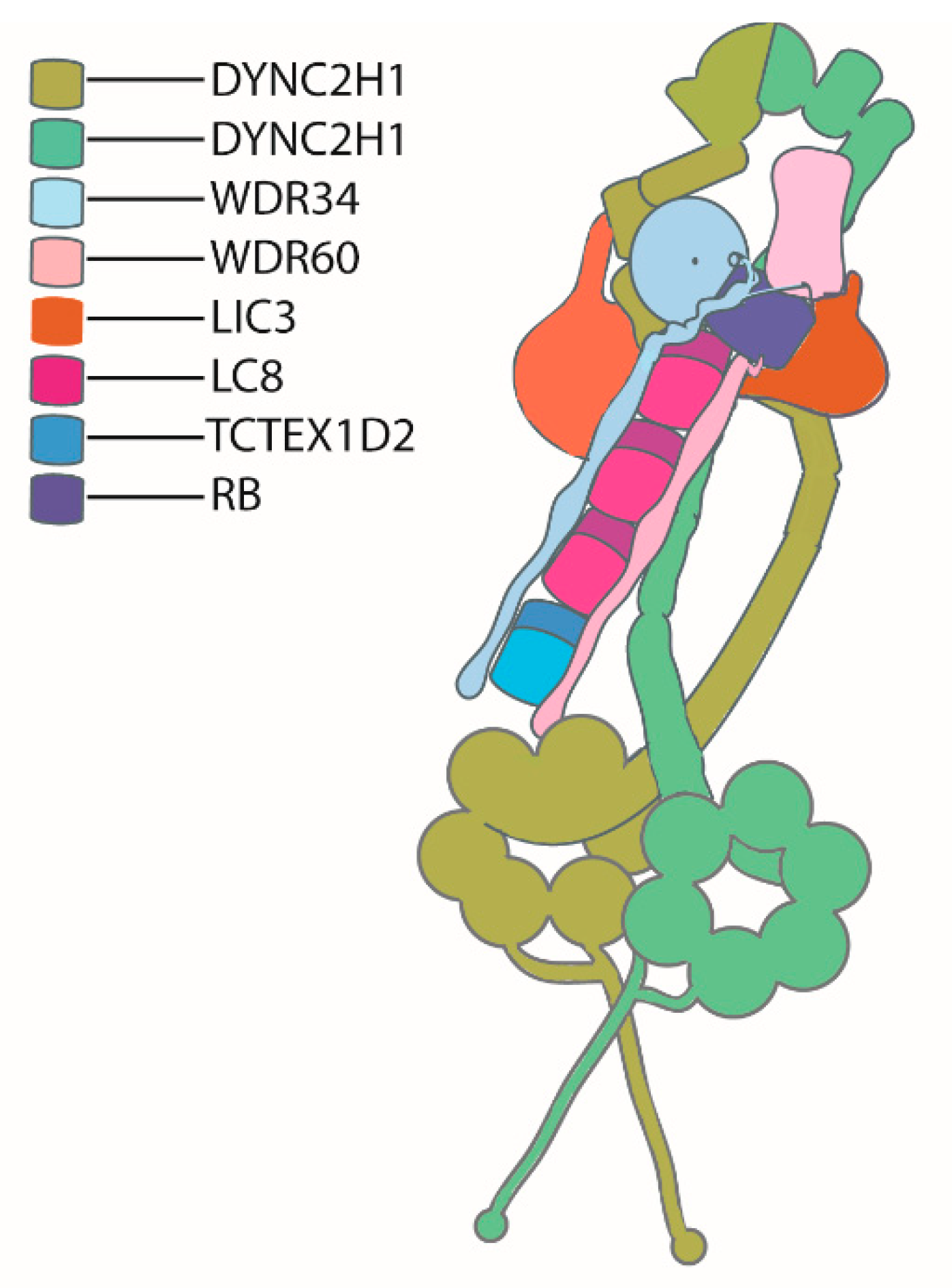
4.2. IFT Dynein Complex Structure
5. Motile Cilia
5.1. Motile Cilia Function
5.2. Motile Ciliogenesis
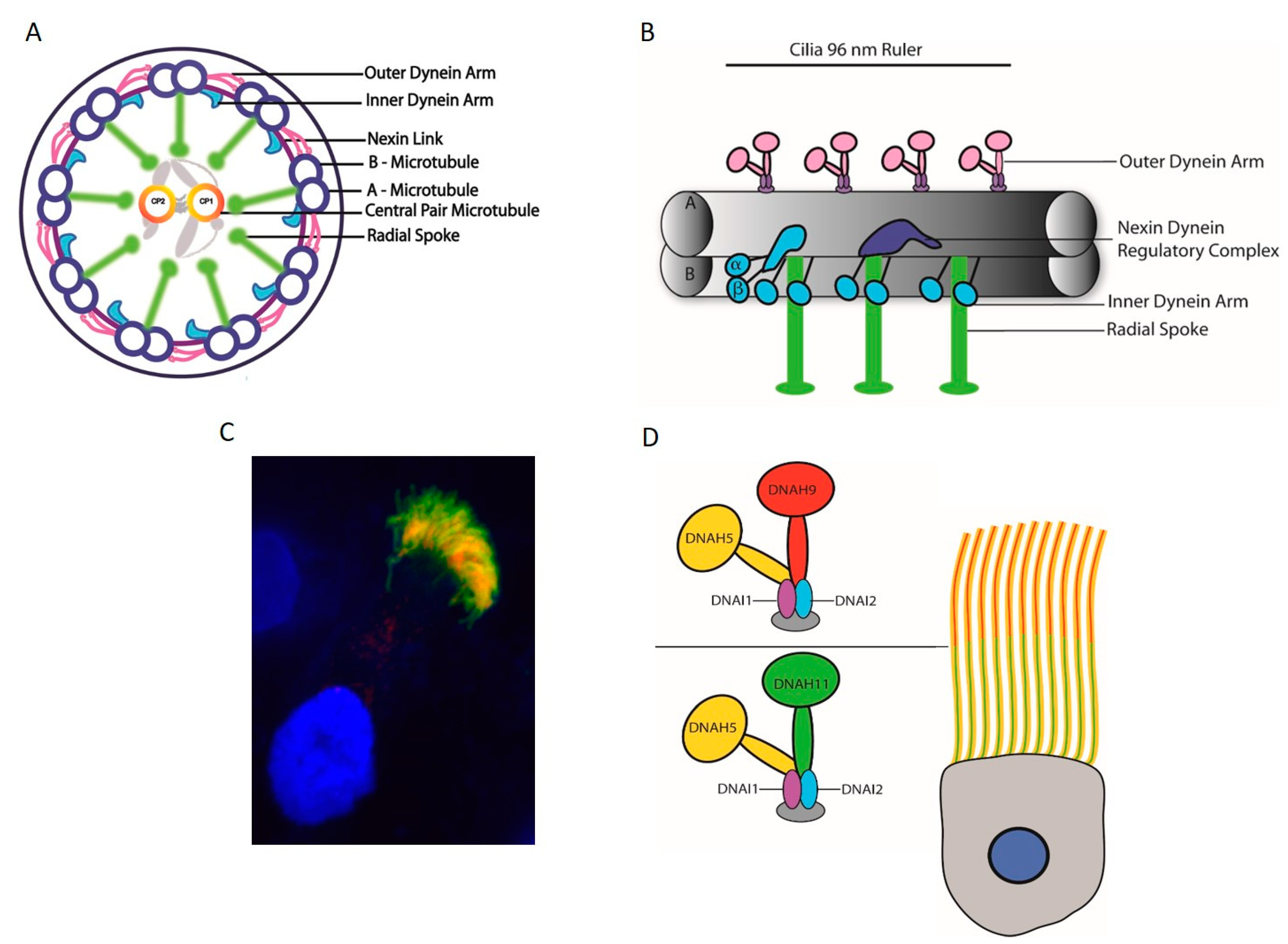
5.3. Motile Cilia Structure
6. Axonemal Dyneins
6.1. Axonemal Dynein Movement to Generate Force for Cilia Beating
6.2. Axonemal Motor Complexes
7. Ciliopathies
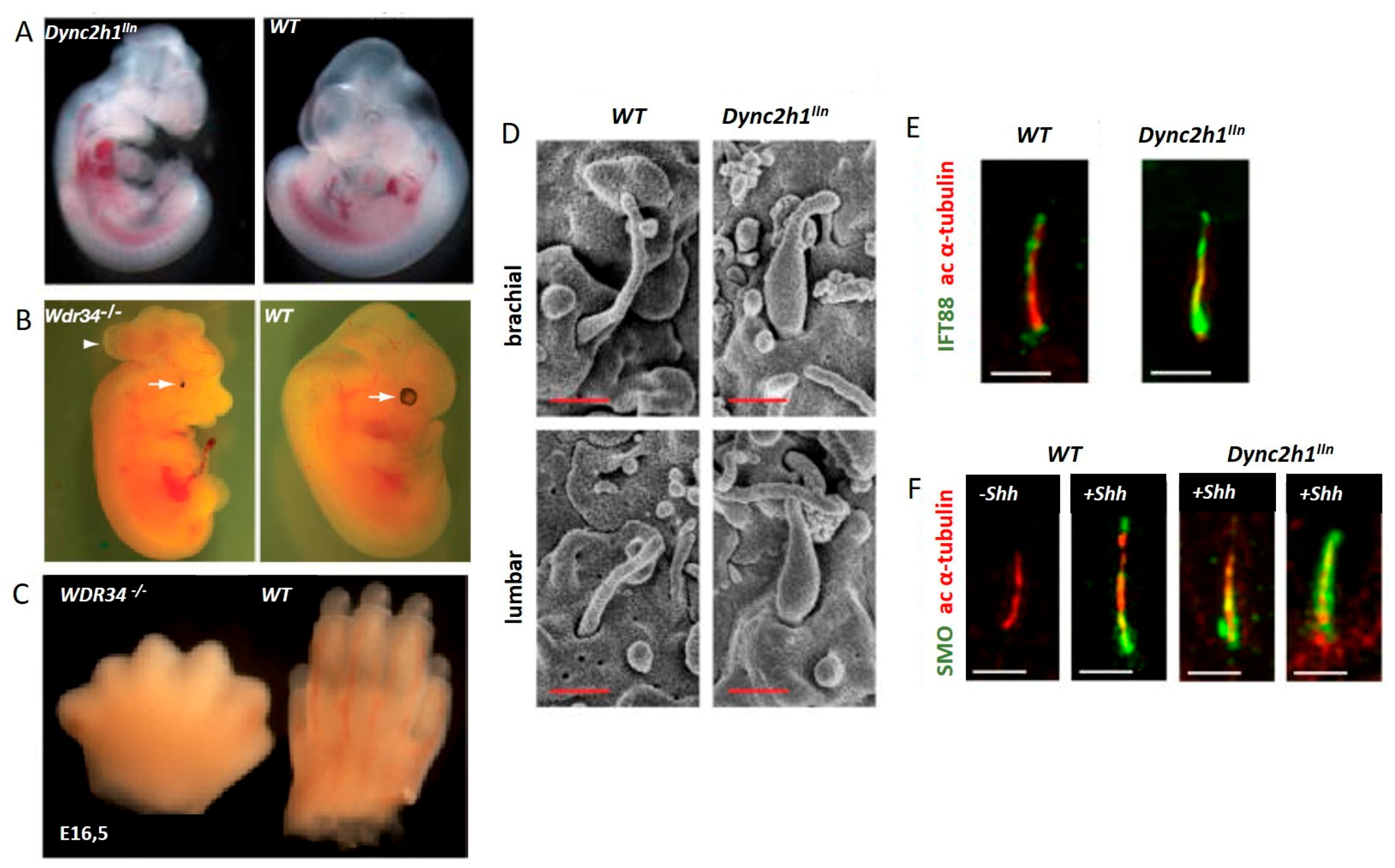
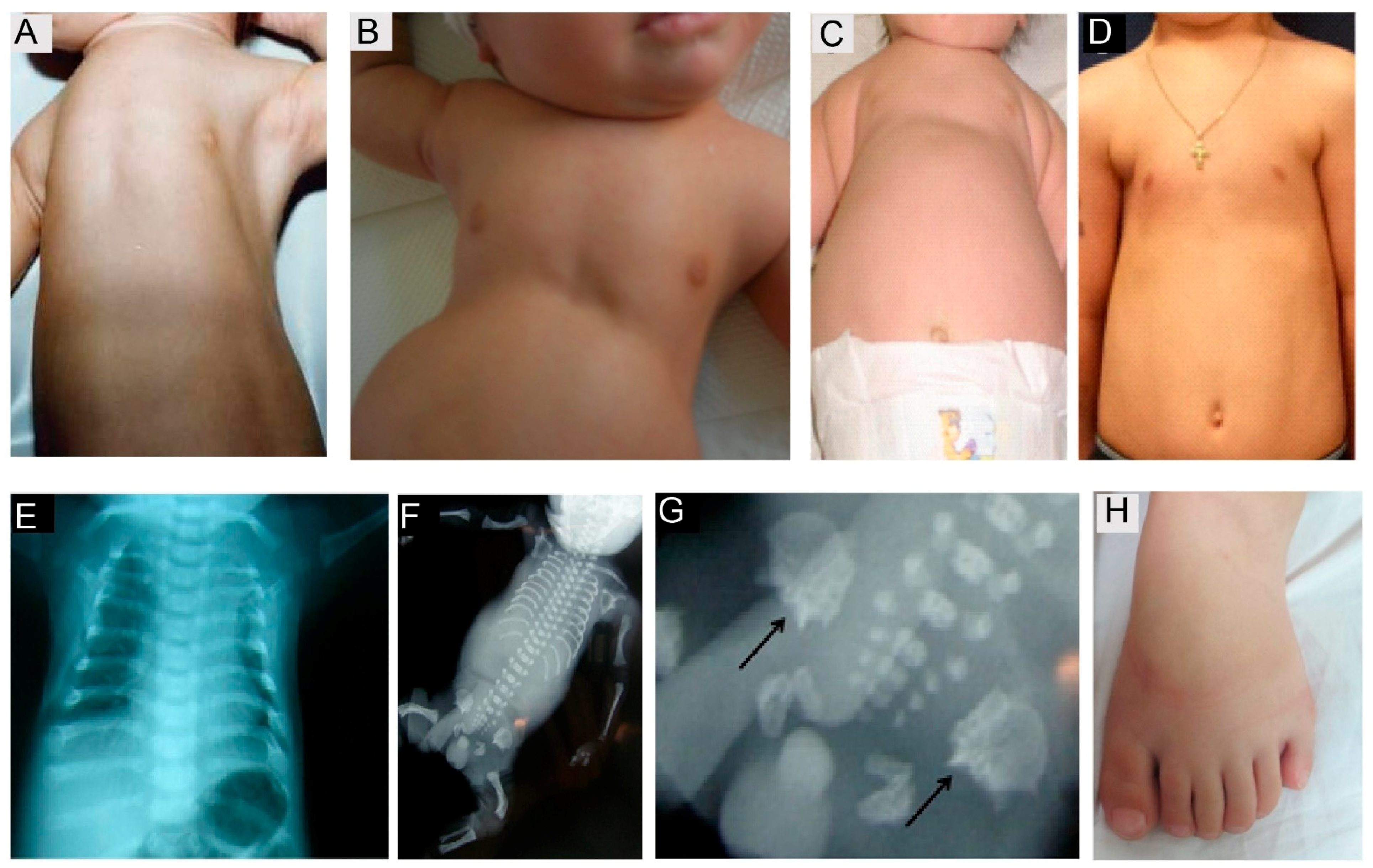
7.1. IFT Dynein Related Ciliopathies
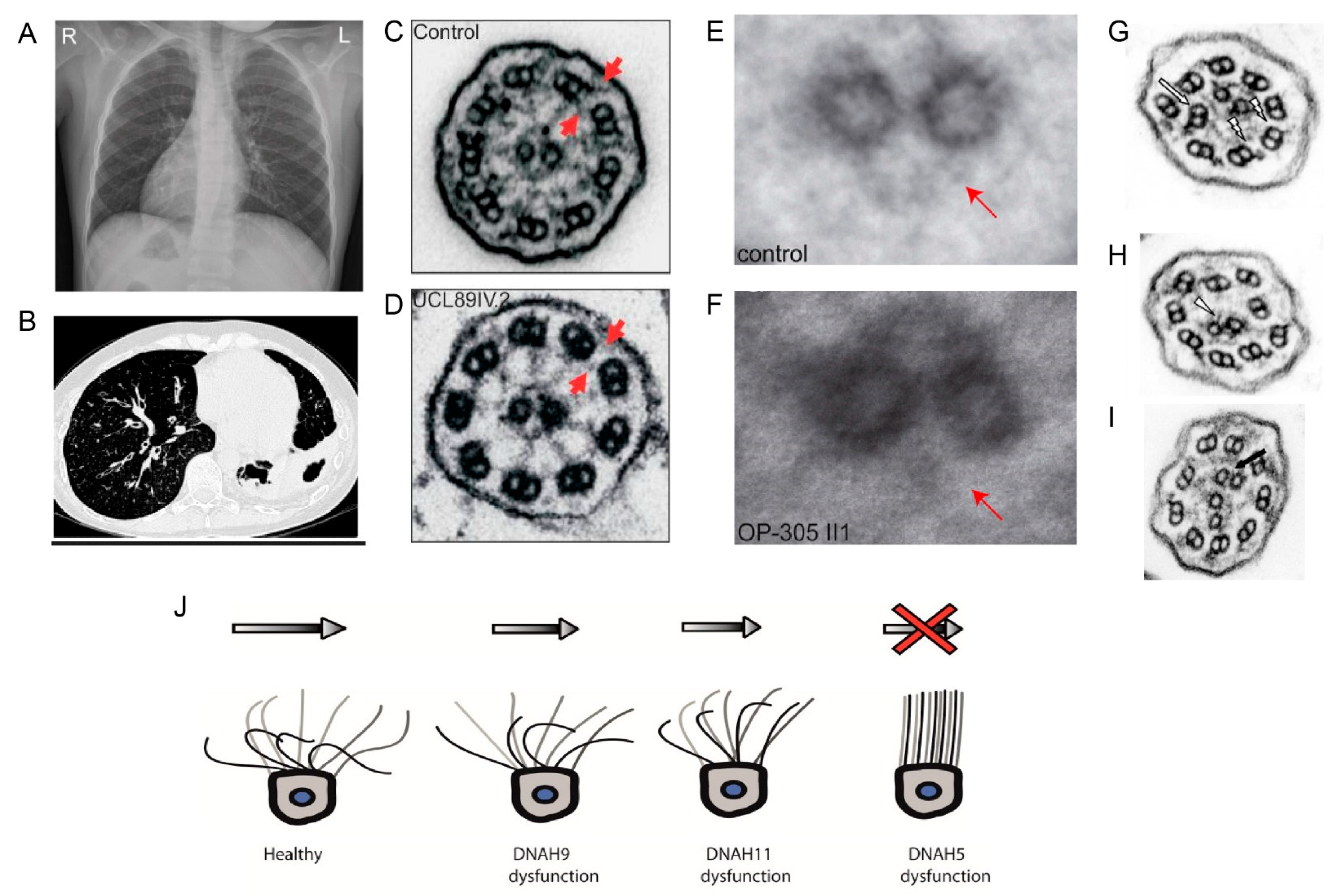
7.2. Axonemal Dynein Related Ciliopathies
8. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Satir, P. Landmarks in cilia research from leeuwenhoek to US. Cell Motil. Cytoskelet. 1995, 32, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.H.; Brugmann, S.A. Sending mixed signals: Cilia-dependent signaling during development and disease. Dev. Biol. 2019, 447, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Choksi, S.P.; Lauter, G.; Swoboda, P.; Roy, S. Switching on cilia: Transcriptional networks regulating ciliogene-sis. Development 2014, 141, 1427–1441. [Google Scholar] [CrossRef]
- Mitchison, H.M.; Valente, E.M. Motile and non-motile cilia in human pathology: From function to phenotypes. J. Pathol. 2016, 241, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Sironen, A.; Shoemark, A.; Patel, M.; Loebinger, M.R.; Mitchison, H.M. Sperm defects in primary ciliary dyskinesia and related causes of male infertility. Cell Mol. Life Sci. 2020, 77, 2029–2048. [Google Scholar] [CrossRef]
- Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 1967, 14, 225–274. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. The evolutionary origin and phylogeny of microtubules, mitotic spindles and eukaryote flagella. Biosystems 1978, 10, 93–114. [Google Scholar] [CrossRef]
- Satir, P.; Guerra, C.; Bell, A.J. Evolution and persistence of the cilium. Cell Motil. Cytoskelet. 2007, 64, 906–913. [Google Scholar] [CrossRef]
- Vincensini, L.; Blisnick, T.; Bastin, P. 1001 model organisms to study cilia and flagella. Biol. Cell 2011, 103, 109–130. [Google Scholar] [CrossRef]
- Malicki, J.J.; Johnson, C.A. The Cilium: Cellular Antenna and Central Processing Unit. Trends Cell Biol. 2017, 27, 126–140. [Google Scholar] [CrossRef]
- Ishikawa, T. Axoneme Structure from Motile Cilia. Cold Spring Harb. Perspect. Biol. 2016, 9, a028076. [Google Scholar] [CrossRef]
- Kempeneers, C.; Chilvers, M.A. To beat, or not to beat, that is question! The spectrum of ciliopathies. Pediatr. Pulmonol. 2018, 53, 1122–1129. [Google Scholar] [CrossRef]
- Behnke, O.; Forer, A. Evidence for Four Classes of Microtubules in Individual Cells. J. Cell Sci. 1967, 2, 169–192. [Google Scholar] [CrossRef]
- Wloga, D.; Gaertig, J. Post-translational modifications of microtubules. J. Cell Sci. 2010, 123, 3447–3455. [Google Scholar] [CrossRef] [PubMed]
- Orbach, R.; Howard, J. The dynamic and structural properties of axonemal tubulins support the high length stability of cilia. Nat. Commun. 2019, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Kozminski, K.G.; Johnson, K.A.; Forscher, P.; Rosenbaum, J.L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 1993, 90, 5519–5523. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Marshall, W.F. Intraflagellar Transport and Ciliary Dynamics. Cold Spring Harb. Perspect. Biol. 2017, 9, a021998. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Rosenbaum, J.L. Intraflagellar transport (ift) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 2008, 85, 23–61. [Google Scholar]
- Prevo, B.; Scholey, J.M.; Peterman, E.J.G. Intraflagellar transport: Mechanisms of motor action, cooperation, and cargo delivery. FEBS J. 2017, 284, 2905–2931. [Google Scholar] [CrossRef]
- Nachury, M.V.; Loktev, A.V.; Zhang, Q.; Westlake, C.J.; Peränen, J.; Merdes, A.; Slusarski, D.; Scheller, R.H.; Bazan, J.F.; Sheffield, V.; et al. A Core Complex of BBS Proteins Cooperates with the GTPase Rab8 to Promote Ciliary Membrane Biogenesis. Cell 2007, 129, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.-F.; Johnson, E.C.; Sakai, T.; Cochran, D.; Ballif, B.A.; Rush, J.; Pazour, G.; Ikebe, M.; Witman, G.B. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 2009, 187, 1117–1132. [Google Scholar] [CrossRef]
- Wingfield, J.; Lechtreck, K.-F.; Lorentzen, E. Trafficking of ciliary membrane proteins by the intraflagellar transport/BBSome machinery. Essays Biochem. 2018, 62, 753–763. [Google Scholar] [CrossRef]
- Benmerah, A. The ciliary pocket. Curr. Opin. Cell Biol. 2013, 25, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, S.P. Reconstructions of Centriole Formation and Ciliogenesis in Mammalian Lungs. J. Cell Sci. 1968, 3, 207–230. [Google Scholar] [CrossRef]
- Brown, J.M.; Witman, G.B. Cilia and diseases. Bioscience 2014, 64, 1126–1137. [Google Scholar] [CrossRef]
- Pazour, G.; Dickert, B.L.; Vucica, Y.; Seeley, E.S.; Rosenbaum, J.L.; Witman, G.; Cole, D.G. Chlamydomonas IFT88 and Its Mouse Homologue, Polycystic Kidney Disease Gene Tg737, Are Required for Assembly of Cilia and Flagella. J. Cell Biol. 2000, 151, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Anvarian, Z.; Mykytyn, K.; Mukhopadhyay, S.; Pedersen, L.; Christensen, S.T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 2019, 15, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Guemez-Gamboa, A.; Coufal, N.; Gleeson, J.G. Primary Cilia in the Developing and Mature Brain. Neuron 2014, 82, 511–521. [Google Scholar] [CrossRef] [PubMed]
- May-Simera, H.; Nagel-Wolfrum, K.; Wolfrum, U. Cilia—The sensory antennae in the eye. Prog. Retin. Eye Res. 2017, 60, 144–180. [Google Scholar] [CrossRef]
- Pala, R.; A lOmari, N.; Nauli, S.M. Primary Cilium-Dependent Signaling Mechanisms. Int. J. Mol. Sci. 2017, 18, 2272. [Google Scholar] [CrossRef]
- Falk, N.; Lösl, M.; Schröder, N.; Gießl, A. Specialized Cilia in Mammalian Sensory Systems. Cells 2015, 4, 500. [Google Scholar] [CrossRef]
- Okamoto, S.; Chaya, T.; Omori, Y.; Kuwahara, R.; Kubo, S.; Sakaguchi, H.; Furukawa, T. Ick ciliary kinase is essential for planar cell polarity formation in inner ear hair cells and hearing function. J. Neurosci. 2017, 37, 2073–2085. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Somlo, S.; Makova, S.; Tian, X.; Brueckner, M. Two Populations of Node Monocilia Initiate Left-Right Asymmetry in the Mouse. Cell 2003, 114, 61–73. [Google Scholar] [CrossRef]
- Nonaka, S.; Tanaka, Y.; Okada, Y.; Takeda, S.; Harada, A.; Kanai, Y.; Kido, M.; Hirokawa, N. Randomization of Left–Right Asymmetry due to Loss of Nodal Cilia Generating Leftward Flow of Extraembryonic Fluid in Mice Lacking KIF3B Motor Protein. Cell 1998, 95, 829–837. [Google Scholar] [CrossRef]
- Sun, S.; Fisher, R.L.; Bowser, S.S.; Pentecost, B.T.; Sui, H. Three-dimensional architecture of epithelial primary cilia. Proc. Natl. Acad. Sci. USA 2019, 116, 9370–9379. [Google Scholar] [CrossRef] [PubMed]
- Bernabé-Rubio, M.; Alonso, M.A. Routes and machinery of primary cilium biogenesis. Cell. Mol. Life Sci. 2017, 74, 4077–4095. [Google Scholar] [CrossRef]
- Gibbons, I.R. Studies on the protein components of cilia from tetrahymena pyriformis. Proc. Natl. Acad. Sci. USA 1963, 50, 1002–1010. [Google Scholar] [CrossRef]
- Gibbons, I.R.; Rowe, A.J. Dynein: A Protein with Adenosine Triphosphatase Activity from Cilia. Science 1965, 149, 424–426. [Google Scholar] [CrossRef] [PubMed]
- Toropova, K.; Zalyte, R.; Mukhopadhyay, A.; Mladenov, M.; Carter, A.P.; Roberts, A.J. Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nat. Struct. Mol. Biol. 2019, 26, 823–829. [Google Scholar] [CrossRef]
- King, S.M. Axonemal dynein arms. Cold Spring Harb. Persp. Biol. 2016, 8, a028100. [Google Scholar] [CrossRef]
- Roberts, A.J.; Kon, T.; Knight, P.J.; Sutoh, K.; Burgess, S.A. Functions and mechanics of dynein motor proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 713–726. [Google Scholar] [CrossRef]
- Reck-Peterson, S.L.; Redwine, W.B.; Vale, R.D.; Carter, A.P. The cytoplasmic dynein transport machinery and its many cargoes. Nat. Rev. Mol. Cell Biol. 2018, 19, 382–398. [Google Scholar] [CrossRef]
- Roberts, A.J. Emerging mechanisms of dynein transport in the cytoplasm versus the cilium. Biochem. Soc. Trans. 2018, 46, 967–982. [Google Scholar] [CrossRef]
- Grotjahn, D.A.; Lander, G.C. Setting the dynein motor in motion: New insights from electron tomography. J. Biol. Chem. 2019, 294, 13202–13217. [Google Scholar] [CrossRef] [PubMed]
- Pazour, G.J.; Wilkerson, C.G.; Witman, G.B. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (ift). J. Cell Biol. 1998, 141, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Ocbina, P.J.; Eggenschwiler, J.T.; Moskowitz, I.; Anderson, K.V. Complex interactions between genes controlling trafficking in primary cilia. Nat. Genet. 2011, 6, 547–553. [Google Scholar] [CrossRef]
- Nachury, M.V. How do cilia organize signalling cascades? Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130465. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.L.; Lambacher, N.J.; Li, C.; Mohan, S.; Williams, C.L.; Inglis, P.N.; Yoder, B.K.; Blacque, O.E.; Leroux, M.R. Role for intraflagellar transport in building a functional transition zone. EMBO Rep. 2018, 19, e45862. [Google Scholar] [CrossRef]
- Vuolo, L.; Stevenson, N.L.; Heesom, K.J.; Stephens, D.J. Dynein-2 intermediate chains play crucial but distinct roles in primary cilia formation and function. eLife 2018, 7. [Google Scholar] [CrossRef]
- Stepanek, L.; Pigino, G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science 2016, 352, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Diener, D.R.; Stepanek, L.; Pigino, G. The cryo-em structure of intraflagellar transport trains reveals how dynein is inactivated to ensure unidirectional anterograde movement in cilia. Nat. Cell Biol. 2018, 20, 1250–1255. [Google Scholar] [CrossRef]
- Pazour, G.J.; Dickert, B.L.; Witman, G.B. The dhc1b (dhc2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 1999, 144, 473–481. [Google Scholar] [CrossRef]
- Hou, Y.; Witman, G.B. Dynein and intraflagellar transport. Exp. Cell Res. 2015, 334, 26–34. [Google Scholar] [CrossRef]
- Blisnick, T.; Buisson, J.; Absalon, S.; Marie, A.; Cayet, N.; Bastin, P. The intraflagellar transport dynein complex of trypanosomes is made of a heterodimer of dynein heavy chains and of light and intermediate chains of distinct functions. Mol. Biol. Cell 2014, 25, 2620–2633. [Google Scholar] [CrossRef]
- Perrone, C.A.; Tritschler, D.; Taulman, P.; Bower, R.; Yoder, B.K.; Porter, M.E. A novel dynein light intermediate chain colocalizes with the retrograde motor for intraflagellar transport at sites of axoneme assembly in chlamydomonas and mammalian cells. Mol. Biol. Cell 2003, 14, 2041–2056. [Google Scholar] [CrossRef]
- Grissom, P.M.; Vaisberg, E.A.; McIntosh, J.R. Identification of a novel light intermediate chain (d2lic) for mammalian cytoplasmic dynein. Mol. Biol. Cell 2002, 13, 817–829. [Google Scholar]
- Mikami, A.; Tynan, S.H.; Hama, T.; Luby-Phelps, K.; Saito, T.; Crandall, J.E.; Besharse, J.C.; Vallee, R.B. Molecular structure of cytoplasmic dynein 2 and its distribution in neuronal and ciliated cells. J. Cell Sci. 2002, 115, 4801–4808. [Google Scholar] [CrossRef]
- Huber, C.; Wu, S.; Kim, A.S.; Sigaudy, S.; Sarukhanov, A.; Serre, V.; Baujat, G.; Le Quan Sang, K.H.; Rimoin, D.L.; Cohn, D.H.; et al. Wdr34 mutations that cause short-rib polydactyly syndrome type iii/severe asphyxiating thoracic dysplasia reveal a role for the nf-kappab pathway in cilia. Am. J. Hum. Genet. 2013, 93, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wang, R.; Li, B.; Yang, Y.; Zhai, Z.; Chen, D.Y. Wdr34 is a novel tak1-associated suppressor of the il-1r/tlr3/tlr4-induced nf-kappab activation pathway. Cell Mol. Life Sci. 2009, 66, 2573–2584. [Google Scholar] [CrossRef] [PubMed]
- McInerney-Leo, A.; Schmidts, M.; Cortes, C.; Leo, P.J.; Gener, B.; Courtney, A.D.; Gardiner, B.; Harris, J.A.; Lu, Y.; Marshall, M.; et al. Short-Rib Polydactyly and Jeune Syndromes Are Caused by Mutations in WDR60. Am. J. Hum. Genet. 2013, 93, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Pedersen, L.; Patel-King, R.S.; King, S.M. Chlamydomonas FAP133 is a dynein intermediate chain associated with the retrograde intraflagellar transport motor. J. Cell Sci. 2007, 120, 3653–3665. [Google Scholar] [CrossRef] [PubMed]
- Patel-King, R.S.; Gilberti, R.M.; Hom, E.F.Y.; King, S.M. WD60/FAP163 is a dynein intermediate chain required for retrograde intraflagellar transport in cilia. Mol. Biol. Cell 2013, 24, 2668–2677. [Google Scholar] [CrossRef] [PubMed]
- Asante, D.; Stevenson, N.; Stephens, D. Subunit composition of the human cytoplasmic dynein-2 complex. J. Cell Sci. 2014, 127, 4774–4787. [Google Scholar] [CrossRef]
- Gholkar, A.; Senese, S.; Lo, Y.-C.; Capri, J.; Deardorff, W.J.; Dharmarajan, H.; Contreras, E.; Hodara, E.; Whitelegge, J.P.; Jackson, P.K.; et al. Tctex1d2 associates with short-rib polydactyly syndrome proteins and is required for ciliogenesis. Cell Cycle 2015, 14, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.P. Cilia, calcium and the basis of left-right asymmetry. BMC Biol. 2012, 10, 102. [Google Scholar] [CrossRef]
- Spassky, N.; Meunier, A. The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol. 2017, 18, 423–436. [Google Scholar] [CrossRef]
- Spassky, N.; Merkle, F.T.; Flames, N.; Tramontin, A.D.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 2005, 25, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Houtmeyers, E.; Gosselink, R.; Gayan-Ramirez, G.; Decramer, M. Regulation of mucociliary clearance in health and disease. Eur. Respir. J. 1999, 13, 1177–1188. [Google Scholar] [CrossRef]
- Afzelius, B.A. Cilia-related diseases. J. Pathol. 2004, 204, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Hanasoge, S.; Hesketh, P.J.; Alexeev, A. Metachronal motion of artificial magnetic cilia. Soft Matter 2018, 14, 3689–3693. [Google Scholar] [CrossRef]
- Bloodgood, R.A. Sensory reception is an attribute of both primary cilia and motile cilia. J. Cell Sci. 2010, 123, 505–509. [Google Scholar] [CrossRef]
- Lorenzo, I.M.; Liedtke, W.; Sanderson, M.J.; Valverde, M.A. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc. Natl. Acad. Sci. USA 2008, 105, 12611–12616. [Google Scholar] [CrossRef]
- Shah, A.S.; Ben-Shahar, Y.; Moninger, T.O.; Kline, J.N.; Welsh, M.J. Motile Cilia of Human Airway Epithelia Are Chemosensory. Science 2009, 325, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Teilmann, S.C.; Clement, C.A.; Thorup, J.; Byskov, A.G.; Christensen, S.T. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J. Endocrinol. 2006, 191, 525–535. [Google Scholar] [CrossRef]
- Shao, R.; Weijdegard, B.; Fernandez-Rodriguez, J.; Egecioglu, E.; Zhu, C.; Andersson, N.; Thurin-Kjellberg, A.; Bergh, C.; Billig, H. Ciliated epithelial-specific and regional-specific expression and regulation of the estrogen receptor-beta2 in the fallopian tubes of immature rats: A possible mechanism for estrogen-mediated transport process in vivo. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E147–E158. [Google Scholar] [CrossRef][Green Version]
- Shao, R.; Nutu, M.; Karlsson-Lindahl, L.; Benrick, A.; Weijdegard, B.; Lager, S.; Egecioglu, E.; Fernandez-Rodriguez, J.; Gemzell-Danielsson, K.; Ohlsson, C.; et al. Downregulation of cilia-localized il-6r alpha by 17beta-estradiol in mouse and human fallopian tubes. Am. J. Physiol. Cell Physiol. 2009, 297, C140–C151. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Liu, Z.; Cheng, H.T.; Winters, N.; Bader, D.; Kopan, R. Canonical notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of clara versus ciliated cell fate. J. Cell Sci. 2010, 123, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Meunier, A.; Azimzadeh, J. Multiciliated cells in animals. Cold Spring Harb. Persp. Biol. 2016, 8, a028233. [Google Scholar] [CrossRef]
- Cibois, M.; Luxardi, G.; Chevalier, B.; Thomé, V.; Mercey, O.; Zaragosi, L.-E.; Barbry, P.; Pasini, A.; Marcet, B.; Kodjabachian, L. BMP signalling controls the construction of vertebrate mucociliary epithelia. Development 2015, 142, 2352–2363. [Google Scholar] [CrossRef]
- Hiraoka, K.; Inada, H.; Yanai, K.; Osumi, N. Bone Morphogenetic Proteins Inhibit Ciliogenesis of Ependymal Cells in Vitro. Tohoku J. Exp. Med. 2020, 252, 199–208. [Google Scholar] [CrossRef]
- Ma, L.; Quigley, I.; Omran, H.; Kintner, C. Multicilin drives centriole biogenesis via E2f proteins. Genes Dev. 2014, 28, 1461–1471. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, L.; Zhu, Y.; Cao, J.; Li, S.; Huang, Q.; Xu, T.; Huang, X.; Yan, X.; Zhu, X. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat. Cell Biol. 2013, 15, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Wallmeier, J.; Al-Mutairi, D.A.; Chen, C.-T.; Loges, N.T.; Pennekamp, P.; Menchen, T.; Ma, L.; Shamseldin, H.E.; Olbrich, H.; Dougherty, G.W.; et al. Mutations in CCNO result in congenital mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat. Genet. 2014, 46, 646–651. [Google Scholar] [CrossRef]
- Purvis, T.L.; Hearn, T.; Spalluto, C.; Knorz, V.J.; Hanley, K.P.; Sanchez-Elsner, T.; Hanley, N.A.; Wilson, D.I. Transcriptional regulation of the alstrom syndrome gene alms1 by members of the rfx family and sp1. Gene 2010, 460, 20–29. [Google Scholar] [CrossRef]
- Lemeille, S.; Paschaki, M.; Baas, D.; Morlé, L.; Duteyrat, J.-L.; Ait-Lounis, A.; Barras, E.; Soulavie, F.; Jerber, J.; Thomas, J.; et al. Interplay of RFX transcription factors 1, 2 and 3 in motile ciliogenesis. Nucleic Acids Res. 2020, 48, 9019–9036. [Google Scholar] [CrossRef] [PubMed]
- Lalioti, M.-E.; Arbi, M.; Loukas, I.; Kaplani, K.; Kalogeropoulou, A.; Lokka, G.; Kyrousi, C.; Mizi, A.; Georgomanolis, T.; Josipovic, N.; et al. GemC1 governs multiciliogenesis through direct interaction and transcriptional regulation of p73. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed]
- Dehring, D.A.K.; Vladar, E.; Werner, M.E.; Mitchell, J.W.; Hwang, P.; Mitchell, B. Deuterosome-Mediated Centriole Biogenesis. Dev. Cell 2013, 27, 103–112. [Google Scholar] [CrossRef]
- Al Jord, A.; Lemaître, A.-I.; Delgehyr, N.; Faucourt, M.; Spassky, N.; Meunier, A. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nat. Cell Biol. 2014, 516, 104–107. [Google Scholar] [CrossRef]
- Mercey, O.; Levine, M.S.; Lo Mastro, G.M.; Rostaing, P.; Brotslaw, E.; Gomez, V.; Kumar, A.; Spassky, N.; Mitchell, B.J.; Meunier, A.; et al. Massive centriole production can occur in the absence of deuterosomes in multiciliated cells. Nat. Cell Biol. 2019, 21, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Gomperts, B.N.; Gong-Cooper, X.; Hackett, B.P. Foxj1 regulates basal body anchoring to the cytoskeleton of ciliated pulmonary epithelial cells. J. Cell Sci. 2004, 117, 1329–1337. [Google Scholar] [CrossRef]
- Odate, T.; Takeda, S.; Narita, K.; Kawahara, T.D. 9 + 0 and 9 + 2 cilia are randomly dispersed in the mouse node. Microscop 2015, 65, 119–126. [Google Scholar] [CrossRef]
- Loreng, T.D.; Smith, E.F. The Central Apparatus of Cilia and Eukaryotic Flagella. Cold Spring Harb. Perspect. Biol. 2016, 9, a028118. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, Y.; McNeill, N.A.; Witman, G.B. The unity and diversity of the ciliary central apparatus. Philos. Trans. R. Soc. B Biol. Sci. 2019, 375, 20190164. [Google Scholar] [CrossRef]
- Oda, T.; Yanagisawa, H.; Yagi, T.; Kikkawa, M. Mechanosignaling between central apparatus and radial spokes controls axonemal dynein activity. J. Cell Biol. 2014, 204, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Ma, M.; Sze-Tu, E.; Wang, X.; Koh, F.; Zhong, E.D.; Berger, B.; Davis, J.H.; Dutcher, S.K.; Zhang, R.; et al. Structures of radial spokes and associated complexes important for ciliary motility. Nat. Struct. Mol. Biol. 2020, 28, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Osinka, A.; Poprzeczko, M.; Zielińska, M.; Fabczak, H.; Joachimiak, E.; Wloga, D. Ciliary Proteins: Filling the Gaps. Recent Advances in Deciphering the Protein Composition of Motile Ciliary Complexes. Cells 2019, 8, 730. [Google Scholar] [CrossRef]
- Afzelius, B. Electron Microscopy of the Sperm Tail Results Obtained with a New Fixative. J. Biophys. Biochem. Cytol. 1959, 5, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Wais-Steider, J.; Satir, P. Effect of vanadate on gill cilia: Switching mechanism in ciliary beat. J. Supramol. Struct. 1979, 11, 339–347. [Google Scholar] [CrossRef]
- Lin, J.; Nicastro, D. Asymmetric distribution and spatial switching of dynein activity generates ciliary motility. Science 2018, 360, eaar1968. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, R.; Yagi, T. Functional Diversity of Axonemal Dyneins as Assessed by in Vitro and in Vivo Motility Assays of Chlamydomonas Mutants. Zool. Sci. 2014, 31, 633–644. [Google Scholar] [CrossRef]
- Pazour, G.; Agrin, N.S.; Walker, B.L.; Witman, G.B. Identification of predicted human outer dynein arm genes: Candidates for primary ciliary dyskinesia genes. J. Med. Genet. 2005, 43, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Fabczak, H.; Osinka, A. Role of the Novel Hsp90 Co-Chaperones in Dynein Arms’ Preassembly. Int. J. Mol. Sci. 2019, 20, 6174. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, G.W.; Loges, N.T.; Klinkenbusch, J.A.; Olbrich, H.; Pennekamp, P.; Menchen, T.; Raidt, J.; Wallmeier, J.; Werner, C.; Westermann, C.; et al. Dnah11 localization in the proximal region of respiratory cilia defines distinct outer dynein arm complexes. Am. J. Respir. Cell Mol. Biol. 2016, 55, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Fliegauf, M.; Olbrich, H.; Horvath, J.; Wildhaber, J.H.; Zariwala, M.A.; Kennedy, M.; Knowles, M.R.; Omran, H. Mislocalization of DNAH5 and DNAH9 in Respiratory Cells from Patients with Primary Ciliary Dyskinesia. Am. J. Respir. Crit. Care Med. 2005, 171, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Viswanadha, R.; Sale, W.S.; Porter, M.E. Ciliary Motility: Regulation of Axonemal Dynein Motors. Cold Spring Harb. Perspect. Biol. 2017, 9, a018325. [Google Scholar] [CrossRef] [PubMed]
- Höben, I.M.; Hjeij, R.; Olbrich, H.; Dougherty, G.W.; Nöthe-Menchen, T.; Aprea, I.; Frank, D.; Pennekamp, P.; Dworniczak, B.; Wallmeier, J.; et al. Mutations in C11orf70 Cause Primary Ciliary Dyskinesia with Randomization of Left/Right Body Asymmetry Due to Defects of Outer and Inner Dynein Arms. Am. J. Hum. Genet. 2018, 102, 973–984. [Google Scholar] [CrossRef]
- Pazour, G.J.; Quarmby, L.; Smith, A.O.; Desai, P.B.; Schmidts, M. Cilia in cystic kidney and other diseases. Cell Sign. 2020, 69, 109519. [Google Scholar] [CrossRef]
- Chen, X.; Garcelon, N.; Neuraz, A.; Billot, K.; Lelarge, M.; Bonald, T.; Garcia, H.; Martin, Y.; Benoit, V.; Vincent, M.; et al. Phenotypic similarity for rare disease: Ciliopathy diagnoses and subtyping. J. Biomed. Inf. 2019, 100, 103308. [Google Scholar] [CrossRef]
- Wheway, G.; Mitchison, H.M.; Genomics England Research, C. Opportunities and challenges for molecular understanding of ciliopathies-the 100,000 genomes project. Front. Genet. 2019, 10, 127. [Google Scholar] [CrossRef]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef]
- Kulaga, H.M.; Leitch, C.C.; Eichers, E.R.; Badano, J.; Lesemann, A.; Hoskins, B.E.; Lupski, J.R.; Beales, P.L.; Reed, R.R.; Katsanis, N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat. Genet. 2004, 36, 994–998. [Google Scholar] [CrossRef]
- Tsang, S.H.; Aycinena, A.R.P.; Sharma, T. Ciliopathy: Usher Syndrome. Atlas Inher. Ret. Deas. 2018, 1085, 167–170. [Google Scholar] [CrossRef]
- Pazour, G.J. Intraflagellar transport and cilia-dependent renal disease: The ciliary hypothesis of polycystic kidney disease. J. Am. Soc. Nephrol. 2004, 15, 2528–2536. [Google Scholar] [CrossRef]
- Yoder, B.K.; Hou, X.; Guay-Woodford, L.M. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 2002, 13, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Z.; Mai, W.; Li, C.; Cho, S.-Y.; Hao, C.; Moeckel, G.; Zhao, R.; Kim, I.; Wang, J.; Xiong, H.; et al. PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc. Natl. Acad. Sci. USA 2004, 101, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Otto, E.A.; Schermer, B.; Obara, T.; O’Toole, J.F.; Hiller, K.S.; Mueller, A.M.; Ruf, R.G.; Hoefele, J.; Beekmann, F.; Landau, D.; et al. Mutations in invs encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat. Genet. 2003, 34, 413–420. [Google Scholar] [CrossRef]
- Sheffield, V.C.; Carml, R.; Kwltek-Black, A.; Rokhlina, T.; Nishlmura, D.; Duyk, G.M.; Elbedour, K.; Sunden, S.L.; Stone, E.M. Identification of a Bardet-Biedl syndrome locus on chromosome 3 and evaluation of an efficient approach to homozygosity mapping. Hum. Mol. Genet. 1994, 3, 1331–1335. [Google Scholar] [CrossRef]
- Waters, A.M.; Beales, P.L. Ciliopathies: An expanding disease spectrum. Pediatr. Nephrol. 2011, 26, 1039–1056. [Google Scholar] [CrossRef]
- Antony, D.; Nampoory, N.; Bacchelli, C.; Melhem, M.; Wu, K.; James, C.; Beales, P.L.; Hubank, M.; Thomas, D.; Mashankar, A.; et al. Exome sequencing for the differential diagnosis of ciliary chondrodysplasias: Example of a WDR35 mutation case and review of the literature. Eur. J. Med. Genet. 2017, 60, 658–666. [Google Scholar] [CrossRef]
- Patel, H.; Li, J.; Herrero, A.; Kroboth, J.; Byron, A.; Von Kriegsheim, A.; Brunton, V.; Carragher, N.; Hurd, T.; Frame, M. Novel roles of PRK1 and PRK2 in cilia and cancer biology. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Knowles, M.R.; Zariwala, M.; Leigh, M. Primary Ciliary Dyskinesia. Clin. Chest Med. 2016, 37, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Wallmeier, J.; Frank, D.; Shoemark, A.; Nothe-Menchen, T.; Cindric, S.; Olbrich, H.; Loges, N.T.; Aprea, I.; Dougherty, G.W.; Pennekamp, P.; et al. De novo mutations in foxj1 result in a motile ciliopathy with hydrocephalus and randomization of left/right body asymmetry. Am. J. Hum. Genet. 2019, 105, 1030–1039. [Google Scholar] [CrossRef]
- Powles-Glover, N. Cilia and ciliopathies: Classic examples linking phenotype and genotype—An overview. Reprod. Toxicol. 2014, 48, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Oud, M.M.; Lamers, I.J.C.; Arts, H.H. Ciliopathies: Genetics in Pediatric Medicine. J. Pediatr. Genet. 2016, 6, 018–029. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.J.; Krentz, A.D.; Finta, K.M.; Okorie, U.C.; Haws, R.M. Thoraco-Abdominal Abnormalities in Bardet-Biedl Syndrome: Situs Inversus and Heterotaxy. J. Pediatr. 2019, 204, 31–37. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, K.H.; Ma, J.; Gao, X.; Yu, K.; Zhang, H.Y.; Wang, Y.; Zhang, Z.X.; Li, W.G.; Liu, Y.; et al. Clinical and genetic analysis of a family with Joubert syndrome type 10 caused by OFD1 gene mutation. Zhonghua er ke za zhi Chin. J. Pediatr. 2017, 55, 131–134. [Google Scholar]
- Porter, M.E.; Bower, R.; Knott, J.A.; Byrd, P.; Dentler, W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in chlamydomonas. Mol. Biol. Cell 1999, 10, 693–712. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Peterson, A.; Tao, K.; Wang, B. Loss of dynein-2 intermediate chain Wdr34 results in defects in retrograde ciliary protein trafficking and Hedgehog signaling in the mouse. Hum. Mol. Genet. 2017, 26, 2386–2397. [Google Scholar] [CrossRef]
- Baujat, G.; Huber, C.; El Hokayem, J.; Caumes, R.; Thanh, C.D.N.; David, A.; Delezoide, A.-L.; Dieux-Coeslier, A.; Estournet, B.; Francannet, C.; et al. Asphyxiating thoracic dysplasia: Clinical and molecular review of 39 families. J. Med. Genet. 2013, 50, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Dagoneau, N.; Goulet, M.; Genevieve, D.; Sznajer, Y.; Martinovic, J.; Smithson, S.; Huber, C.; Baujat, G.; Flori, E.; Tecco, L.; et al. Dync2h1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syn-drome, type iii. Am. J. Hum. Genet. 2009, 84, 706–711. [Google Scholar] [CrossRef]
- Schmidts, M.; Vodopiutz, J.; Christou-Savina, S.; Cortes, C.; McInerney-Leo, A.M.; Emes, R.; Arts, H.H.; Tüysüz, B.; D’Silva, J.; Leo, P.J.; et al. Mutations in the Gene Encoding IFT Dynein Complex Component WDR34 Cause Jeune Asphyxiating Thoracic Dystrophy. Am. J. Hum. Genet. 2013, 93, 932–944. [Google Scholar] [CrossRef]
- Taylor, S.P.; University of Washington Center for Mendelian Genomics Consortium; Dantas, T.J.; Duran, I.; Wu, S.; Lachman, R.S.; Nelson, S.F.; Cohn, D.H.; Vallee, R.B.; Krakow, D. Mutations in DYNC2LI1 disrupt cilia function and cause short rib polydactyly syndrome. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, M.; Hou, Y.; Cortes, C.R.; Mans, D.A.; Huber, C.; Boldt, K.; Patel, M.; van Reeuwijk, J.; Plaza, J.M.; van Beersum, S.E.; et al. Tctex1d2 mutations underlie jeune asphyxiating thoracic dystrophy with impaired retrograde intraflagellar transport. Nat. Commun. 2015, 6, 7074. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, M. Clinical genetics and pathobiology of ciliary chondrodysplasias. J. Pediatr. Genet. 2015, 3, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Schmidts, M.; Arts, H.H.; Bongers, E.M.; Yap, Z.; Oud, M.M.; Antony, D.; Duijkers, L.; Emes, R.D.; Stalker, J.; Yntema, J.B.; et al. Exome sequencing identifies dync2h1 mutations as a common cause of asphyxiating thoracic dystrophy (jeune syndrome) without major polydactyly, renal or retinal involvement. J. Med. Genet. 2013, 50, 309–323. [Google Scholar] [CrossRef]
- Halbritter, J.; Bizet, A.; Schmidts, M.; Porath, J.D.; Braun, D.A.; Gee, H.Y.; McInerney-Leo, A.; Krug, P.; Filhol, E.; Davis, E.; et al. Defects in the IFT-B Component IFT172 Cause Jeune and Mainzer-Saldino Syndromes in Humans. Am. J. Hum. Genet. 2013, 93, 915–925. [Google Scholar] [CrossRef]
- Vig, A.; Genomics England Research Consortium; Poulter, J.A.; Ottaviani, D.; Tavares, E.; Toropova, K.; Tracewska, A.M.; Mollica, A.; Kang, J.; Kehelwathugoda, O.; et al. DYNC2H1 hypomorphic or retina-predominant variants cause nonsyndromic retinal degeneration. Genet. Med. 2020, 22, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Thiel, C.; Kessler, K.; Gießl, A.; Dimmler, A.; Shalev, S.A.; von der Haar, S.; Zenker, M.; Zahnleiter, D.; Stöss, H.; Beinder, E.; et al. NEK1 Mutations Cause Short-Rib Polydactyly Syndrome Type Majewski. Am. J. Hum. Genet. 2011, 88, 106–114. [Google Scholar] [CrossRef]
- Mill, P.; Lockhart, P.J.; Fitzpatrick, E.; Mountford, H.; Hall, E.; Reijns, M.; Keighren, M.; Bahlo, M.; Bromhead, C.J.; Budd, P.; et al. Human and Mouse Mutations in WDR35 Cause Short-Rib Polydactyly Syndromes Due to Abnormal Ciliogenesis. Am. J. Hum. Genet. 2011, 88, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Niceta, M.; Margiotti, K.; Digilio, M.; Guida, V.; Bruselles, A.; Pizzi, S.; Ferraris, A.; Memo, L.; Laforgia, N.; Dentici, M.; et al. Biallelic mutations in DYNC2LI1 are a rare cause of Ellis-van Creveld syndrome. Clin. Genet. 2017, 93, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Solaguren-Beascoa, M.; Bujakowska, K.M.; Mejecase, C.; Emmenegger, L.; Orhan, E.; Neuille, M.; Mohand-Said, S.; Condroyer, C.; Lancelot, M.E.; Michiels, C.; et al. Wdr34, a candidate gene for non-syndromic rod-cone dystrophy. Clin. Genet. 2020, 99, 298–302. [Google Scholar] [CrossRef]
- Schmidts, M.; Frank, V.; Eisenberger, T.; Al Turki, S.; Bizet, A.A.; Antony, D.; Rix, S.; Decker, C.; Bachmann, N.; Bald, M.; et al. Combined ngs approaches identify mutations in the intraflagellar transport gene ift140 in skeletal ciliopathies with early progressive kidney disease. Hum. Mut. 2013, 34, 714–724. [Google Scholar] [CrossRef]
- Echelard, Y.; Epstein, D.J.; St-Jacques, B.; Shen, L.; Mohler, J.; McMahon, J.A.; McMahon, A.P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993, 75, 1417–1430. [Google Scholar] [CrossRef]
- Bitgood, M.J.; Shen, L.; McMahon, A.P. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr. Biol. 1996, 6, 298–304. [Google Scholar] [CrossRef]
- Wijgerde, M.; Ooms, M.; Hoogerbrugge, J.W.; Grootegoed, J.A. Hedgehog Signaling in Mouse Ovary: Indian Hedgehog and Desert Hedgehog from Granulosa Cells Induce Target Gene Expression in Developing Theca Cells. Endocrinology 2005, 146, 3558–3566. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Litingtung, Y.; Lee, E.; Young, K.E.; Corden, J.L.; Westphal, H.; Beachy, P.A. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nat. Cell Biol. 1996, 383, 407–413. [Google Scholar] [CrossRef] [PubMed]
- St-Jacques, B.; Hammerschmidt, M.; McMahon, A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999, 13, 2072–2086. [Google Scholar] [CrossRef]
- Tempe, D.; Casas, M.; Karaz, S.; Blanchet-Tournier, M.F.; Concordet, J.P. Multisite protein kinase a and glycogen synthase kinase 3beta phosphorylation leads to gli3 ubiquitination by scfbetatrcp. Mol. Cell Biol. 2006, 26, 4316–4326. [Google Scholar] [CrossRef] [PubMed]
- Carney, T.J.; Ingham, P.W. Drugging Hedgehog: Signaling the pathway to translation. BMC Biol. 2013, 11, 37. [Google Scholar] [CrossRef][Green Version]
- Onoufriadis, A.; Paff, T.; Antony, D.; Shoemark, A.; Micha, D.; Kuyt, B.; Schmidts, M.; Petridi, S.; Dankert-Roelse, J.E.; Haarman, E.G.; et al. Splice-Site Mutations in the Axonemal Outer Dynein Arm Docking Complex Gene CCDC114 Cause Primary Ciliary Dyskinesia. Am. J. Hum. Genet. 2013, 92, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Fliegauf, M.; Benzing, T.; Omran, H. When cilia go bad: Cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007, 8, 880–893. [Google Scholar] [CrossRef]
- Noone, P.G.; Leigh, M.W.; Sannuti, A.; Minnix, S.L.; Carson, J.L.; Hazucha, M.; Zariwala, M.A.; Knowles, M.R. Primary ciliary dyskinesia: Diagnostic and phenotypic features. Am. J. Respir. Crit. Care Med. 2004, 169, 459–467. [Google Scholar] [CrossRef]
- Best, S.; Shoemark, A.; Rubbo, B.; Patel, M.P.; Fassad, M.; Dixon, M.; Rogers, A.V.; Hirst, R.A.; Rutman, A.; Ollosson, S.; et al. Risk factors for situs defects and congenital heart disease in primary ciliary dyskinesia. Thorax 2018, 74, 203–205. [Google Scholar] [CrossRef]
- Kennedy, M.P.; Omran, H.; Leigh, M.W.; Dell, S.; Morgan, L.; Molina, P.L.; Robinson, B.V.; Minnix, S.L.; Olbrich, H.; Severin, T.; et al. Congenital Heart Disease and Other Heterotaxic Defects in a Large Cohort of Patients With Primary Ciliary Dyskinesia. Circulation 2007, 115, 2814–2821. [Google Scholar] [CrossRef]
- Shapiro, A.J.; Davis, S.D.; Ferkol, T.; Dell, S.D.; Rosenfeld, M.; Olivier, K.N.; Sagel, S.D.; Milla, C.; Zariwala, M.A.; Wolf, W.; et al. Laterality defects other than situs inversus totalis in primary ciliary dyskinesia: Insights into situs ambiguus and heterotaxy. Chest 2014, 146, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xia, H.; Deng, S. Genetic basis of human left–right asymmetry disorders. Expert Rev. Mol. Med. 2014, 16, e19. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, H.S.; Wurtzebach, L.R. Kartagener’s triad, review of the literature and report of a case. Dis. Chest 1951, 19, 92–97. [Google Scholar] [CrossRef][Green Version]
- Loges, N.T.; Antony, D.; Maver, A.; Deardorff, M.A.; Gulec, E.Y.; Gezdirici, A.; Nothe-Menchen, T.; Hoben, I.M.; Jelten, L.; Frank, D.; et al. Recessive dnah9 loss-of-function mutations cause laterality defects and subtle respiratory ciliary-beating defects. Am. J. Hum. Genet. 2018, 103, 955–1008. [Google Scholar] [CrossRef]
- Mitchison, H.M.; Schmidts, M.; Loges, N.T.; Freshour, J.; Dritsoula, A.; Hirst, R.A.; O’Callaghan, C.; Blau, H.; Al Dabbagh, M.; Olbrich, H.; et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat. Genet. 2012, 44, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, H.; Schmidts, M.; Werner, C.; Onoufriadis, A.; Loges, N.T.; Raidt, J.; Banki, N.F.; Shoemark, A.; Burgoyne, T.; Al Turki, S.; et al. Recessive HYDIN Mutations Cause Primary Ciliary Dyskinesia without Randomization of Left-Right Body Asymmetry. Am. J. Hum. Genet. 2012, 91, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Antony, D.; Becker-Heck, A.; Zariwala, M.A.; Schmidts, M.; Onoufriadis, A.; Forouhan, M.; Wilson, R.; Taylor-Cox, T.; Dewar, A.; Jackson, C.; et al. Mutations in CCDC39 and CCDC 40 are the Major Cause of Primary Ciliary Dyskinesia with Axonemal Disorganization and Absent Inner Dynein Arms. Hum. Mutat. 2013, 34, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, B.H.; Gibbons, I.R. The Effect of Partial Extraction of Dynein Arms on the Movement of Reactivated Sea-Urchin Sperm. J. Cell Sci. 1973, 13, 337–357. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, R.; Okamoto, M. A mutant of Chlamydomonas reinhardtii that lacks the flagellar outer dynein arm but can swim. J. Cell Sci. 1985, 74, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, C.J.; Kamiya, R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskelet. 1987, 8, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, C.G.; King, S.M.; Koutoulis, A.; Pazour, G.; Witman, G. The 78,000 M(r) intermediate chain of Chlamydomonas outer arm dynein isa WD-repeat protein required for arm assembly. J. Cell Biol. 1995, 129, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Pennarun, G.; Escudier, E.; Chapelin, C.; Bridoux, A.-M.; Cacheux, V.; Roger, G.; Clément, A.; Goossens, M.; Amselem, S.; Duriez, B. Loss-of-Function Mutations in a Human Gene Related to Chlamydomonas reinhardtii Dynein IC78 Result in Primary Ciliary Dyskinesia. Am. J. Hum. Genet. 1999, 65, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Omran, H.; Häffner, K.; Völkel, A.; Kuehr, J.; Ketelsen, U.-P.; Ross, U.-H.; Konietzko, N.; Wienker, T.; Brandis, M.; Hildebrandt, F. Homozygosity Mapping of a Gene Locus for Primary Ciliary Dyskinesia on Chromosome 5p and Identification of the Heavy Dynein ChainDNAH5as a Candidate Gene. Am. J. Respir. Cell Mol. Biol. 2000, 23, 696–702. [Google Scholar] [CrossRef]
- Poprzeczko, M.; Bicka, M.; Farahat, H.; Bazan, R.; Osinka, A.; Fabczak, H.; Joachimiak, E.; Wloga, D. Rare human diseases: Model organisms in deciphering the molecular basis of primary ciliary dyskinesia. Cells 2019, 8, 1614. [Google Scholar] [CrossRef]
- Leigh, M.W.; Horani, A.; Kinghorn, B.; O’Connor, M.G.; Zariwala, M.A.; Knowles, M.R. Primary ciliary dyskinesia (PCD): A genetic disorder of motile cilia. Transl. Sci. Rare Dis. 2019, 4, 51–75. [Google Scholar] [CrossRef]
- Rossman, C.M.; Forrest, J.B.; Lee, R.M.; Newhouse, M.T. The dyskinetic cilia syndrome. Ciliary motility in immotile cilia syndrome. Chest 1980, 78, 580–582. [Google Scholar] [CrossRef]
- Olbrich, H.; Haffner, K.; Kispert, A.; Volkel, A.; Volz, A.; Sasmaz, G.; Reinhardt, R.; Hennig, S.; Lehrach, H.; Konietzko, N.; et al. Mutations in dnah5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat. Genet. 2002, 30, 143–144. [Google Scholar] [CrossRef]
- Duriez, B.; Duquesnoy, P.; Escudier, E.; Bridoux, A.-M.; Escalier, D.; Rayet, I.; Marcos, E.; Vojtek, A.-M.; Bercher, J.-F.; Amselem, S. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc. Natl. Acad. Sci. USA 2007, 104, 3336–3341. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Horani, A.; Stoyanova, M.; Charng, W.-L.; Bottier, M.; Sears, P.R.; Yin, W.-N.; Daniels, L.A.; Bowen, H.; Conrad, D.F.; et al. Mutation of CFAP57, a protein required for the asymmetric targeting of a subset of inner dynein arms in Chlamydomonas, causes primary ciliary dyskinesia. PLoS Genet. 2020, 16, e1008691. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.; Rutman, A.; Williams, G.M.; Hirst, R.A. Inner dynein arm defects causing primary ciliary dyskinesia: Repeat testing required. Eur. Respir. J. 2011, 38, 603–607. [Google Scholar] [CrossRef]
- Merveille, A.C.; Davis, E.E.; Becker-Heck, A.; Legendre, M.; Amirav, I.; Bataille, G.; Belmont, J.; Beydon, N.; Billen, F.; Clement, A.; et al. Ccdc39 is required for assembly of inner dynein arms and the dynein regulatory com-plex and for normal ciliary motility in humans and dogs. Nat. Genet. 2011, 43, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Becker-Heck, A.; Zohn, I.E.; Okabe, N.; Pollock, A.; Lenhart, K.B.; Sullivan-Brown, J.; McSheene, J.; Loges, N.T.; Olbrich, H.; Haeffner, K.; et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat. Genet. 2010, 43, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ibañez-Tallon, I.; Pagenstecher, A.; Fliegauf, M.; Olbrich, H.; Kispert, A.; Ketelsen, U.-P.; North, A.; Heintz, N.; Omran, H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum. Mol. Genet. 2004, 13, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.; Adam, E.C.; Goggin, P.M.; Jackson, C.; Powles-Glover, N.; Patel, S.H.; Humphreys, J.; Fray, M.D.; Falconnet, E.; Blouin, J.-L.; et al. Static respiratory cilia associated with mutations in Dnahc11/DNAH11: A mouse model of PCD. Hum. Mut. 2011, 33, 495–503. [Google Scholar] [CrossRef]
- Tan, S.; Rosenthal, J.; Zhao, X.-Q.; Francis, R.; Chatterjee, B.; Sabol, S.L.; Linask, K.L.; Bracero, L.; Connelly, P.S.; Daniels, M.P.; et al. Heterotaxy and complex structural heart defects in a mutant mouse model of primary ciliary dyskinesia. J. Clin. Investig. 2007, 117, 3742–3752. [Google Scholar] [CrossRef]
- Bartoloni, L.; Blouin, J.-L.; Pan, Y.; Gehrig, C.; Maiti, A.K.; Scamuffa, N.; Rossier, C.; Jorissen, M.; Armengot, M.; Meeks, M.; et al. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc. Natl. Acad. Sci. USA 2002, 99, 10282–10286. [Google Scholar] [CrossRef]
- Lee, L.; Ostrowski, L.E. Motile cilia genetics and cell biology: Big results from little mice. Cell. Mol. Life Sci. 2020, 78, 769–797. [Google Scholar] [CrossRef] [PubMed]
- Coles, J.L.; Thompson, J.; Horton, K.L.; Hirst, R.A.; Griffin, P.; Williams, G.M.; Goggin, P.; Doherty, R.; Lackie, P.M.; Harris, A.; et al. A Revised Protocol for Culture of Airway Epithelial Cells as a Diagnostic Tool for Primary Ciliary Dyskinesia. J. Clin. Med. 2020, 9, 3753. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.A.; Gove, K.; Walker, W.; Lucas, J.S. Nasal nitric oxide screening for primary ciliary dyskinesia: Systematic review and meta-analysis. Eur. Respir. J. 2014, 44, 1589–1599. [Google Scholar] [CrossRef]
- Dalrymple, R.A.; Kenia, P. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia: A guideline review. Arch. Dis. Child. Educ. Pr. Ed. 2018, 104, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.; Smits, A.; Cuppens, H.; Jaspers, M.; Proesmans, M.; Dupont, L.J.; Vermeulen, F.L.; Van Daele, S.; Malfroot, A.; Godding, V.; et al. Primary ciliary dyskinesia: Critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J. Rare Dis. 2014, 9, 11. [Google Scholar] [CrossRef]
- Shoemark, A.; Frost, E.; Dixon, M.; Ollosson, S.; Kilpin, K.; Patel, M.; Scully, J.; Rogers, A.V.; Mitchison, H.M.; Bush, A.; et al. Accuracy of Immunofluorescence in the Diagnosis of Primary Ciliary Dyskinesia. Am. J. Respir. Crit. Care Med. 2017, 196, 94–101. [Google Scholar] [CrossRef]
| Sl. No | Gene Name | Protein Function | EM Description and Beat Pattern of Respiratory Cilia | Laterality Defects | OMIM |
|---|---|---|---|---|---|
| 1 | DNAH5 | ODA Heavy Chain | Absence of ODAs Immotile cilia | yes | 603335 |
| 2 | DNAH11 | ODA Heavy Chain | Partial reduction of ODAs in the proximal region Stiffness and reduced beating in the proximal region of the cilia | yes | 603339 |
| 3 | DNAH9 | ODA Heavy Chain | Partial reduction of ODAs in the distal region Impaired ciliary bending at the distal end the of cilia | yes | 603330 |
| 4 | DNAI1 | ODA Intermediate Chain | Absence or truncated ODAs Immotile cilia | yes | 604366 |
| 5 | DNAI2 | ODA Intermediate Chain | Absence of ODAs Minimal residual motility | yes | 605483 |
| 6 | DNAL1 | ODA Light chain | Absence of ODAs Reduced/absent motility | yes | 610062 |
| 7 | NME8 | ODA Intermediate Chain | Shortened or absent ODAs Normal beat frequency | yes | 607421 |
| 8 | CCDC114 | ODA docking complex | Absence of ODAs Large areas of static cilia with 1–2 cilia showing stiff movement | yes | 615038 |
| 9 | ARMC4 | ODA docking complex | Reduction of ODAs Reduce beat frequency and amplitude or static cilia | yes | 615408 |
| 10 | CCDC151 | ODA docking complex | Absence of ODAs Static cilia | yes | 615956 |
| 11 | TTC25 | ODA docking complex | Absence of ODAs Static cilia | yes | 617095 |
| 12 | MNS1 * | ODA docking complex | Slight reduction of ODAs Ciliary motility is not altered significantly | Yes | 610766 |
| 13 | CCDC103 | Dynein arm attachment | Absent or defective ODAs and IDAs Static cilia | yes | 614677 |
| 14 | DNAAF1 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 613190 |
| 15 | DNAAF2 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 612517 |
| 16 | DNAAF3 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 614566 |
| 17 | DNAAF4 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 608706 |
| 18 | DNAAF5 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 614864 |
| 19 | LRRC6 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 614930 |
| 20 | ZMYND10 | Dynein preassembly factor | Absent of ODAs and IDAs Static cilia | yes | 607070 |
| 21 | SPAG1 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 603395 |
| 22 | C21ORF59 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 615494 |
| 23 | PIH1D3 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 300933 |
| 24 | CFAP300 | Dynein preassembly factor | Absent or defective ODAs and IDAs Static cilia | yes | 618058 |
| 25 | CCDC164 | Nexin Dynein Regulatory Complex (N-DRC) | Missing N-DRC links Reduced amplitude and stiff compared with the control | no | 615288 |
| 26 | CCDC65 | Nexin Dynein Regulatory Complex (N-DRC) | Normal ODAs, reduction in IDAs and nexin links, and MT disorganization Dyskinetic or hyperkinetic cilia | no | 611088 |
| 27 | GAS8 | Nexin Dynein Regulatory Complex (N-DRC) | Misaligned outer doublet MTs Subtle reduction in the beating amplitude compared with the control | no | 605178 |
| 28 | CCDC39 | 96-nm Axonemal ruler | Axonemal disorganization with mislocalized peripheral MTs, absence of the central pair, supernumerary central pairs, reduction or absence of inner dynein arms, and defective radial spokes and nexin links Reduced amplitude with rigid axonemes that showed fast, flickery movements and defective beat regulation | yes | 613798 |
| 29 | CCDC40 | 96-nm Axonemal ruler | Axonemal disorganization with mislocalized peripheral MTs, absence of the central pair, supernumerary central pairs, reduction or absence of inner dynein arms, and defective radial spokes and nexin links Reduced amplitude with rigid axonemes that showed fast, flickery movements and defective beat regulation | yes | 613799 |
| 30 | CFAP57 | Important for assembly of IDAs | Normal EM Heterogeneous waveforms in ciliary movement with reduced frequency | Single case, no laterality defect reported | 614259 |
| 31 | MCIDAS | Transcription factor | Reduced number of cilia and basal bodies, and immotile cilia | No | 614086 |
| 32 | FOXJ1 | Motile ciliogenesis | Reduced number of cilia, and mislocalized basal bodies Stiff beating pattern with a reduced beat amplitude | yes | 602291 |
| 33 | CCNO | Amplification of centrioles | Reduced number of cilia and the few cilia present are motile | No | 607752 |
| 34 | RSPH1 | Radial spoke head | Central complex and radial spoke abnormalities, different ciliary beating patterns (cilia with a normal beat frequency combined with abnormal motion, immotile cilia and cilia with a slowed beat frequency) | No | 609314 |
| 35 | RSPH4A | Radial spoke head | Central complex abnormality and abnormal circular movement | No | 612647 |
| 36 | RSPH9 | Radial spoke head | Central complex abnormality and abnormal circular movement | No | 612648 |
| 37 | RSPH3 | Radial spoke stalk | Central complex abnormality, absence of radial spokes, coexistence of immotile cilia, and motile cilia with movements of a reduced amplitude | No | 615876 |
| 38 | DNAJB13 | Radial spoke neck | Central complex abnormality, lower beating frequency with reduced amplitude | No | 610263 |
| 39 | HYDIN | Central apparatus | Lacking C2b projection of the central pair, central pair number abnormality, and reduced beating amplitudes | No | 610812 |
| 40 | STK36 | Between central pair and radial spoke | Central complex and basal foot abnormality, lacked coordinated beating, and stiff beating pattern with a reduced amplitude | No | 607652 |
| 41 | SPEF2 | Central apparatus | Central complex abnormality and reduced beating amplitudes | No | 610172 |
| 42 | CFAP221 | Central pair projection component | Normal ciliary structure and reduced beat frequency with abnormal circular movement | No | 618704 |
| 43 | RPGR | Transitional zone | Mixture of motile and immotile cilia and absence of various axonemal structures (dynein arms, the central pair, and nexin links) | No | 312610 |
| 44 | OFD1 | Basal body | Normal ciliary structure Impaired ciliary motility | No | 300170 |
| 45 | GAS2L2 | Basal body, basal feet, rootlets, and actin filaments | Normal ciliary structure, and defects in the orientation of cilia with asynchronous and hyperkinetic beat patterns | No | 611398 |
| 46 | LRRC56 | Intraflagellar transport | Normal ciliary structure and severely dyskinetic cilia | Yes | 618227 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antony, D.; Brunner, H.G.; Schmidts, M. Ciliary Dyneins and Dynein Related Ciliopathies. Cells 2021, 10, 1885. https://doi.org/10.3390/cells10081885
Antony D, Brunner HG, Schmidts M. Ciliary Dyneins and Dynein Related Ciliopathies. Cells. 2021; 10(8):1885. https://doi.org/10.3390/cells10081885
Chicago/Turabian StyleAntony, Dinu, Han G. Brunner, and Miriam Schmidts. 2021. "Ciliary Dyneins and Dynein Related Ciliopathies" Cells 10, no. 8: 1885. https://doi.org/10.3390/cells10081885
APA StyleAntony, D., Brunner, H. G., & Schmidts, M. (2021). Ciliary Dyneins and Dynein Related Ciliopathies. Cells, 10(8), 1885. https://doi.org/10.3390/cells10081885






