Tuning Monocytes and Macrophages for Personalized Therapy and Diagnostic Challenge in Rheumatoid Arthritis
Abstract
1. Introduction
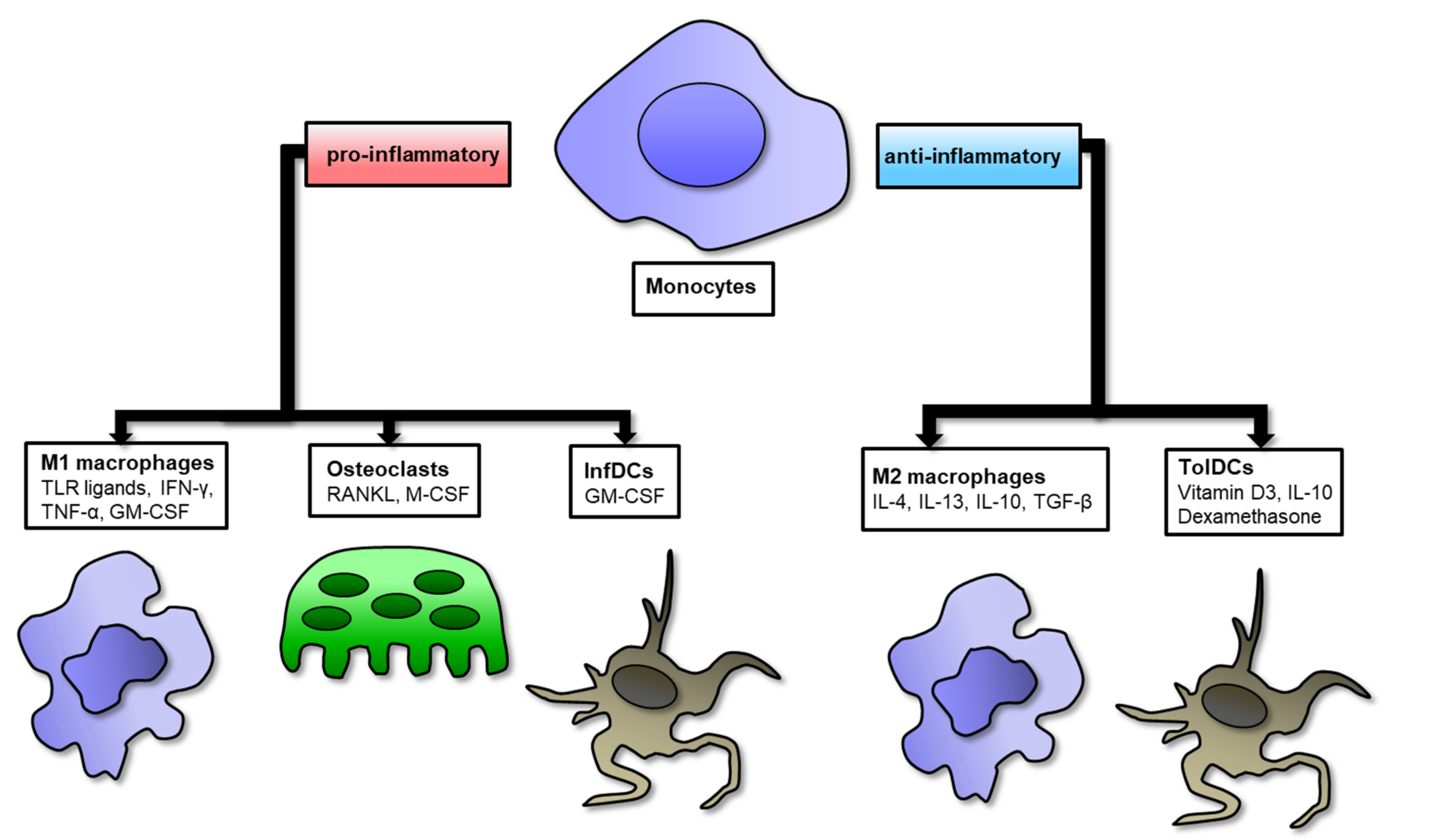
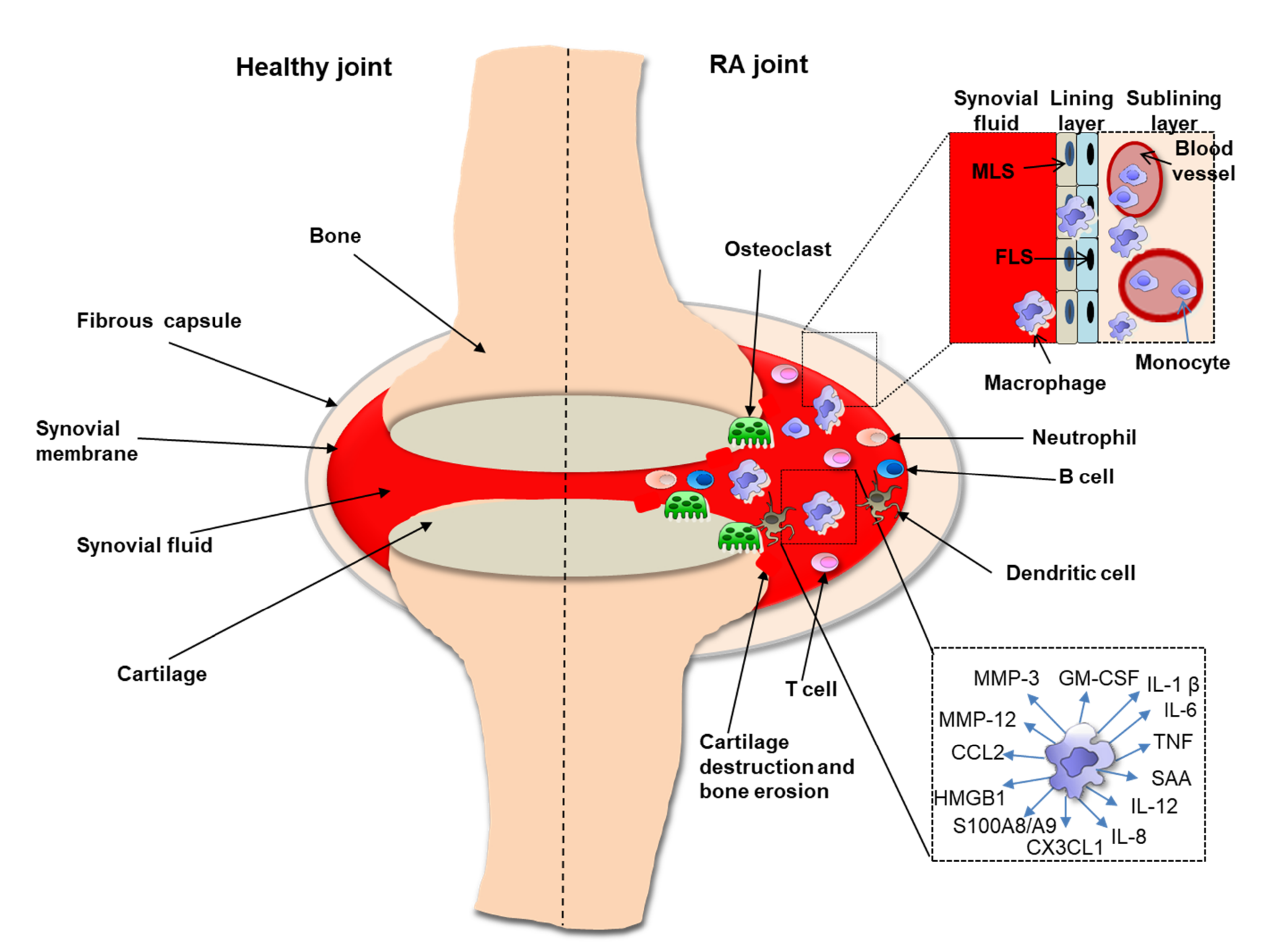
2. Role of Macrophages in RA Synovium
3. Genetic, Epigenetic and Environmental Factors Modulating Monocytes/Macrophages in RA
3.1. Role of DNA Methylation
3.2. Role of miRNA
3.3. Role of Histone Modification
4. Targeting Monocyte/Macrophage Biomarkers in RA Diagnostics
4.1. Targeting Toll-Like Receptors (TLRs)
4.2. Targeting Calprotectin (CLP)/S100A8/A9
4.3. Targeting High Mobility Group Box-1 (HMGB1) Protein
4.4. Targeting Serum Amyloid A (SAA)
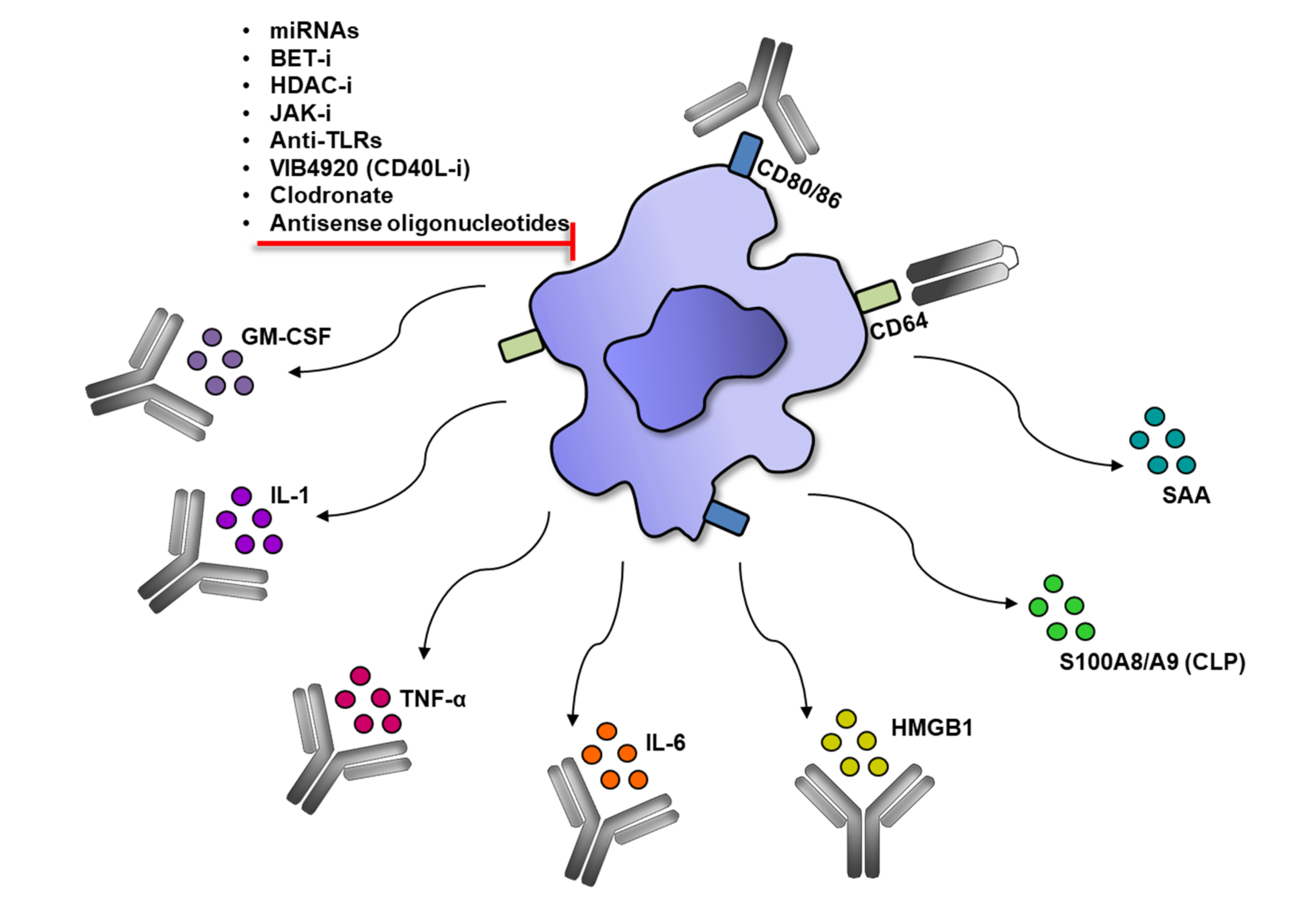
5. Modulation of Monocytes/Macrophage in RA Therapies
5.1. Modulation of Monocytes/Macrophage by Biologics
5.2. Modulation of Monocytes/Macrophages by Small Molecules
5.3. Modulation of Monocytes/Macrophages by Other Therapeutic Modalities
Tolerogenic Dendritic Cells (tolDCs) Induction
6. Application of Single-Cell Technologies in RA Monocytes/Macrophages Phenotyping
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Horizon 2020 Framework Programme EULAR’s Position and Recommendations. Available online: https://www.eular.org/myUploadData/files/EU_Horizon_2020_EULAR_position_paper.pdf (accessed on 11 November 2011).
- Haringman, J.J.; Gerlag, D.M.; Zwinderman, A.H.; Smeets, T.J.; Kraan, M.C.; Baeten, D.; McInnes, I.B.; Bresnihan, B.; Tak, P.P. Synovial tissue macrophages: A sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2005, 64, 834–838. [Google Scholar] [CrossRef]
- Kinne, R.W.; Stuhlmuller, B.; Burmester, G.R. Cells of the synovium in rheumatoid arthritis. Macrophages. Arthritis Res. Ther. 2007, 9, 224. [Google Scholar] [CrossRef] [PubMed]
- Kotsovilis, S.; Andreakos, E. Therapeutic human monoclonal antibodies in inflammatory diseases. Methods Mol. Biol. 2014, 1060, 37–59. [Google Scholar] [CrossRef]
- Van Roon, J.A.; Bijlsma, J.W.; Van de Winkel, J.G.; Lafeber, F.P. Depletion of synovial macrophages in rheumatoid arthritis by an anti-FcgammaRI-calicheamicin immunoconjugate. Ann. Rheum. Dis. 2005, 64, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, R.; Li, C.; Wei, W.; Shen, W.; Cui, Y.; Yuan, X. Macrophage depletion with clodronate-containing liposomes affects the incidence and development of rheumatoid arthritis. Z. Rheumatol. 2019, 78, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Harris, R.A.; Ley, K. Macrophage Polarization: Decisions That Affect Health. J. Clin. Cell. Immunol. 2015, 6. [Google Scholar] [CrossRef]
- Fukui, S.; Iwamoto, N.; Takatani, A.; Igawa, T.; Shimizu, T.; Umeda, M.; Nishino, A.; Horai, Y.; Hirai, Y.; Koga, T.; et al. M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front. Immunol. 2017, 8, 1958. [Google Scholar] [CrossRef]
- Chara, L.; Sanchez-Atrio, A.; Perez, A.; Cuende, E.; Albarran, F.; Turrion, A.; Chevarria, J.; Sanchez, M.A.; Monserrat, J.; De la Hera, A.; et al. Monocyte populations as markers of response to adalimumab plus MTX in rheumatoid arthritis. Arthritis Res. 2012, 14, R175. [Google Scholar] [CrossRef]
- Chara, L.; Sanchez-Atrio, A.; Perez, A.; Cuende, E.; Albarran, F.; Turrion, A.; Chevarria, J.; Del Barco, A.A.; Sanchez, M.A.; Monserrat, J.; et al. The number of circulating monocytes as biomarkers of the clinical response to methotrexate in untreated patients with rheumatoid arthritis. J. Transl. Med. 2015, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Allard-Chamard, H.; Carrier, N.; Dufort, P.; Durand, M.; De Brum-Fernandes, A.J.; Boire, G.; Komarova, S.V.; Dixon, S.J.; Harrison, R.E.; Manolson, M.F.; et al. Osteoclasts and their circulating precursors in rheumatoid arthritis: Relationships with disease activity and bone erosions. Bone Rep. 2020, 12, 100282. [Google Scholar] [CrossRef] [PubMed]
- Gerlag, D.M.; Tak, P.P. Novel approaches for the treatment of rheumatoid arthritis: Lessons from the evaluation of synovial biomarkers in clinical trials. Best Pract. Res. Clin. Rheumatol. 2008, 22, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.; Gibbon, J.R.; Pratt, A.G.; Wood, M.J.; Coady, D.; Raftery, G.; Lorenzi, A.R.; Gray, A.; Filer, A.; Buckley, C.D.; et al. Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Hilkens, C.M.; Isaacs, J.D. Tolerogenic dendritic cell therapy for rheumatoid arthritis: Where are we now? Clin. Exp. Immunol. 2013, 172, 148–157. [Google Scholar] [CrossRef]
- Bain, C.C.; Hawley, C.A.; Garner, H.; Scott, C.L.; Schridde, A.; Steers, N.J.; Mack, M.; Joshi, A.; Guilliams, M.; Mowat, A.M.; et al. Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities. Nat. Commun. 2016, 7, ncomms11852. [Google Scholar] [CrossRef]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef]
- De la Rica, L.; Garcia-Gomez, A.; Comet, N.R.; Rodriguez-Ubreva, J.; Ciudad, L.; Vento-Tormo, R.; Company, C.; Alvarez-Errico, D.; Garcia, M.; Gomez-Vaquero, C.; et al. NF-kappaB-direct activation of microRNAs with repressive effects on monocyte-specific genes is critical for osteoclast differentiation. Genome Biol. 2015, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, L.; Yu, C.; Yang, X.F.; Wang, H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark. Res. 2014, 2, 1. [Google Scholar] [CrossRef]
- Yoon, B.R.; Yoo, S.J.; Choi, Y.; Chung, Y.H.; Kim, J.; Yoo, I.S.; Kang, S.W.; Lee, W.W. Functional phenotype of synovial monocytes modulating inflammatory T-cell responses in rheumatoid arthritis (RA). PLoS ONE 2014, 9, e109775. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Tacke, R.; Hedrick, C.C.; Hanna, R.N. Nonclassical patrolling monocyte function in the vasculature. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Ambarus, C.A.; Noordenbos, T.; De Hair, M.J.; Tak, P.P.; Baeten, D.L. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res. Ther. 2012, 14, R74. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.D.; Barg, E.; Weedon, H.; Papengelis, V.; Smeets, T.; Tak, P.P.; Kraan, M.; Coleman, M.; Ahern, M.J. Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints. Ann. Rheum. Dis. 2003, 62, 303–307. [Google Scholar] [CrossRef]
- You, S.; Yoo, S.A.; Choi, S.; Kim, J.Y.; Park, S.J.; Ji, J.D.; Kim, T.H.; Kim, K.J.; Cho, C.S.; Hwang, D.; et al. Identification of key regulators for the migration and invasion of rheumatoid synoviocytes through a systems approach. Proc. Natl. Acad. Sci. USA 2014, 111, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.C.; Willoughby, D.A. Demonstration of bone marrow derived cells in synovial lining by means of giant intracellular granules as genetic markers. Ann. Rheum. Dis. 1982, 41, 177–182. [Google Scholar] [CrossRef]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, A.K.; Duke, O.; Poulter, L.W. Macrophage-like cells of the pannus area in rheumatoid arthritic joints. Scand. J. Rheumatol. 1987, 16, 263–272. [Google Scholar] [CrossRef]
- Koch, A.E. Angiogenesis as a target in rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62 (Suppl. 2), ii60–ii67. [Google Scholar] [CrossRef]
- Cauli, A.; Yanni, G.; Panayi, G.S. Interleukin-1, interleukin-1 receptor antagonist and macrophage populations in rheumatoid arthritis synovial membrane. Br. J. Rheumatol. 1997, 36, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Deng, C.; Hu, C.; Li, J.; Wen, X.; Wu, Z.; Li, Y.; Zhang, F.; Li, Y. Association of MCP-1-2518A/G polymorphism with susceptibility to autoimmune diseases: A meta-analysis. Clin. Rheumatol. 2016, 35, 1169–1179. [Google Scholar] [CrossRef]
- Diaz-Gallo, L.M.; Ramskold, D.; Shchetynsky, K.; Folkersen, L.; Chemin, K.; Brynedal, B.; Uebe, S.; Okada, Y.; Alfredsson, L.; Klareskog, L.; et al. Systematic approach demonstrates enrichment of multiple interactions between non-HLA risk variants and HLA-DRB1 risk alleles in rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000196126-HLA-DRB1/celltype (accessed on 24 February 2021).
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000108691-CCL2/blood (accessed on 24 February 2021).
- Zhao, Y.; Chen, B.; Li, S.; Yang, L.; Zhu, D.; Wang, Y.; Wang, H.; Wang, T.; Shi, B.; Gai, Z.; et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci. Rep. 2018, 8, 14305. [Google Scholar] [CrossRef]
- Smiljanovic, B.; Grutzkau, A.; Sorensen, T.; Grun, J.R.; Vogl, T.; Bonin, M.; Schendel, P.; Stuhlmuller, B.; Claussnitzer, A.; Hermann, S.; et al. Synovial tissue transcriptomes of long-standing rheumatoid arthritis are dominated by activated macrophages that reflect microbial stimulation. Sci. Rep. 2020, 10, 7907. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Bae, J.B.; Lee, S.; Kim, B.J.; Han, B.G.; Kwok, S.K.; Roh, T.Y. Epigenetic analysis in rheumatoid arthritis synoviocytes. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Hammaker, D.; Firestein, G.S. Epigenetics of inflammatory arthritis. Curr. Opin. Rheumatol. 2018, 30, 188–196. [Google Scholar] [CrossRef]
- Ciechomska, M.; Roszkowski, L.; Maslinski, W. DNA Methylation as a Future Therapeutic and Diagnostic Target in Rheumatoid Arthritis. Cells 2019, 8, 953. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ubreva, J.; De la Calle-Fabregat, C.; Li, T.; Ciudad, L.; Ballestar, M.L.; Catala-Moll, F.; Morante-Palacios, O.; Garcia-Gomez, A.; Celis, R.; Humby, F.; et al. Inflammatory cytokines shape a changing DNA methylome in monocytes mirroring disease activity in rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 1505–1516. [Google Scholar] [CrossRef]
- Fang, Q.; Li, T.; Chen, P.; Wu, Y.; Wang, T.; Mo, L.; Ou, J.; Nandakumar, K.S. Comparative Analysis on Abnormal Methylome of Differentially Expressed Genes and Disease Pathways in the Immune Cells of RA and SLE. Front. Immunol. 2021, 12, 668007. [Google Scholar] [CrossRef]
- Mok, A.; Rhead, B.; Holingue, C.; Shao, X.; Quach, H.L.; Quach, D.; Sinclair, E.; Graf, J.; Imboden, J.; Link, T.; et al. Hypomethylation of CYP2E1 and DUSP22 Promoters Associated with Disease Activity and Erosive Disease Among Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2018, 70, 528–536. [Google Scholar] [CrossRef]
- Dobbs, K.R.; Embury, P.; Koech, E.; Ogolla, S.; Munga, S.; Kazura, J.W.; Dent, A.E. Age-related differences in monocyte DNA methylation and immune function in healthy Kenyan adults and children. Immun. Ageing 2021, 18, 11. [Google Scholar] [CrossRef]
- Tao, W.; Concepcion, A.N.; Vianen, M.; Marijnissen, A.C.A.; Lafeber, F.; Radstake, T.; Pandit, A. Multiomics and Machine Learning Accurately Predict Clinical Response to Adalimumab and Etanercept Therapy in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 212–222. [Google Scholar] [CrossRef]
- Niu, X.; Schulert, G.S. Functional Regulation of Macrophage Phenotypes by MicroRNAs in Inflammatory Arthritis. Front. Immunol. 2019, 10, 2217. [Google Scholar] [CrossRef]
- Elmesmari, A.; Fraser, A.R.; Wood, C.; Gilchrist, D.; Vaughan, D.; Stewart, L.; McSharry, C.; McInnes, I.B.; Kurowska-Stolarska, M. MicroRNA-155 regulates monocyte chemokine and chemokine receptor expression in Rheumatoid Arthritis. Rheumatology 2016, 55, 2056–2065. [Google Scholar] [CrossRef]
- Paoletti, A.; Rohmer, J.; Ly, B.; Pascaud, J.; Riviere, E.; Seror, R.; Le Goff, B.; Nocturne, G.; Mariette, X. Monocyte/Macrophage Abnormalities Specific to Rheumatoid Arthritis Are Linked to miR-155 and Are Differentially Modulated by Different TNF Inhibitors. J. Immunol. 2019, 203, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Schulert, G.S.; Fall, N.; Harley, J.B.; Shen, N.; Lovell, D.J.; Thornton, S.; Grom, A.A. Monocyte MicroRNA Expression in Active Systemic Juvenile Idiopathic Arthritis Implicates MicroRNA-125a-5p in Polarized Monocyte Phenotypes. Arthritis Rheumatol. 2016, 68, 2300–2313. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Liu, J.; Wu, K.; Zhang, J.; Lv, Y.; Wang, S.; Liu, L.; Liu, D. TNF-alpha-elicited miR-29b potentiates resistance to apoptosis in peripheral blood monocytes from patients with rheumatoid arthritis. Apoptosis 2019, 24, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Ammari, M.; Presumey, J.; Ponsolles, C.; Roussignol, G.; Roubert, C.; Escriou, V.; Toupet, K.; Mausset-Bonnefont, A.L.; Cren, M.; Robin, M.; et al. Delivery of miR-146a to Ly6C(high) Monocytes Inhibits Pathogenic Bone Erosion in Inflammatory Arthritis. Theranostics 2018, 8, 5972–5985. [Google Scholar] [CrossRef] [PubMed]
- Ciechomska, M.; Wojtas, B.; Bonek, K.; Roszkowski, L.; Gluszko, P.; Benes, V.; Maslinski, W. Comprehensive microRNA and transcriptomic profiling of rheumatoid arthritis monocytes: Role of microRNA-146b in proinflammatory progression. Rheumatology 2021. [Google Scholar] [CrossRef] [PubMed]
- Ciechomska, M.; Wojtas, B.; Swacha, M.; Olesinska, M.; Benes, V.; Maslinski, W. Global miRNA and mRNA expression profiles identify miRNA-26a-2-3p-dependent repression of IFN signature in systemic sclerosis human monocytes. Eur. J. Immunol. 2020, 50, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Nemtsova, M.V.; Zaletaev, D.V.; Bure, I.V.; Mikhaylenko, D.S.; Kuznetsova, E.B.; Alekseeva, E.A.; Beloukhova, M.I.; Deviatkin, A.A.; Lukashev, A.N.; Zamyatnin, A.A., Jr. Epigenetic Changes in the Pathogenesis of Rheumatoid Arthritis. Front. Genet. 2019, 10, 570. [Google Scholar] [CrossRef]
- Chan, C.H.; Fang, C.; Yarilina, A.; Prinjha, R.K.; Qiao, Y.; Ivashkiv, L.B. BET bromodomain inhibition suppresses transcriptional responses to cytokine-Jak-STAT signaling in a gene-specific manner in human monocytes. Eur. J. Immunol. 2015, 45, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Klein, K. Bromodomain protein inhibition: A novel therapeutic strategy in rheumatic diseases. RMD Open 2018, 4, e000744. [Google Scholar] [CrossRef]
- Wang, N.; Wu, R.; Tang, D.; Kang, R. The BET family in immunity and disease. Signal Transduct. Target 2021, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Kato, M.; Frank-Bertoncelj, M.; Kolling, C.; Ciurea, A.; Gay, S.; Ospelt, C. Evaluating the bromodomain protein BRD1 as a therapeutic target in rheumatoid arthritis. Sci. Rep. 2018, 8, 11125. [Google Scholar] [CrossRef]
- Cribbs, A.P.; Filippakopoulos, P.; Philpott, M.; Wells, G.; Penn, H.; Oerum, H.; Valge-Archer, V.; Feldmann, M.; Oppermann, U. Dissecting the Role of BET Bromodomain Proteins BRD2 and BRD4 in Human NK Cell Function. Front. Immunol. 2021, 12, 626255. [Google Scholar] [CrossRef]
- Wei, S.; Sun, Y.; Sha, H. Therapeutic targeting of BET protein BRD4 delays murine lupus. Int. Immunopharmacol. 2015, 29, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Grabiec, A.M.; Krausz, S.; De Jager, W.; Burakowski, T.; Groot, D.; Sanders, M.E.; Prakken, B.J.; Maslinski, W.; Eldering, E.; Tak, P.P.; et al. Histone deacetylase inhibitors suppress inflammatory activation of rheumatoid arthritis patient synovial macrophages and tissue. J. Immunol. 2010, 184, 2718–2728. [Google Scholar] [CrossRef]
- Ciechomska, M.; O'Reilly, S.; Przyborski, S.; Oakley, F.; Bogunia-Kubik, K.; Van Laar, J.M. Histone Demethylation and Toll-like Receptor 8-Dependent Cross-Talk in Monocytes Promotes Transdifferentiation of Fibroblasts in Systemic Sclerosis Via Fra-2. Arthritis Rheumatol. 2016, 68, 1493–1504. [Google Scholar] [CrossRef]
- Ciechomska, M.; Zarecki, P.; Merdas, M.; Swierkot, J.; Morgiel, E.; Wiland, P.; Maslinski, W.; Bogunia-Kubik, K. The role of microRNA-5196 in the pathogenesis of systemic sclerosis. Eur. J. Clin. Invest. 2017, 47, 555–564. [Google Scholar] [CrossRef]
- Okamato, Y.; Ghosh, T.; Okamoto, T.; Schuyler, R.P.; Seifert, J.; Charry, L.L.; Visser, A.; Feser, M.; Fleischer, C.; Pedrick, C.; et al. Subjects at-risk for future development of rheumatoid arthritis demonstrate a PAD4-and TLR-dependent enhanced histone H3 citrullination and proinflammatory cytokine production in CD14(hi) monocytes. J. Autoimmun. 2021, 117, 102581. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, Y.C.; Huang, M.Y.; Kuo, P.L.; Wu, C.C.; Lee, M.S.; Hsieh, C.C.; Kuo, H.F.; Kuo, C.H.; Tsai, W.C.; et al. Tumor necrosis factor-alpha inhibitors suppress CCL2 chemokine in monocytes via epigenetic modification. Mol. Immunol. 2017, 83, 82–91. [Google Scholar] [CrossRef]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T. Biomarkers as a treatment guide in rheumatoid arthritis. Clin. Immunol. 2018, 186, 59–62. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Sallenave, J.M.; Guillot, L. Innate Immune Signaling and Proteolytic Pathways in the Resolution or Exacerbation of SARS-CoV-2 in Covid-19: Key Therapeutic Targets? Front. Immunol. 2020, 11, 1229. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, M.; Yamamura, M.; Aita, T.; Okamoto, A.; Ueno, A.; Ogawa, N.; Akashi, S.; Miyake, K.; Godowski, P.J.; Makino, H. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004, 50, 1457–1467. [Google Scholar] [CrossRef]
- Lacerte, P.; Brunet, A.; Egarnes, B.; Duchene, B.; Brown, J.P.; Gosselin, J. Overexpression of TLR2 and TLR9 on monocyte subsets of active rheumatoid arthritis patients contributes to enhance responsiveness to TLR agonists. Arthritis Res. Ther. 2016, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Harre, U.; Georgess, D.; Bang, H.; Bozec, A.; Axmann, R.; Ossipova, E.; Jakobsson, P.J.; Baum, W.; Nimmerjahn, F.; Szarka, E.; et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Investig. 2012, 122, 1791–1802. [Google Scholar] [CrossRef]
- Sokolove, J.; Zhao, X.; Chandra, P.E.; Robinson, W.H. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis Rheum. 2011, 63, 53–62. [Google Scholar] [CrossRef]
- Lu, M.C.; Lai, N.S.; Yu, H.C.; Huang, H.B.; Hsieh, S.C.; Yu, C.L. Anti-citrullinated protein antibodies bind surface-expressed citrullinated Grp78 on monocyte/macrophages and stimulate tumor necrosis factor alpha production. Arthritis Rheum. 2010, 62, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Ospelt, C.; Brentano, F.; Rengel, Y.; Stanczyk, J.; Kolling, C.; Tak, P.P.; Gay, R.E.; Gay, S.; Kyburz, D. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: Toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008, 58, 3684–3692. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, M.F.; Joosten, L.A.; Abdollahi-Roodsaz, S.; Van Lieshout, A.W.; Sprong, T.; Van den Hoogen, F.H.; Van den Berg, W.B.; Radstake, T.R. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005, 52, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Ultaigh, S.N.; Saber, T.P.; McCormick, J.; Connolly, M.; Dellacasagrande, J.; Keogh, B.; McCormack, W.; Reilly, M.; O’Neill, L.A.; McGuirk, P.; et al. Blockade of Toll-like receptor 2 prevents spontaneous cytokine release from rheumatoid arthritis ex vivo synovial explant cultures. Arthritis Res. Ther. 2011, 13, R33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, D.; Yao, C.; Zheng, F.; Zhou, T.; Cao, Q.; Zhu, X.; Wang, M.; Zhu, J. Human monoclonal antiTLR4 antibody negatively regulates lipopolysaccharideinduced inflammatory responses in mouse macrophages. Mol. Med. Rep. 2020, 22, 4125–4134. [Google Scholar] [CrossRef]
- Samarpita, S.; Kim, J.Y.; Rasool, M.K.; Kim, K.S. Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug. Arthritis Res. Ther. 2020, 22, 16. [Google Scholar] [CrossRef]
- Monnet, E.; Choy, E.H.; McInnes, I.; Kobakhidze, T.; De Graaf, K.; Jacqmin, P.; Lapeyre, G.; De Min, C. Efficacy and safety of NI-0101, an anti-toll-like receptor 4 monoclonal antibody, in patients with rheumatoid arthritis after inadequate response to methotrexate: A phase II study. Ann. Rheum. Dis. 2020, 79, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Sunahori, K.; Yamamura, M.; Yamana, J.; Takasugi, K.; Kawashima, M.; Yamamoto, H.; Chazin, W.J.; Nakatani, Y.; Yui, S.; Makino, H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R69. [Google Scholar] [CrossRef]
- Rammes, A.; Roth, J.; Goebeler, M.; Klempt, M.; Hartmann, M.; Sorg, C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J. Biol. Chem. 1997, 272, 9496–9502. [Google Scholar] [CrossRef]
- Van Lent, P.L.; Grevers, L.; Blom, A.B.; Sloetjes, A.; Mort, J.S.; Vogl, T.; Nacken, W.; Van den Berg, W.B.; Roth, J. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann. Rheum. Dis. 2008, 67, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.C.; Lee, Y.H. Calprotectin levels in rheumatoid arthritis and their correlation with disease activity: A meta-analysis. Postgrad. Med. 2017, 129, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Narvaez, J.A.; Ruiz-Esquide, V.; Hernandez-Ganan, J.; Cuervo, A.; Inciarte-Mundo, J.; Hernandez, M.V.; Sampayo-Cordero, M.; Pablos, J.L.; Sanmarti, R.; et al. Clinical and sonographic biomarkers of structural damage progression in RA patients in clinical remission: A prospective study with 12 months follow-up. Semin. Arthritis Rheum. 2017, 47, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arias, M.; Pascual-Salcedo, D.; Ramiro, S.; Ueberschlag, M.E.; Jermann, T.M.; Cara, C.; Martin-Mola, E.; Balsa, A. Calprotectin in rheumatoid arthritis: Association with disease activity in a cross-sectional and a longitudinal cohort. Mol. Diagn. Ther. 2013, 17, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hurnakova, J.; Hulejova, H.; Zavada, J.; Hanova, P.; Komarc, M.; Mann, H.; Klein, M.; Sleglova, O.; Olejarova, M.; Forejtova, S.; et al. Relationship between serum calprotectin (S100A8/9) and clinical, laboratory and ultrasound parameters of disease activity in rheumatoid arthritis: A large cohort study. PLoS ONE 2017, 12, e0183420. [Google Scholar] [CrossRef]
- Nordal, H.H.; Brokstad, K.A.; Solheim, M.; Halse, A.K.; Kvien, T.K.; Hammer, H.B. Calprotectin (S100A8/A9) has the strongest association with ultrasound-detected synovitis and predicts response to biologic treatment: Results from a longitudinal study of patients with established rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 3. [Google Scholar] [CrossRef]
- Mahler, M.; Meroni, P.L.; Infantino, M.; Buhler, K.A.; Fritzler, M.J. Circulating Calprotectin as a Biomarker of COVID-19 Severity. Expert Rev. Clin. Immunol. 2021, 17, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef]
- Austermann, J.; Zenker, S.; Roth, J. S100-alarmins: Potential therapeutic targets for arthritis. Expert Opin. Ther. Targets 2017, 21, 739–751. [Google Scholar] [CrossRef]
- Andersson, U.; Yang, H.; Harris, H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin. Ther. Targets 2018, 22, 263–277. [Google Scholar] [CrossRef]
- Pullerits, R.; Urbonaviciute, V.; Voll, R.E.; Forsblad-D'Elia, H.; Carlsten, H. Serum levels of HMGB1 in postmenopausal patients with rheumatoid arthritis: Associations with proinflammatory cytokines, acute-phase reactants, and clinical disease characteristics. J. Rheumatol. 2011, 38, 1523–1525. [Google Scholar] [CrossRef][Green Version]
- Schaper, F.; Westra, J.; Bijl, M. Recent developments in the role of high-mobility group box 1 in systemic lupus erythematosus. Mol. Med. 2014, 20, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Catellani, C.; Sartori, C.; Lazzeroni, P.; Morini, D.; Nicoli, A.; Giorgi-Rossi, P.; Amarri, S.; La Sala, G.B.; Street, M.E. CFTR and FOXO1 gene expression are reduced and high mobility group box 1 (HMGB1) is increased in the ovaries and serum of women with polycystic ovarian syndrome. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2019, 35, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Rando, M.M.; Nardella, E.; Cecchini, A.L.; Pecorini, G.; Landolfi, R.; Flex, A. High Mobility Group Box-1 and Diabetes Mellitus Complications: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Zhang, S.; Yu, Q.; Xiong, F.; Huang, K.; Wang, C.Y.; Yang, P. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol. Cell. Endocrinol. 2017, 454, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E. HMGB1: A Possible Crucial Therapeutic Target for COVID-19? Horm. Res. Paediatr. 2020, 93, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Kokkola, R.; Sundberg, E.; Ulfgren, A.K.; Palmblad, K.; Li, J.; Wang, H.; Ulloa, L.; Yang, H.; Yan, X.J.; Furie, R.; et al. High mobility group box chromosomal protein 1: A novel proinflammatory mediator in synovitis. Arthritis Rheum. 2002, 46, 2598–2603. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Kawahara, K.; Yone, K.; Hashiguchi, T.; Yamakuchi, M.; Goto, M.; Inoue, K.; Yamada, S.; Ijiri, K.; Matsunaga, S.; et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 2003, 48, 971–981. [Google Scholar] [CrossRef]
- Goldstein, R.S.; Bruchfeld, A.; Yang, L.; Qureshi, A.R.; Gallowitsch-Puerta, M.; Patel, N.B.; Huston, B.J.; Chavan, S.; Rosas-Ballina, M.; Gregersen, P.K.; et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol. Med. 2007, 13, 210–215. [Google Scholar] [CrossRef]
- Garcia-Arnandis, I.; Guillen, M.I.; Gomar, F.; Pelletier, J.P.; Martel-Pelletier, J.; Alcaraz, M.J. High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1beta in osteoarthritic synoviocytes. Arthritis Res. Ther. 2010, 12, R165. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ma, Y.; Adebayo, A.; Pope, R.M. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007, 56, 2192–2201. [Google Scholar] [CrossRef]
- Kaur, I.; Behl, T.; Bungau, S.; Kumar, A.; Mehta, V.; Setia, D.; Uddin, M.S.; Zengin, G.; Aleya, L.; Arora, S. Exploring the therapeutic promise of targeting HMGB1 in rheumatoid arthritis. Life Sci. 2020, 258, 118164. [Google Scholar] [CrossRef]
- Jumeau, C.; Awad, F.; Assrawi, E.; Cobret, L.; Duquesnoy, P.; Giurgea, I.; Valeyre, D.; Grateau, G.; Amselem, S.; Bernaudin, J.F.; et al. Expression of SAA1, SAA2 and SAA4 genes in human primary monocytes and monocyte-derived macrophages. PLoS ONE 2019, 14, e0217005. [Google Scholar] [CrossRef] [PubMed]
- Vallon, R.; Freuler, F.; Desta-Tsedu, N.; Robeva, A.; Dawson, J.; Wenner, P.; Engelhardt, P.; Boes, L.; Schnyder, J.; Tschopp, C.; et al. Serum amyloid A (apoSAA) expression is up-regulated in rheumatoid arthritis and induces transcription of matrix metalloproteinases. J. Immunol. 2001, 166, 2801–2807. [Google Scholar] [CrossRef] [PubMed]
- Cakan, M.; Karadag, S.G.; Tanatar, A.; Sonmez, H.E.; Ayaz, N.A. The Value of Serum Amyloid A Levels in Familial Mediterranean Fever to Identify Occult Inflammation During Asymptomatic Periods. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2021, 27, 1–4. [Google Scholar] [CrossRef]
- Wang, C.M.; Deng, J.H.; Mao, G.F.; He, Y.L.; Shi, X. Serum Amyloid A: A Potential Biomarker Assessing Disease Activity in Systemic Lupus Erythematosus. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e923290. [Google Scholar] [CrossRef] [PubMed]
- Lis-Swiety, A.; Widuchowska, M.; Brzezinska-Wcislo, L.; Kucharz, E. High acute phase protein levels correlate with pulmonary and skin involvement in patients with diffuse systemic sclerosis. J. Int. Med. Res. 2018, 46, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Boyd, T.A.; Eastman, P.S.; Huynh, D.H.; Qureshi, F.; Sasso, E.H.; Bolce, R.; Temple, J.; Hillman, J.; Boyle, D.L.; Kavanaugh, A. Correlation of serum protein biomarkers with disease activity in psoriatic arthritis. Expert Rev. Clin. Immunol. 2020, 16, 335–341. [Google Scholar] [CrossRef]
- Bargagli, E.; Magi, B.; Olivieri, C.; Bianchi, N.; Landi, C.; Rottoli, P. Analysis of serum amyloid A in sarcoidosis patients. Respir. Med. 2011, 105, 775–780. [Google Scholar] [CrossRef]
- Dev, S.; Singh, A. Study of role of serum amyloid A (SAA) as a marker of disease activity in juvenile idiopathic arthritis. J. Fam. Med. Prim. Care 2019, 8, 2129–2133. [Google Scholar] [CrossRef]
- Liu, S.; Ji, W.; Lu, J.; Tang, X.; Guo, Y.; Ji, M.; Xu, T.; Gu, W.; Kong, D.; Shen, Q.; et al. Discovery of Potential Serum Protein Biomarkers in Ankylosing Spondylitis Using Tandem Mass Tag-Based Quantitative Proteomics. J. Proteome Res. 2020, 19, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Rigante, D.; Lopalco, G.; Brizi, M.G.; Caso, F.; Franceschini, R.; Denaro, R.; Galeazzi, M.; Punzi, L.; Iannone, F.; et al. Serum amyloid-A in Behcet’s disease. Clin. Rheumatol. 2014, 33, 1165–1167. [Google Scholar] [CrossRef][Green Version]
- Dartevel, A.; Toussaint, B.; Trocme, C.; Arnaud, M.; Simon, N.; Faure, P.; Bouillet, L. Serum amyloid A as a marker of disease activity in Giant cell arteritis. Autoimmun. Rev. 2020, 19, 102428. [Google Scholar] [CrossRef]
- Nair, A.M.; Goel, R.; Hindhumati, M.; Jayakanthan, K.; Visalakshi, J.; Joseph, G.; Danda, S.; Danda, D. Serum amyloid A as a marker of disease activity and treatment response in Takayasu arteritis. Rheumatol. Int. 2017, 37, 1643–1649. [Google Scholar] [CrossRef]
- Mo, X.N.; Su, Z.Q.; Lei, C.L.; Chen, D.F.; Peng, H.; Chen, R.C.; Sang, L.; Wu, H.K.; Li, S.Y. Serum amyloid A is a predictor for prognosis of COVID-19. Respirology 2020, 25, 764–765. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.E.; MacFarlane, D.G.; Whicher, J.T.; Dieppe, P.A. Serum amyloid-A protein concentration in rheumatoid arthritis and its role in monitoring disease activity. Ann. Rheum. Dis. 1983, 42, 665–667. [Google Scholar] [CrossRef] [PubMed]
- De Seny, D.; Cobraiville, G.; Charlier, E.; Neuville, S.; Esser, N.; Malaise, D.; Malaise, O.; Calvo, F.Q.; Relic, B.; Malaise, M.G. Acute-phase serum amyloid a in osteoarthritis: Regulatory mechanism and proinflammatory properties. PLoS ONE 2013, 8, e66769. [Google Scholar] [CrossRef] [PubMed]
- Targonska-Stepniak, B.; Majdan, M. Serum amyloid A as a marker of persistent inflammation and an indicator of cardiovascular and renal involvement in patients with rheumatoid arthritis. Mediat. Inflamm. 2014, 2014, 793628. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Lee, S.K.; Lim, M.; Sheen, D.; Choi, E.H.; Kim, S.A. Exosomal amyloid A and lymphatic vessel endothelial hyaluronic acid receptor-1 proteins are associated with disease activity in rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 119. [Google Scholar] [CrossRef]
- Ostensen, M.; Marhaug, G.; Husby, G. Amyloid-related serum protein (SAA) during and after pregnancy in healthy women and women with rheumatic disease. Acta Pathol. Microbiol. Et Immunol. Scandinavica. Sect. C Immunol. 1985, 93, 1–5. [Google Scholar] [CrossRef]
- Hwang, Y.G.; Balasubramani, G.K.; Metes, I.D.; Levesque, M.C.; Bridges, S.L., Jr.; Moreland, L.W. Differential response of serum amyloid A to different therapies in early rheumatoid arthritis and its potential value as a disease activity biomarker. Arthritis Res. Ther. 2016, 18, 108. [Google Scholar] [CrossRef]
- Connolly, M.; Mullan, R.H.; McCormick, J.; Matthews, C.; Sullivan, O.; Kennedy, A.; FitzGerald, O.; Poole, A.R.; Bresnihan, B.; Veale, D.J.; et al. Acute-phase serum amyloid A regulates tumor necrosis factor alpha and matrix turnover and predicts disease progression in patients with inflammatory arthritis before and after biologic therapy. Arthritis Rheum. 2012, 64, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Migita, K.; Izumi, Y.; Jiuchi, Y.; Kozuru, H.; Kawahara, C.; Izumi, M.; Sakai, T.; Nakamura, M.; Motokawa, S.; Nakamura, T.; et al. Effects of Janus kinase inhibitor tofacitinib on circulating serum amyloid A and interleukin-6 during treatment for rheumatoid arthritis. Clin. Exp. Immunol. 2014, 175, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Centola, M.; Cavet, G.; Shen, Y.; Ramanujan, S.; Knowlton, N.; Swan, K.A.; Turner, M.; Sutton, C.; Smith, D.R.; Haney, D.J.; et al. Development of a multi-biomarker disease activity test for rheumatoid arthritis. PLoS ONE 2013, 8, e60635. [Google Scholar] [CrossRef]
- Ma, M.H.Y.; Defranoux, N.; Li, W.; Sasso, E.H.; Ibrahim, F.; Scott, D.L.; Cope, A.P. A multi-biomarker disease activity score can predict sustained remission in rheumatoid arthritis. Arthritis Res. Ther. 2020, 22, 158. [Google Scholar] [CrossRef] [PubMed]
- Soric Hosman, I.; Kos, I.; Lamot, L. Serum Amyloid A in Inflammatory Rheumatic Diseases: A Compendious Review of a Renowned Biomarker. Front. Immunol. 2020, 11, 631299. [Google Scholar] [CrossRef]
- Kuret, T.; Lakota, K.; Mali, P.; Cucnik, S.; Praprotnik, S.; Tomsic, M.; Sodin-Semrl, S. Naturally occurring antibodies against serum amyloid A reduce IL-6 release from peripheral blood mononuclear cells. PLoS ONE 2018, 13, e0195346. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Kapoor, S.R.; Filer, A.; Fitzpatrick, M.A.; Fisher, B.A.; Taylor, P.C.; Buckley, C.D.; McInnes, I.B.; Raza, K.; Young, S.P. Metabolic profiling predicts response to anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2013, 65, 1448–1456. [Google Scholar] [CrossRef]
- Ehrenstein, M.R.; Evans, J.G.; Singh, A.; Moore, S.; Warnes, G.; Isenberg, D.A.; Mauri, C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 2004, 200, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.C.; Peters, A.M.; Paleolog, E.; Chapman, P.T.; Elliott, M.J.; McCloskey, R.; Feldmann, M.; Maini, R.N. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 2000, 43, 38–47. [Google Scholar] [CrossRef]
- Degboe, Y.; Rauwel, B.; Baron, M.; Boyer, J.F.; Ruyssen-Witrand, A.; Constantin, A.; Davignon, J.L. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Front. Immunol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Cutolo, M.; Bisso, A.; Sulli, A.; Felli, L.; Briata, M.; Pizzorni, C.; Villaggio, B. Antiproliferative and antiinflammatory effects of methotrexate on cultured differentiating myeloid monocytic cells (THP-1) but not on synovial macrophages from patients with rheumatoid arthritis. J. Rheumatol. 2000, 27, 2551–2557. [Google Scholar] [PubMed]
- Jain, S.; Tran, T.H.; Amiji, M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials 2015, 61, 162–177. [Google Scholar] [CrossRef]
- Bonek, K.; Roszkowski, L.; Massalska, M.; Maslinski, W.; Ciechomska, M. Biologic Drugs for Rheumatoid Arthritis in the Context of Biosimilars, Genetics, Epigenetics and COVID-19 Treatment. Cells 2021, 10, 323. [Google Scholar] [CrossRef]
- Obeng, J.A.; Amoruso, A.; Camaschella, G.L.; Sola, D.; Brunelleschi, S.; Fresu, L.G. Modulation of human monocyte/macrophage activity by tocilizumab, abatacept and etanercept: An in vitro study. Eur. J. Pharmacol. 2016, 780, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tono, T.; Aihara, S.; Hoshiyama, T.; Arinuma, Y.; Nagai, T.; Hirohata, S. Effects of anti-IL-6 receptor antibody on human monocytes. Mod. Rheumatol. 2015, 25, 79–84. [Google Scholar] [CrossRef]
- Furst, D.E.; Keystone, E.C.; So, A.K.; Braun, J.; Breedveld, F.C.; Burmester, G.R.; De Benedetti, F.; Dorner, T.; Emery, P.; Fleischmann, R.; et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2012. Ann. Rheum. Dis. 2013, 72 (Suppl. 2), ii2–ii34. [Google Scholar] [CrossRef]
- Burmester, G.R.; McInnes, I.B.; Kremer, J.M.; Miranda, P.; Vencovsky, J.; Godwood, A.; Albulescu, M.; Michaels, M.A.; Guo, X.; Close, D.; et al. Mavrilimumab, a Fully Human Granulocyte-Macrophage Colony-Stimulating Factor Receptor alpha Monoclonal Antibody: Long-Term Safety and Efficacy in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 679–689. [Google Scholar] [CrossRef]
- Behrens, F.; Tak, P.P.; Ostergaard, M.; Stoilov, R.; Wiland, P.; Huizinga, T.W.; Berenfus, V.Y.; Vladeva, S.; Rech, J.; Rubbert-Roth, A.; et al. MOR103, a human monoclonal antibody to granulocyte-macrophage colony-stimulating factor, in the treatment of patients with moderate rheumatoid arthritis: Results of a phase Ib/IIa randomised, double-blind, placebo-controlled, dose-escalation trial. Ann. Rheum. Dis. 2015, 74, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Greven, D.E.; Cohen, E.S.; Gerlag, D.M.; Campbell, J.; Woods, J.; Davis, N.; Van Nieuwenhuijze, A.; Lewis, A.; Heasmen, S.; McCourt, M.; et al. Preclinical characterisation of the GM-CSF receptor as a therapeutic target in rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, 1924–1930. [Google Scholar] [CrossRef]
- Korhonen, R.; Moilanen, E. Abatacept, a novel CD80/86-CD28 T cell co-stimulation modulator, in the treatment of rheumatoid arthritis. Basic Clin. Pharmacol. Toxicol. 2009, 104, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Zaiss, M.M.; Kagwiria, R.; Voll, R.; Rauh, M.; Chen, Z.; Mueller-Schmucker, S.; Kroczek, R.A.; Heinzerling, L.; Moser, M.; et al. T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci. Transl. Med. 2014, 6, 235ra260. [Google Scholar] [CrossRef]
- Cutolo, M.; Nadler, S.G. Advances in CTLA-4-Ig-mediated modulation of inflammatory cell and immune response activation in rheumatoid arthritis. Autoimmun. Rev. 2013, 12, 758–767. [Google Scholar] [CrossRef]
- Ding, C.; Xu, J.; Li, J. ABT-874, a fully human monoclonal anti-IL-12/IL-23 antibody for the potential treatment of autoimmune diseases. Curr. Opin. Investig. Drugs 2008, 9, 515–522. [Google Scholar]
- Baslund, B.; Tvede, N.; Danneskiold-Samsoe, B.; Larsson, P.; Panayi, G.; Petersen, J.; Petersen, L.J.; Beurskens, F.J.; Schuurman, J.; Van de Winkel, J.G.; et al. Targeting interleukin-15 in patients with rheumatoid arthritis: A proof-of-concept study. Arthritis Rheum. 2005, 52, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- Plater-Zyberk, C.; Joosten, L.A.; Helsen, M.M.; Sattonnet-Roche, P.; Siegfried, C.; Alouani, S.; Van De Loo, F.A.; Graber, P.; Aloni, S.; Cirillo, R.; et al. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J. Clin. Investig. 2001, 108, 1825–1832. [Google Scholar] [CrossRef]
- Smolen, J.S.; Agarwal, S.K.; Ilivanova, E.; Xu, X.L.; Miao, Y.; Zhuang, Y.; Nnane, I.; Radziszewski, W.; Greenspan, A.; Beutler, A.; et al. A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann. Rheum. Dis. 2017, 76, 831–839. [Google Scholar] [CrossRef]
- Massalska, M.; Maslinski, W.; Ciechomska, M. Small Molecule Inhibitors in the Treatment of Rheumatoid Arthritis and Beyond: Latest Updates and Potential Strategy for Fighting COVID-19. Cells 2020, 9, 1876. [Google Scholar] [CrossRef]
- Favalli, E.G.; Matucci-Cerinic, M.; Szekanecz, Z. The Giants (biologicals) against the Pigmies (small molecules), pros and cons of two different approaches to the disease modifying treatment in rheumatoid arthritis. Autoimmun. Rev. 2020, 19, 102421. [Google Scholar] [CrossRef] [PubMed]
- Harrington, R.; Al Nokhatha, S.A.; Conway, R. JAK Inhibitors in Rheumatoid Arthritis: An Evidence-Based Review on the Emerging Clinical Data. J. Inflamm. Res. 2020, 13, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Tuckwell, K.; Katsumoto, T.R.; Zhao, R.; Galanter, J.; Lee, C.; Rae, J.; Toth, B.; Ramamoorthi, N.; Hackney, J.A.; et al. Fenebrutinib versus Placebo or Adalimumab in Rheumatoid Arthritis: A Randomized, Double-Blind, Phase II Trial (ANDES Study). Arthritis Rheumatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Mohanty, M.; Mangla, A.; George, A.; Bal, V.; Rath, S.; Ravindran, B. Macrophage effector functions controlled by Bruton's tyrosine kinase are more crucial than the cytokine balance of T cell responses for microfilarial clearance. J. Immunol. 2002, 168, 2914–2921. [Google Scholar] [CrossRef]
- Galapagos Annual Report 2019. Available online: https://reports.glpg.com/annual-report-2019/en/r-d/our-toledo-program.html (accessed on 24 February 2021).
- Stanczyk, J.; Ospelt, C.; Gay, S. Is there a future for small molecule drugs in the treatment of rheumatic diseases? Curr. Opin. Rheumatol. 2008, 20, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Cheng, T.T.; Chindalore, V.; Damjanov, N.; Burgos-Vargas, R.; Delora, P.; Zimany, K.; Travers, H.; Caulfield, J.P. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthritis Rheum. 2009, 60, 335–344. [Google Scholar] [CrossRef]
- Pine, P.R.; Chang, B.; Schoettler, N.; Banquerigo, M.L.; Wang, S.; Lau, A.; Zhao, F.; Grossbard, E.B.; Payan, D.G.; Brahn, E. Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clin. Immunol. 2007, 124, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Clavel, G.; Thiolat, A.; Boissier, M.C. Interleukin newcomers creating new numbers in rheumatology: IL-34 to IL-38. Jt. Bone Spine 2013, 80, 449–453. [Google Scholar] [CrossRef]
- Peng, M.; Qiang, L.; Xu, Y.; Li, C.; Li, T.; Wang, J. IL-35 ameliorates collagen-induced arthritis by promoting TNF-alpha-induced apoptosis of synovial fibroblasts and stimulating M2 macrophages polarization. FEBS J. 2019, 286, 1972–1985. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, C.; Chi, X.; Hu, Y.W.; Zheng, L.; Zeng, T.; Wang, Q. IL-37 Expression is Upregulated in Patients with Tuberculosis and Induces Macrophages Towards an M2-like Phenotype. Scand. J. Immunol. 2015, 82, 370–379. [Google Scholar] [CrossRef]
- Boutet, M.A.; Najm, A.; Bart, G.; Brion, R.; Touchais, S.; Trichet, V.; Layrolle, P.; Gabay, C.; Palmer, G.; Blanchard, F.; et al. IL-38 overexpression induces anti-inflammatory effects in mice arthritis models and in human macrophages in vitro. Ann. Rheum. Dis. 2017, 76, 1304–1312. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Zhao, Y.; Li, S.; Zhao, T.; Tai, Y.; Zhang, B.; Wang, X.; Wang, C.; Chen, J.; et al. GRK2 Mediated Abnormal Transduction of PGE2-EP4-cAMP-CREB Signaling Induces the Imbalance of Macrophages Polarization in Collagen-Induced Arthritis Mice. Cells 2019, 8, 1596. [Google Scholar] [CrossRef]
- Hristodorov, D.; Mladenov, R.; Fischer, R.; Barth, S.; Thepen, T. Fully human MAP-fusion protein selectively targets and eliminates proliferating CD64(+) M1 macrophages. Immunol. Cell Biol. 2016, 94, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; He, Y.; Zhu, Y.; Jiang, L.; Zhang, S.; Qin, J.; Wu, Q.; Dai, W.; Shen, S.; Pang, Z.; et al. Route to Rheumatoid Arthritis by Macrophage-Derived Microvesicle-Coated Nanoparticles. Nano Lett. 2019, 19, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Durie, F.H.; Fava, R.A.; Foy, T.M.; Aruffo, A.; Ledbetter, J.A.; Noelle, R.J. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science 1993, 261, 1328–1330. [Google Scholar] [CrossRef]
- Karnell, J.L.; Rieder, S.A.; Ettinger, R.; Kolbeck, R. Targeting the CD40-CD40L pathway in autoimmune diseases: Humoral immunity and beyond. Adv. Drug Deliv. Rev. 2019, 141, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Andreakos, E.; Rauchhaus, U.; Stavropoulos, A.; Endert, G.; Wendisch, V.; Benahmed, A.S.; Giaglis, S.; Karras, J.; Lee, S.; Gaus, H.; et al. Amphoteric liposomes enable systemic antigen-presenting cell-directed delivery of CD40 antisense and are therapeutically effective in experimental arthritis. Arthritis Rheum. 2009, 60, 994–1005. [Google Scholar] [CrossRef]
- Davignon, J.L.; Hayder, M.; Baron, M.; Boyer, J.F.; Constantin, A.; Apparailly, F.; Poupot, R.; Cantagrel, A. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology 2013, 52, 590–598. [Google Scholar] [CrossRef]
- Courties, G.; Baron, M.; Presumey, J.; Escriou, V.; Van Lent, P.; Scherman, D.; Cantagrel, A.; Van den Berg, W.B.; Jorgensen, C.; Apparailly, F.; et al. Cytosolic phospholipase A2alpha gene silencing in the myeloid lineage alters development of Th1 responses and reduces disease severity in collagen-induced arthritis. Arthritis Rheum. 2011, 63, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Schinnerling, K.; Soto, L.; Garcia-Gonzalez, P.; Catalan, D.; Aguillon, J.C. Skewing dendritic cell differentiation towards a tolerogenic state for recovery of tolerance in rheumatoid arthritis. Autoimmun. Rev. 2015, 14, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Benham, H.; Nel, H.J.; Law, S.C.; Mehdi, A.M.; Street, S.; Ramnoruth, N.; Pahau, H.; Lee, B.T.; Ng, J.; Brunck, M.E.; et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci. Transl. Med. 2015, 7, 290ra287. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.M.; Anderson, A.E.; Diboll, J.; Reece, R.; Eltherington, O.; Harry, R.A.; Fouweather, T.; MacDonald, C.; Chadwick, T.; McColl, E.; et al. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 2017, 76, 227–234. [Google Scholar] [CrossRef] [PubMed]
- García-González, P.A.; Schinnerling, K.; Sepúlveda-Gutiérrez, A.; Maggi, J.; Hoyos, L.; Morales, R.A.; Mehdi, A.M.; Nel, H.J.; Soto, L.; Pesce, B.; et al. Treatment with Dexamethasone and Monophosphoryl Lipid A Removes Disease-Associated Transcriptional Signatures in Monocyte-Derived Dendritic Cells from Rheumatoid Arthritis Patients and Confers Tolerogenic Features. Front. Immunol. 2016, 7, 458. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, N.; Apparailly, F.; Courties, G. New insights into macrophage heterogeneity in rheumatoid arthritis. Jt. Bone Spine 2021, 88, 105091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Alivernini, S.; MacDonald, L.; Elmesmari, A.; Finlay, S.; Tolusso, B.; Gigante, M.R.; Petricca, L.; Di Mario, C.; Bui, L.; Perniola, S.; et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat. Med. 2020, 26, 1295–1306. [Google Scholar] [CrossRef]
- Kuo, D.; Ding, J.; Cohn, I.S.; Zhang, F.; Wei, K.; Rao, D.A.; Rozo, C.; Sokhi, U.K.; Shanaj, S.; Oliver, D.J.; et al. HBEGF(+) macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
| Drugs | Immune Target | Clinical Stage | Main Course of Action on Macrophages/Monocytes |
|---|---|---|---|
| Infliximab | TNF | Approved | Neutralization of TNF, apoptosis of monocytes and macrophages in both peripheral blood and synovial fluid. |
| Adalimumab | Approved | ||
| Etanercept | Approved | ||
| Golimumab | Approved | ||
| Certolizumab pegol | Approved | ||
| Tocilizumab | IL-6 | Approved | Inhibition of IL-6, apoptosis of monocytes. |
| Sarilumab | Approved | ||
| Olokizumab | Phase III | ||
| Clazakizumab | Phase IIb | ||
| Vobarilizumab | Phase III | ||
| Sirukumab | Phase III | ||
| Anakinra | IL-1 | Approved | Inhibition of IL-1. |
| Canakinumab | Phase II | ||
| Rilonacept | Phase II | ||
| Mavrilimumab | GM-CSF | Phase IIb | Blocking of GM-CSF, inhibition of M1 polarization. |
| Gimsilumab | Phase I | ||
| Otilimab | Phase III | ||
| Namilumab | Phase II | ||
| Lenzilumab | Phase II | ||
| Tofacitinib | JAK1/JAK3 | Approved | Blocking of JAK-STAT pathway activity and macrophage-secreted inflammatory factors. |
| Baricitinib | JAK1/JAK2 | Approved | |
| Upadacitinib | JAK1 | Approved | |
| Spebrutinib | BTK | Phase II | Blocking of macrophage-secreted TNF-α, nitric oxide and IL-Iβ. |
| Fenebrutinib | Phase II | ||
| Abatacept | CD80/CD86 | Approved | Prevention of co-stimulation between APCs and T cells. Inhibition of monocyte differentiation into osteoclasts. |
| Ustekinumab | IL-12/IL-23 | Phase II | Inhibition of IL-12/IL-23. |
| Guselkumab | IL-23 | Phase II | Inhibition of IL-23. |
| H22 (scFv) -MAP | CD64 | Preclinical | CD64+ M1 macrophage apoptosis. |
| VIB4920 | CD40L | Phase II | Blocking the access of macrophages to the synovial tissue. |
| Clodronate-containing liposomes | Release chlorophosphate | Preclinical | Macrophage depletion. |
| NI-0101 | TLR-4 | Phase II | Blocking innate inflammatory responses by blocking TLR-4 activation. |
| TAK-242 | TLR-4 | Preclinical | Blocking innate inflammatory responses by blocking TLR-4 activation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roszkowski, L.; Ciechomska, M. Tuning Monocytes and Macrophages for Personalized Therapy and Diagnostic Challenge in Rheumatoid Arthritis. Cells 2021, 10, 1860. https://doi.org/10.3390/cells10081860
Roszkowski L, Ciechomska M. Tuning Monocytes and Macrophages for Personalized Therapy and Diagnostic Challenge in Rheumatoid Arthritis. Cells. 2021; 10(8):1860. https://doi.org/10.3390/cells10081860
Chicago/Turabian StyleRoszkowski, Leszek, and Marzena Ciechomska. 2021. "Tuning Monocytes and Macrophages for Personalized Therapy and Diagnostic Challenge in Rheumatoid Arthritis" Cells 10, no. 8: 1860. https://doi.org/10.3390/cells10081860
APA StyleRoszkowski, L., & Ciechomska, M. (2021). Tuning Monocytes and Macrophages for Personalized Therapy and Diagnostic Challenge in Rheumatoid Arthritis. Cells, 10(8), 1860. https://doi.org/10.3390/cells10081860






