Mitotic Acetylation of Microtubules Promotes Centrosomal PLK1 Recruitment and Is Required to Maintain Bipolar Spindle Homeostasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Cell Culture and Generation of Cell Lines
2.3. siRNAs, Cell Transfection, and Drug Treatments
2.4. Immunofluorescence
2.5. Cell Imaging
2.6. Image Quantification and Statistical Analysis
3. Results
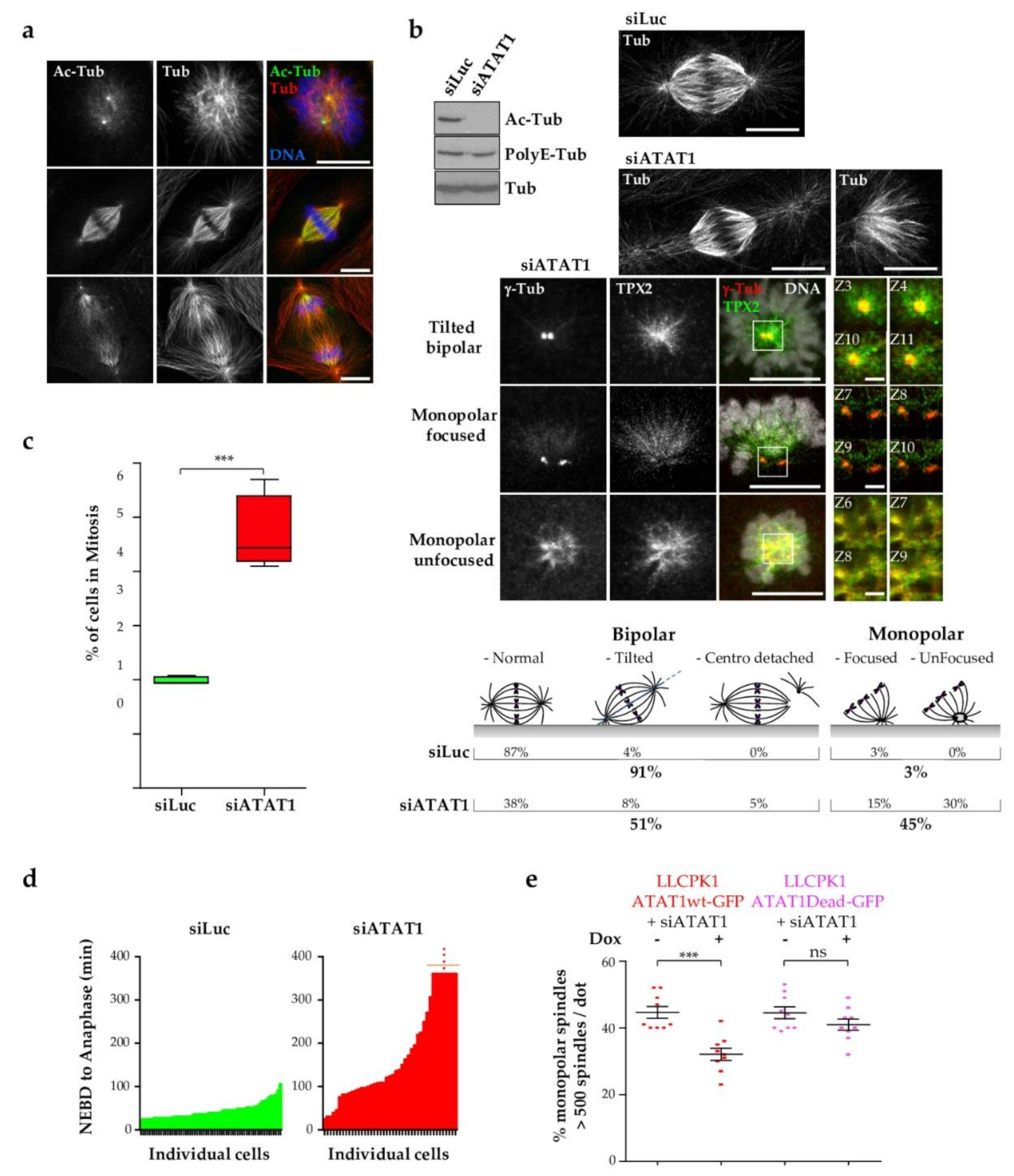
3.1. Loss of ATAT1 Induces Mitotic Defects in LLCPK1 Cells
3.2. Kinesin-5 Activity Is Not Affected in siATAT1 Cells
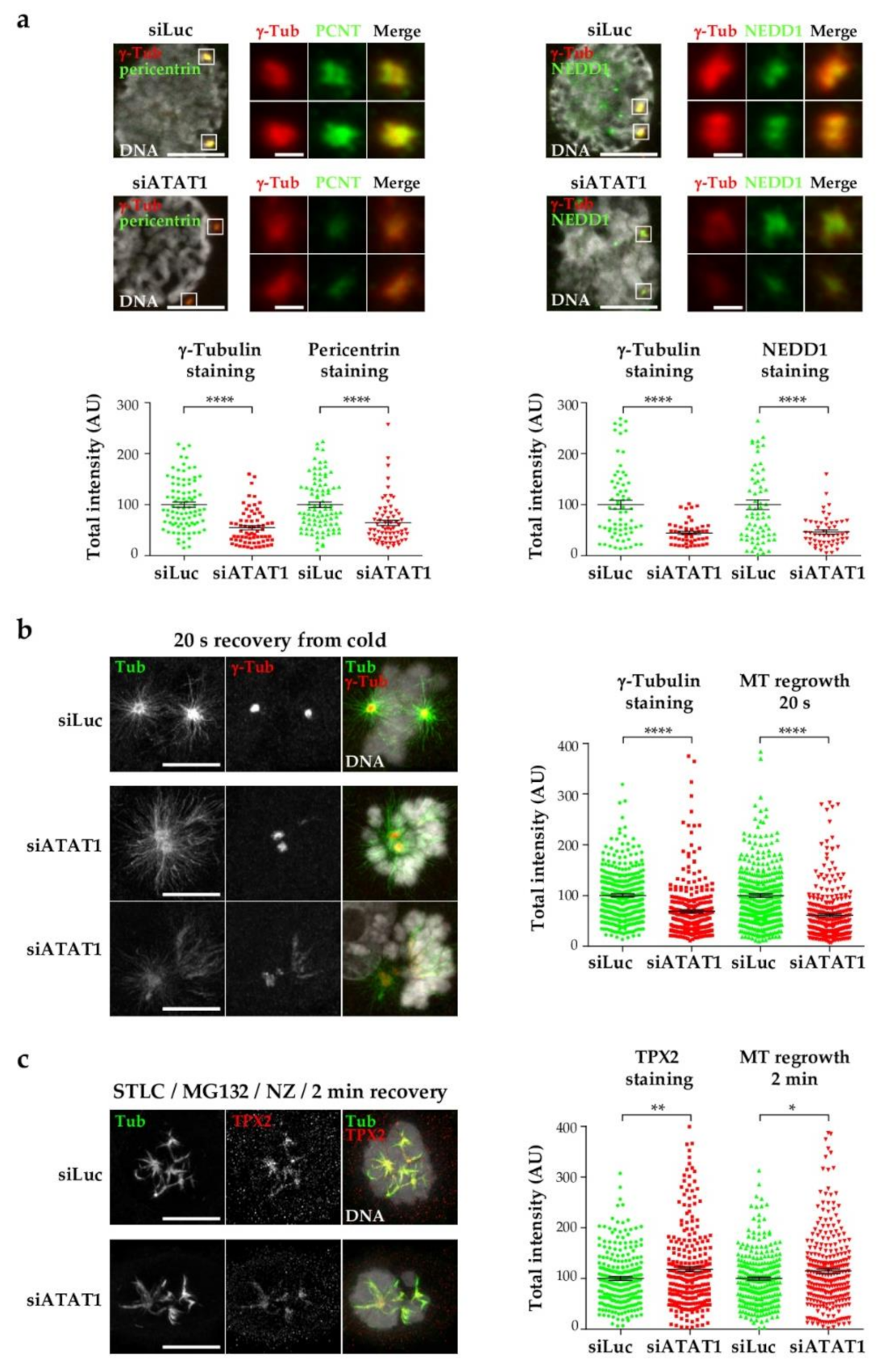
3.3. Centrosome Maturation and Centrosome-Dependent MT Nucleation Are Defective in siATAT1 Mitotic Cells
3.4. PLK1 Recruitment to Centrosomes Is Hampered in siATAT1 Cells
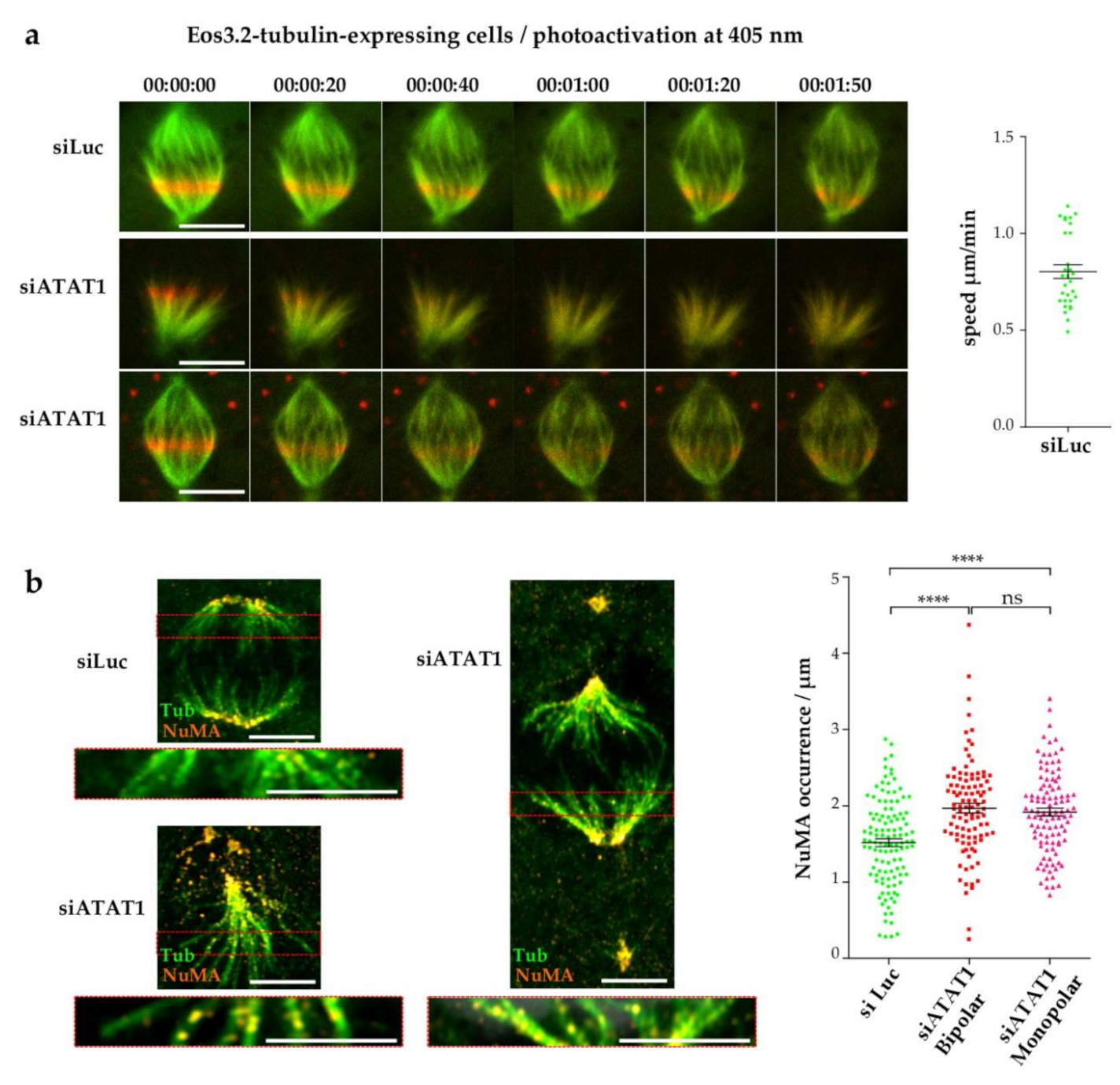
3.5. Loss of MT Acetylation Impedes MT Flux in Mitosis and Induces Breaks in Spindle MT Bundles
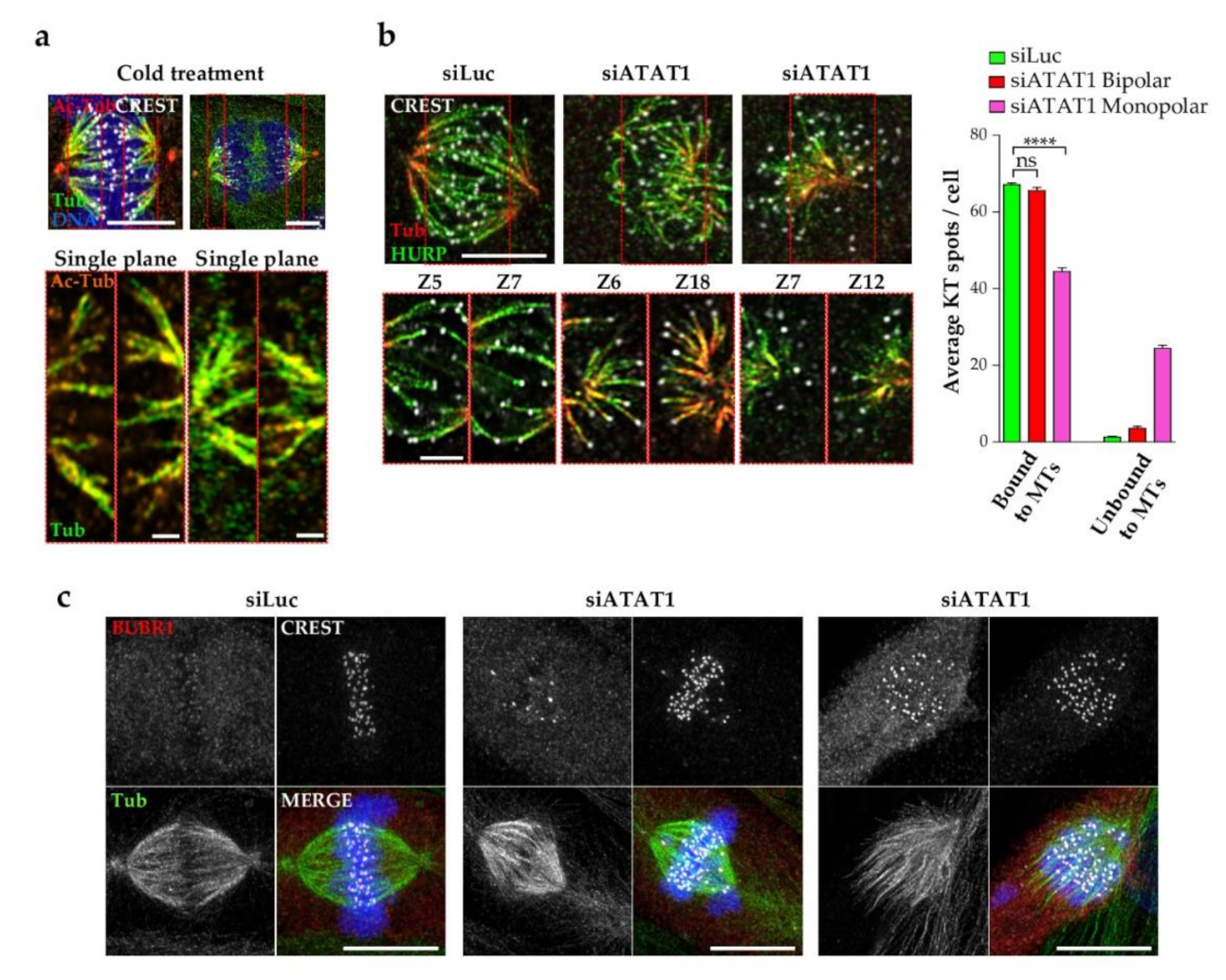
3.6. Kinetochore Fibers Are Acetylated and Loss of MT Acetylation Activates the Spindle Checkpoint
3.7. In siATAT1 Cells, Stabilizing MT Plus-Ends, or Increasing Centrosomal PLK1 Activity Restore Spindle Bipolarity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desai, A.; Mitchison, T.J. Microtubule Polymerization Dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- Hazan, J.; Fonknechten, N.; Mavel, D.; Paternotte, C.; Samson, D.; Artiguenave, F.; Davoine, C.-S.; Cruaud, C.; Dürr, A.; Wincker, P.; et al. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat. Genet. 1999, 23, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, B.; Van Dijk, J.; Gold, N.D.; Guizetti, J.; Aldrian-Herrada, G.; Rogowski, K.; Gerlich, D.W.; Janke, C. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J. Cell Biol. 2010, 189, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Valenstein, M.L.; Roll-Mecak, A. Graded Control of Microtubule Severing by Tubulin Glutamylation. Cell 2016, 164, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Peris, L.; Thery, M.; Fauré, J.; Saoudi, Y.; Lafanechère, L.; Chilton, J.K.; Gordon-Weeks, P.; Galjart, N.; Bornens, M.; Wordeman, L.; et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J. Cell Biol. 2006, 174, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Barisic, M.; Sousa, R.S.; Tripathy, S.K.; Magiera, M.M.; Zaytsev, A.V.; Pereira, A.L.; Janke, C.; Grishchuk, E.L.; Maiato, H. Microtubule detyrosination guides chromosomes during mitosis. Science 2015, 348, 799–803. [Google Scholar] [CrossRef]
- Ferreira, L.T.; Orr, B.; Rajendraprasad, G.; Pereira, A.J.; Lemos, C.; Lima, J.T.; Boldú, C.G.; Ferreira, J.G.; Barisic, M.; Maiato, H. α-Tubulin detyrosination impairs mitotic error correction by suppressing MCAK centromeric activity. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef]
- L’Hernault, S.W.; Rosenbaum, J.L. Chlamydomonas.alpha.-tubulin is posttranslationally modified by acetylation on the.epsilon.-amino group of a lysine. Biochemistry 1985, 24, 473–478. [Google Scholar] [CrossRef]
- Xu, Z.; Schaedel, L.; Portran, D.; Aguilar, A.; Gaillard, J.; Marinkovich, M.P.; Théry, M.; Nachury, M.V. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 2017, 356, 328–332. [Google Scholar] [CrossRef]
- Portran, D.; Schaedel, L.; Xu, Z.; Théry, L.S.M.; Nachury, D.P.Z.X.M.V. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 2017, 19, 391–398. [Google Scholar] [CrossRef]
- Kim, G.-W.; Li, L.; Gorbani, M.; You, L.; Yang, X.-J. Mice Lacking α-Tubulin Acetyltransferase 1 Are Viable but Display α-Tubulin Acetylation Deficiency and Dentate Gyrus Distortion. J. Biol. Chem. 2013, 288, 20334–20350. [Google Scholar] [CrossRef]
- Aguilar, A.; Becker, L.; Tedeschi, T.; Heller, S.; Iomini, C.; Nachury, M.V. α-Tubulin K40 acetylation is required for contact inhibition of proliferation and cell–substrate adhesion. Mol. Biol. Cell 2014, 25, 1854–1866. [Google Scholar] [CrossRef]
- Kalebic, N.; Sorrentino, S.; Perlas, E.; Bolasco, G.; Martinez, C.; Heppenstall, P.A. αTAT1 is the major α-tubulin acetyltransferase in mice. Nat. Commun. 2013, 4, 1962. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, T.; Nemoto, T.; Suzuki, T.; Asano-Hoshino, A.; Tanaka, H.; Arisawa, K.; Nishijima, Y.; Kiuchi, Y.; Hagiwara, H. Adrenalectomy facilitates ATAT1 expression and α-tubulin acetylation in ACTH-producing corticotrophs. Cell Tissue Res. 2016, 366, 363–370. [Google Scholar] [CrossRef]
- Wu, K.; Wang, L.; Chen, Y.; Pirooznia, M.; Singh, K.; Wälde, S.; Kehlenbach, R.H.; Scott, I.; Gucek, M.; Sack, M.N. GCN5L1 interacts with αTAT1 and RanBP2 to regulate hepatic α-tubulin acetylation and lysosome trafficking. J. Cell Sci. 2018, 131, jcs.221036. [Google Scholar] [CrossRef] [PubMed]
- Montagnac, G.; Meas-Yedid, V.; Irondelle, M.; Castro-Castro, A.; Franco, M.; Shida, T.; Nachury, M.; Benmerah, A.; Olivo-Marin, J.-C.; Chavrier, P. αTAT1 catalyses microtubule acetylation at clathrin-coated pits. Nat. Cell Biol. 2013, 502, 567–570. [Google Scholar] [CrossRef]
- Bance, B.; Seetharaman, S.; Leduc, C.; Boëda, B.; Etienne-Manneville, S. Microtubule acetylation but not detyrosination promotes focal adhesion dynamics and astrocyte migration. J. Cell Sci. 2019, 132, jcs.225805. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, G.; Calogero, A.M.; Rolando, C. Microtubule acetylation: A reading key to neural physiology and degeneration. Neurosci. Lett. 2021, 755, 135900. [Google Scholar] [CrossRef]
- Castro-Castro, A.; Janke, C.; Montagnac, G.; Paul-Gilloteaux, P.; Chavrier, P. ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur. J. Cell Biol. 2012, 91, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; You, E.; Ko, P.; Jeong, J.; Keum, S.; Rhee, S. Genetic disruption of tubulin acetyltransferase, αTAT1, inhibits proliferation and invasion of colon cancer cells through decreases in Wnt1/β-catenin signaling. Biochem. Biophys. Res. Commun. 2017, 482, 8–14. [Google Scholar] [CrossRef]
- Tan, H.-F.; Tan, S.-M. The focal adhesion protein kindlin-2 controls mitotic spindle assembly by inhibiting histone deacetylase 6 and maintaining α-tubulin acetylation. J. Biol. Chem. 2020, 295, 5928–5943. [Google Scholar] [CrossRef]
- Wolf, K.W. Acetylation of α-tubilin in different bovine cell types: Implications for microtubule dynamics in interphase and mitosis. Cell Biol. Int. 1995, 19, 43–52. [Google Scholar] [CrossRef]
- Piperno, G.; Ledizet, M.; Chang, X.J. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 1987, 104, 289–302. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Chen, H.; Zhan, Y.-Q.; Yin, R.-H.; Li, C.-Y.; Ge, C.-H.; Yu, M.; Yang, X.-M. EWSR1 regulates mitosis by dynamically influencing microtubule acetylation. Cell Cycle 2016, 15, 2202–2215. [Google Scholar] [CrossRef]
- Patel, H.; Stavrou, I.; Shrestha, R.L.; Draviam, V.; Frame, M.C.; Brunton, V.G. Kindlin1 regulates microtubule function to ensure normal mitosis. J. Mol. Cell Biol. 2016, 8, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Steinhäuser, K.; Klöble, P.; Kreis, N.-N.; Ritter, A.; Friemel, A.; Roth, S.; Reichel, J.M.; Michaelis, J.; Rieger, M.; Louwen, F.; et al. Deficiency of RITA results in multiple mitotic defects by affecting microtubule dynamics. Oncogene 2016, 36, 2146–2159. [Google Scholar] [CrossRef]
- Combes, G.; Alharbi, I.; Braga, L.G.; Elowe, S. Playing polo during mitosis: PLK1 takes the lead. Oncogene 2017, 36, 4819–4827. [Google Scholar] [CrossRef] [PubMed]
- Colicino, E.G.; Hehnly, H. Regulating a key mitotic regulator, polo-like kinase 1 (PLK1). Cytoskeleton 2018, 75, 481–494. [Google Scholar] [CrossRef]
- Shimizu, H.; Ito, M.; Miyahara, M.; Ichikawa, K.; Okubo, S.; Konishi, T.; Naka, M.; Tanaka, T.; Hirano, K.; Hartshorne, D.J. Characteri-zation of the myosin-binding subunit of smooth muscle myosin phosphatase. J. Biol. Chem. 1994, 269, 30407–30411. [Google Scholar] [CrossRef]
- Yamashiro, S.; Yamakita, Y.; Totsukawa, G.; Goto, H.; Kaibuchi, K.; Ito, M.; Hartshorne, D.J.; Matsumura, F. Myosin Phosphatase-Targeting Subunit 1 Regulates Mitosis by Antagonizing Polo-like Kinase 1. Dev. Cell 2008, 14, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Bompard, G.; Rabeharivelo, G.; Frank, M.; Cau, J.; Delsert, C.; Morin, N. Subgroup II PAK-mediated phosphorylation regulates Ran activity during mitosis. J. Cell Biol. 2010, 190, 807–822. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, J.; Rogowski, K.; Miro, J.; Lacroix, B.; Eddé, B.; Janke, C. A Targeted Multienzyme Mechanism for Selective Microtubule Polyglutamylation. Mol. Cell 2007, 26, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Rusan, N.M.; Tulu, U.S.; Fagerstrom, C.; Wadsworth, P. Reorganization of the microtubule array in prophase/prometaphase requires cytoplasmic dynein-dependent microtubule transport. J. Cell Biol. 2002, 158, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Durisic, N.; Laparra-Cuervo, L.; Sandoval-Álvarez, Á.; Borbely, J.; Lakadamyali, M. Single-molecule evaluation of fluorescent protein photoactivation efficiency using an in vivo nanotemplate. Nat. Methods 2014, 11, 156–162. [Google Scholar] [CrossRef]
- Kowarz, E.; Loescher, D.; Marschalek, R. Optimized Sleeping Beauty transposons rapidly generate stable transgenic cell lines. Biotechnol. J. 2015, 10, 647–653. [Google Scholar] [CrossRef]
- Shida, T.; Cueva, J.G.; Xu, Z.; Goodman, M.B.; Nachury, M.V. The major -tubulin K40 acetyltransferase TAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA 2010, 107, 21517–21522. [Google Scholar] [CrossRef]
- Nagai, T.; Ikeda, M.; Chiba, S.; Kanno, S.-I.; Mizuno, K. Furry promotes acetylation of microtubules in the mitotic spindle via inhibition of SIRT2 tubulin-deacetylase. J. Cell Sci. 2013, 126, 4369–4380. [Google Scholar] [CrossRef]
- Ferenz, N.P.; Gable, A.; Wadsworth, P. Mitotic functions of kinesin-5. Semin. Cell Dev. Biol. 2010, 21, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Eibes, S.; Gallisà-Suñé, N.; Rosas-Salvans, M.; Martínez-Delgado, P.; Vernos, I.; Roig, J. Nek9 Phosphorylation Defines a New Role for TPX2 in Eg5-Dependent Centrosome Separation before Nuclear Envelope Breakdown. Curr. Biol. 2018, 28, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sawin, K.; Leguellec, K.; Philippe, M.; Mitchison, T.J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nat. Cell Biol. 1992, 359, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Blangy, A.; Lane, H.A.; D’Hérin, P.; Harper, M.; Kress, M.; Nigg, E. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 1995, 83, 1159–1169. [Google Scholar] [CrossRef]
- Torosantucci, L.; De Luca, M.; Guarguaglini, G.; LaVia, P.; Degrassi, F. Localized RanGTP Accumulation Promotes Microtubule Nucleation at Kinetochores in Somatic Mammalian Cells. Mol. Biol. Cell 2008, 19, 1873–1882. [Google Scholar] [CrossRef]
- Bastiaens, P.; Caudron-Herger, M.; Niethammer, P.; Karsenti, E. Gradients in the self-organization of the mitotic spindle. Trends Cell Biol. 2006, 16, 125–134. [Google Scholar] [CrossRef]
- Lenart, P.; Petronczki, M.; Steegmaier, M.; Di Fiore, B.; Lipp, J.J.; Hoffmann, M.; Rettig, W.J.; Kraut, N.; Peters, J.-M. The Small-Molecule Inhibitor BI 2536 Reveals Novel Insights into Mitotic Roles of Polo-like Kinase 1. Curr. Biol. 2007, 17, 304–315. [Google Scholar] [CrossRef]
- Dudka, D.; Castrogiovanni, C.; Liaudet, N.; Vassal, H.; Meraldi, P. Spindle-Length-Dependent HURP Localization Allows Centrosomes to Control Kinetochore-Fiber Plus-End Dynamics. Curr. Biol. 2019, 29, 3563–3578.e6. [Google Scholar] [CrossRef]
- Wu, G.; Wei, R.; Cheng, E.; Ngo, B.; Lee, W.-H. Hec1 Contributes to Mitotic Centrosomal Microtubule Growth for Proper Spindle Assembly through Interaction with Hice1. Mol. Biol. Cell 2009, 20, 4686–4695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ganem, N.; Compton, D.A. The KinI kinesin Kif2a is required for bipolar spindle assembly through a functional relationship with MCAK. J. Cell Biol. 2004, 166, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Maffini, S.; Maia, A.R.; Manning, A.L.; Maliga, Z.; Pereira, A.L.; Junqueira, M.; Shevchenko, A.; Hyman, A.; Yates, J.R.; Galjart, N.; et al. Motor-Independent Targeting of CLASPs to Kinetochores by CENP-E Promotes Microtubule Turnover and Poleward Flux. Curr. Biol. 2009, 19, 1566–1572. [Google Scholar] [CrossRef]
- Steblyanko, Y.; Rajendraprasad, G.; Osswald, M.; Eibes, S.; Jacome, A.; Geley, S.; Pereira, A.J.; Maiato, H.; Barisic, M. Microtubule poleward flux in human cells is driven by the coordinated action of four kinesins. EMBO J. 2020, 39, e105432. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.-Y.; Coppinger, J.; Seki, A.; Yates, J.R.; Fang, G. Plk1 and Aurora A regulate the depolymerase activity and the cellular localization of Kif2a. J. Cell Sci. 2009, 122, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Sanhaji, M.; Steinhäuser, K.; Roth, S.; Louwen, F.; Yuan, J. The activity regulation of the mitotic centromere-associated kinesin by Polo-like kinase 1. Oncotarget 2014, 6, 6641–6655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shao, H.; Huang, Y.; Zhang, L.; Yuan, K.; Chu, Y.; Dou, Z.; Jin, C.; Garcia-Barrio, M.; Liu, X.; Yao, X. Spatiotemporal dynamics of Aurora B-PLK1-MCAK signaling axis orchestrates kinetochore bi-orientation and faithful chromosome segregation. Sci. Rep. 2015, 5, 12204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shao, H.; Huang, Y.; Yan, F.; Chu, Y.; Hou, H.; Zhu, M.; Fu, C.; Aikhionbare, F.; Fang, G.; et al. PLK1 Phosphorylates Mitotic Centromere-associated Kinesin and Promotes Its Depolymerase Activity. J. Biol. Chem. 2011, 286, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Barisic, M.; Rajendraprasad, G.; Steblyanko, Y. The metaphase spindle at steady state—Mechanism and functions of microtubule poleward flux. Semin. Cell Dev. Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Taulet, N.; Vitre, B.; Anguille, C.; Douanier, A.; Rocancourt, M.; Taschner, M.; Lorentzen, E.; Echard, A.; Delaval, B. IFT proteins spatially control the geometry of cleavage furrow ingression and lumen positioning. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, J.; Bibikova, M.; Leverson, J.D.; Bossy-Wetzel, E.; Fan, J.-B.; Abraham, R.T.; Jiang, W. Functional Analysis of Human Microtubule-based Motor Proteins, the Kinesins and Dyneins, in Mitosis/Cytokinesis Using RNA Interference. Mol. Biol. Cell 2005, 16, 3187–3199. [Google Scholar] [CrossRef] [PubMed]
- Cueva, J.G.; Hsin, J.; Huang, K.C.; Goodman, M.B. Posttranslational Acetylation of α-Tubulin Constrains Protofilament Number in Native Microtubules. Curr. Biol. 2012, 22, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Polak, B.; Simunić, J.; Boban, Z.; Kuzmić, B.; Thomae, A.; Tolić, I.M.; Pavin, N. The mitotic spindle is chiral due to torques within microtubule bundles. Nat. Commun. 2018, 9, 3571. [Google Scholar] [CrossRef]
- Smith, E.; Hégarat, N.; Vesely, C.; Roseboom, I.; Larch, C.; Streicher, H.; Straatman, K.; Flynn, H.; Skehel, M.; Hirota, T.; et al. Differential control of Eg5-dependent centrosome separation by Plk1 and Cdk1. EMBO J. 2011, 30, 2233–2245. [Google Scholar] [CrossRef]
- Tanenbaum, M.E.; Macůrek, L.; Janssen, A.; Geers, E.F.; Fernández, M.A.; Medema, R.H. Kif15 Cooperates with Eg5 to Promote Bipolar Spindle Assembly. Curr. Biol. 2009, 19, 1703–1711. [Google Scholar] [CrossRef]
- Raaijmakers, J.; Van Heesbeen, R.G.H.P.; Meaders, J.L.; Geers, E.F.; Fernandez-Garcia, B.; Medema, R.H.; Tanenbaum, M. Nuclear envelope-associated dynein drives prophase centrosome separation and enables Eg5-independent bipolar spindle formation. EMBO J. 2012, 31, 4179–4190. [Google Scholar] [CrossRef]
- Gemble, S.; Simon, A.; Pennetier, C.; Dumont, M.; Hervé, S.; Meitinger, F.; Oegema, K.; Rodriguez, R.; Almouzni, G.; Fachinetti, D.; et al. Centromere Dysfunction Compromises Mitotic Spindle Pole Integrity. Curr. Biol. 2019, 29, 3072–3080. [Google Scholar] [CrossRef]
- Zimmerman, W.C.; Sillibourne, J.; Rosa, J.; Doxsey, S.J. Mitosis-specific Anchoring of γ Tubulin Complexes by Pericentrin Controls Spindle Organization and Mitotic Entry. Mol. Biol. Cell 2004, 15, 3642–3657. [Google Scholar] [CrossRef] [PubMed]
- Haren, L.; Stearns, T.; Lüders, J. Plk1-Dependent Recruitment of γ-Tubulin Complexes to Mitotic Centrosomes Involves Multiple PCM Components. PLoS ONE 2009, 4, e5976. [Google Scholar] [CrossRef]
- Haren, L.; Remy, M.-H.; Bazin, I.; Callebaut, I.; Wright, M.; Merdes, A. NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 2006, 172, 505–515. [Google Scholar] [CrossRef]
- Fong, K.-W.; Choi, Y.-K.; Rattner, J.B.; Qi, R.Z. CDK5RAP2 Is a Pericentriolar Protein That Functions in Centrosomal Attachment of the γ-Tubulin Ring Complex. Mol. Biol. Cell 2008, 19, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, R.E.; Vogel, J.M.; Schnackenberg, B.J.; Hull, D.R.; Wu, X. Centrosome maturation. Curr. Top. Dev. Biol. 1999, 49, 449–470. [Google Scholar] [CrossRef]
- Mennella, V.; Agard, D.A.; Huang, B.; Pelletier, L. Amorphous no more: Subdiffraction view of the pericentriolar material architecture. Trends Cell Biol. 2014, 24, 188–197. [Google Scholar] [CrossRef]
- Ohta, M.; Zhao, Z.; Wu, D.; Wang, S.; Harrison, J.L.; Gómez-Cavazos, J.S.; Desai, A.; Oegema, K.F. Polo-like kinase 1 independently controls microtubule-nucleating capacity and size of the centrosome. J. Cell Biol. 2021, 220, e202009083. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Rhee, K. PLK1 phosphorylation of pericentrin initiates centrosome maturation at the onset of mitosis. J. Cell Biol. 2011, 195, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Rhee, K. Importance of the CEP215-Pericentrin Interaction for Centrosome Maturation during Mitosis. PLoS ONE 2014, 9, e87016. [Google Scholar] [CrossRef]
- Dobbelaere, J.; Cernohorska, M.S.; Huranova, M.; Slade, D.; Dammermann, A. Cep97 Is Required for Centriole Structural Integrity and Cilia Formation in Drosophila. Curr. Biol. 2020, 30, 3045–3056. [Google Scholar] [CrossRef]
- Gasic, I.; Nerurkar, P.; Meraldi, P. Centrosome age regulates kinetochore–microtubule stability and biases chromosome mis-segregation. eLife 2015, 4, e07909. [Google Scholar] [CrossRef] [PubMed]
- Kishi, K.; van Vugt, M.A.T.M.; Okamoto, K.-I.; Hayashi, Y.; Yaffe, M.B. Functional Dynamics of Polo-Like Kinase 1 at the Centrosome. Mol. Cell. Biol. 2009, 29, 3134–3150. [Google Scholar] [CrossRef]
- Nguyen, A.L.; Fadel, M.D.; Cheeseman, I.M. Differential requirements for the CENP-O complex reveal parallel PLK1 kinetochore recruitment pathways. Mol. Biol. Cell 2021, 32, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Ems-McClung, S.C.; Hainline, S.G.; Devare, J.; Zong, H.; Cai, S.; Carnes, S.K.; Shaw, S.; Walczak, C.E. Aurora B Inhibits MCAK Activity through a Phosphoconformational Switch that Reduces Microtubule Association. Curr. Biol. 2013, 23, 2491–2499. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Kreis, N.-N.; Louwen, F.; Wordeman, L.; Yuan, J. Molecular insight into the regulation and function of MCAK. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 228–245. [Google Scholar] [CrossRef]
- McHugh, T.; Zou, J.; Volkov, V.; Bertin, A.; Rappsilber, J.; Dogterom, M.; Welburn, J.P.I. The depolymerase activity of MCAK shows graded response to Aurora B kinase phosphorylation through allosteric regulation. J. Cell Sci. 2019, 132, jcs228353. [Google Scholar] [CrossRef]
- Xia, P.; Wang, Z.; Liu, X.; Wu, B.; Wang, J.; Ward, T.; Zhang, L.; Ding, X.; Gibbons, G.; Shi, Y.; et al. EB1 acetylation by P300/CBP-associated factor (PCAF) ensures accurate kinetochore-microtubule interactions in mitosis. Proc. Natl. Acad. Sci. USA 2012, 109, 16564–16569. [Google Scholar] [CrossRef]
- Zhao, G.; Cheng, Y.; Gui, P.; Cui, M.; Liu, W.; Wang, W.; Wang, X.; Ali, M.; Dou, Z.; Niu, L.; et al. Dynamic acetylation of the kinetochore-associated protein HEC1 ensures accurate microtubule–kinetochore attachment. J. Biol. Chem. 2019, 294, 576–592. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Choe, H.; Min, J.; Choi, J.Y.; Kim, J.; Lee, H. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J. 2009, 28, 2077–2089. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasamizafy, S.F.; Delsert, C.; Rabeharivelo, G.; Cau, J.; Morin, N.; van Dijk, J. Mitotic Acetylation of Microtubules Promotes Centrosomal PLK1 Recruitment and Is Required to Maintain Bipolar Spindle Homeostasis. Cells 2021, 10, 1859. https://doi.org/10.3390/cells10081859
Rasamizafy SF, Delsert C, Rabeharivelo G, Cau J, Morin N, van Dijk J. Mitotic Acetylation of Microtubules Promotes Centrosomal PLK1 Recruitment and Is Required to Maintain Bipolar Spindle Homeostasis. Cells. 2021; 10(8):1859. https://doi.org/10.3390/cells10081859
Chicago/Turabian StyleRasamizafy, Sylvia Fenosoa, Claude Delsert, Gabriel Rabeharivelo, Julien Cau, Nathalie Morin, and Juliette van Dijk. 2021. "Mitotic Acetylation of Microtubules Promotes Centrosomal PLK1 Recruitment and Is Required to Maintain Bipolar Spindle Homeostasis" Cells 10, no. 8: 1859. https://doi.org/10.3390/cells10081859
APA StyleRasamizafy, S. F., Delsert, C., Rabeharivelo, G., Cau, J., Morin, N., & van Dijk, J. (2021). Mitotic Acetylation of Microtubules Promotes Centrosomal PLK1 Recruitment and Is Required to Maintain Bipolar Spindle Homeostasis. Cells, 10(8), 1859. https://doi.org/10.3390/cells10081859






