Cancer Metabostemness and Metabolic Reprogramming via P2X7 Receptor
Abstract
:1. Introduction
2. The Purinergic Signaling System—Structural Insights Focusing on the P2X7 Receptor
3. Metabolic Pathways Driving Cancer Cell Survival and Stemness
4. P2X7 Receptor Relevance in Metabolism
5. Anti-P2X7 Receptor Drugs in Effectivity Studies or in Use for Cancer Therapy
6. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuahasi, K.K.; Demasi, M.A.; Tamajusuku, A.S.K.; Lenz, G.; Sogayar, M.C.; Fornazari, M.; Lameu, C.; Nascimento, N.I.; Glaser, T.; Schwindt, T.T.; et al. Regulation of Neurogenesis and Gliogenesis of Retinoic Acid-Induced P19 Embryonal Carcinoma Cells by P2X2 and P2X7 Receptors Studied by RNA Interference. Int. J. Dev. Neurosci. 2012, 30, 91–97. [Google Scholar] [CrossRef]
- Andrejew, R.; Oliveira-Giacomelli, Á.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.E.; Palombit, K.; Tavares-de-Lima, W.; Castelucci, P. Enteric Glial Cells Immunoreactive for P2X7 Receptor Are Affected in the Ileum Following Ischemia and Reperfusion. Acta Histochem. 2019, 121, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.S.; Barreira, A.L.; Gomes, C.R.; Ornellas, F.M.; Ornellas, D.S.; Miranda, L.C.; Cardoso, L.R.; Coutinho-Silva, R.; Schanaider, A.; Morales, M.M.; et al. Brilliant Blue G, a P2X7 Receptor Antagonist, Attenuates Early Phase of Renal Inflammation, Interstitial Fibrosis and Is Associated with Renal Cell Proliferation in Ureteral Obstruction in Rats. BMC Nephrol. 2020, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Sampaio, V.F.; Rabelo, I.L.A.; Bento, C.A.; Glaser, T.; Bezerra, J.; Coutinho-Silva, R.; Ulrich, H.; Lameu, C. Using Cytometry for Investigation of Purinergic Signaling in Tumor-Associated Macrophages. Cytom. Part A 2020, 97, 1109–1126. [Google Scholar] [CrossRef]
- Ulrich, H.; Ratajczak, M.Z.; Schneider, G.; Adinolfi, E.; Orioli, E.; Ferrazoli, E.G.; Glaser, T.; Corrêa-Velloso, J.; Martins, P.C.M.; Coutinho, F.; et al. Kinin and Purine Signaling Contributes to Neuroblastoma Metastasis. Front. Pharmacol. 2018, 9, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, R.; Adinolfi, E.; Harwood, C.A.; Philpott, M.; Barden, J.A.; Di Virgilio, F.; McNulty, S. P2X7 in Cancer: From Molecular Mechanisms to Therapeutics. Front. Pharmacol. 2020, 11, 793. [Google Scholar] [CrossRef]
- Amoroso, F.; Salaro, E.; Falzoni, S.; Chiozzi, P.; Lisa, A.; Cavallesco, G.; Maniscalco, P.; Puozzo, A.; Bononi, I. P2X7 Targeting Inhibits Growth of Human Mesothelioma. Oncotarget 2016, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Toombes, G.E.S.; Silberberg, S.D.; Swartz, K.J. Physical Basis of Apparent Pore Dilation of ATP-Activated P2X Receptor Channels. Nat. Neurosci. 2015, 18, 1577–1583. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef]
- Adinolfi, E.; Cirillo, M.; Woltersdorf, R.; Falzoni, S.; Chiozzi, P.; Pellegatti, P.; Callegari, M.G.; Sandona, D.; Markwardt, F.; Schmalzing, G.; et al. Trophic Activity of a Naturally Occurring Truncated Isoform of the P2X7 Receptor. Faseb. J. 2010, 24, 3393–3404. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 Purinergic Signalling in the Tumour Microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Hu, C.G.; Zhu, Z.M.; Luo, H.L. Effect of P2X7 Receptor on Tumorigenesis and Its Pharmacological Properties. Biomed. Pharmacother. 2020, 125, 2019. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Sampaio, V.F.; Rabelo, I.L.A.; Ulrich, H.; Lameu, C. The P2X7 Receptor in the Maintenance of Cancer Stem Cells, Chemoresistance and Metastasis. Stem Cell Rev. Rep. 2020, 16, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.S.H.; Frezza, C. Metabolic Reprogramming and Oncogenesis: One Hallmark, Many Organelles, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 332. [Google Scholar]
- Tang, Z.; Ye, W.; Chen, H.; Kuang, X.; Guo, J.; Xiang, M.; Peng, C.; Chen, X.; Liu, H. Role of Purines in Regulation of Metabolic Reprogramming. Purinergic Signal. 2019, 15, 423–438. [Google Scholar] [CrossRef]
- Adinolfi, E.; Callegari, M.G.; Ferrari, D.; Bolognesi, C.; Minelli, M.; Wieckowski, M.R.; Pinton, P.; Rizzuto, R. Basal Activation of the P2X7 ATP Receptor Elevates Mitochondrial Calcium and Potential, Increases Cellular ATP Levels, and Promotes Serum-Independent Growth. Mol. Biol. Cell 2005, 16, 3260–3272. [Google Scholar] [CrossRef] [Green Version]
- Sarti, A.C.; Vultaggio-Poma, V.; Falzoni, S.; Missiroli, S.; Giuliani, A.L.; Boldrini, P.; Bonora, M.; Faita, F.; Di Lascio, N.; Kusmic, C.; et al. Mitochondrial P2X7 Receptor Localization Modulates Energy Metabolism Enhancing Physical Performance. Function 2021, 2, zqab005. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Adinolfi, E.; Virgilio, F.; Di Adinol, E.; Di Virgilio, F.; Adinolfi, E.; Virgilio, F.; Di Adinol, E. Extracellular Purines, Purinergic Receptors and Tumor Growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, F.; Capece, M.; Rotondo, A.; Cangelosi, D.; Ferracin, M.; Franceschini, A.; Raffaghello, L.; Pistoia, V.; Varesio, L.; Adinol, E.; et al. The P2X7 Receptor Is a Key Modulator of the PI3K/GSK3 β/VEGF Signaling Network: Evidence in Experimental Neuroblastoma. Oncogene 2015, 34, 5240–5251. [Google Scholar] [CrossRef]
- Arguin, G.; Bourzac, J.F.; Placet, M.; Molle, C.M.; Paquette, M.; Beaudoin, J.F.; Rousseau, J.A.; Lecomte, R.; Plourde, M.; Gendron, F.P. The Loss of P2X7 Receptor Expression Leads to Increase Intestinal Glucose Transit and Hepatic Steatosis. Sci. Rep. 2017, 7, 12917. [Google Scholar] [CrossRef] [Green Version]
- Menendez, J.A.; Alarcón, T. Metabostemness: A New Cancer Hallmark. Front. Oncol. 2014, 4, 262. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G. Physiology and Pathophysiology of Purinergic Neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G. Purinergic Signalling: From Discovery to Current Developments. Exp. Physiol. 2014, 99, 16–34. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ramírez, A.S.; Díaz-Muñoz, M.; Butanda-Ochoa, A.; Vázquez-Cuevas, F.G. Nucleotides and Nucleoside Signaling in the Regulation of the Epithelium to Mesenchymal Transition (EMT). Purinergic Signal. 2017, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pelegrín, P. Purinergic Signaling: Methods and Protocols; Humana Press: New York, NY, USA, 2020; p. 2041. [Google Scholar]
- Saul, A.; Hausmann, R.; Kless, A.; Nicke, A. Heteromeric Assembly of P2X Subunits. Front. Cell. Neurosci. 2013, 7, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Kawate, T.; Michel, J.C.; Birdsong, W.T.; Gouaux, E. Crystal Structure of the ATP-Gated P2X4 ion Channel in the Closed State. Nature 2009, 460, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.H.; Baldwin, J.M.; Roger, S.; Baldwin, S. Insights into the Molecular Mechanisms Underlying Mammalian P2X7 Receptor Functions and Contributions in Diseases, Revealed by Structural Modeling and Single Nucleotide Polymorphisms. Front. Pharmacol. 2013, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yu, Y. Insights into the Channel Gating of P2X Receptors from Structures, Dynamics and Small Molecules. Acta Pharmacol. Sin. 2016, 37, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Taly, A.; Grutter, T. Moving through the Gate in ATP-Activated P2X Receptors. Trends Biochem. Sci. 2013, 38, 20–29. [Google Scholar] [CrossRef]
- Jindrichova, M.; Bhattacharya, A.; Rupert, M.; Skopek, P.; Obsil, T.; Zemkova, H. Functional Characterization of Mutants in the Transmembrane Domains of the Rat P2X7 Receptor That Regulate Pore Conductivity and Agonist Sensitivity. J. Neurochem. 2015, 133, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Heid, M.E.; Keyel, P.A.; Salter, R.D. The Second Transmembrane Domain of P2X7 Contributes to Dilated Pore Formation. PLoS ONE 2013, 8, e61886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, L.; Young, M.T. Purinergic P2X Receptors: Structural and Functional Features Depicted by X-Ray and Molecular Modelling Studies. Curr. Med. Chem. 2014, 22, 783–798. [Google Scholar] [CrossRef] [Green Version]
- Bernier, L.-P.; Ase, A.R.; Séguéla, P. Post-Translational Regulation of P2X Receptor Channels: Modulation by Phospholipids. Front. Cell. Neurosci. 2013, 7, 226. [Google Scholar] [CrossRef] [Green Version]
- Habermacher, C.; Dunning, K.; Chataigneau, T.; Grutter, T. Molecular Structure and Function of P2X Receptors. Neuropharmacology 2016, 104, 18–30. [Google Scholar] [CrossRef]
- Stelmashenko, O.; Lalo, U.; Yang, Y.; Bragg, L.; North, R.A.; Compan, V. Activation of Trimeric P2X2 Receptors by Fewer than Three ATP Molecules. Mol. Pharmacol. 2012, 82, 760–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, W.J.; Jiang, L.-H.; Surprenant, A.; North, R.A. Role of Ectodomain Lysines in the Subunits of the Heteromeric P2X2/3 Receptor. Mol. Pharmacol. 2006, 70, 1159–1163. [Google Scholar] [CrossRef] [Green Version]
- Volonté, C.; Apolloni, S.; Skaper, S.D.; Burnstock, G.; Volonte, C. P2X7 Receptors: Channels, Pores and More. CNS Neurol. Disord. Drug Targets 2012, 11, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Young, C.N.J.; Górecki, D.C. P2RX7 Purinoceptor as a Therapeutic Target—The Second Coming? Front. Chem. 2018, 6, 248. [Google Scholar] [CrossRef] [Green Version]
- Ángeles, M.C.M.; Amparo, B.R.M.; Ortega-Luna, R.; Sánchez-López, A.; Álvarez, Á. Molecular Sciences Structural and Functional Basis for Understanding the Biological Significance of P2X7 Receptor. Int. J. Mol. Sci. 2020, 21, 8454. [Google Scholar]
- Pegoraro, A.; De Marchi, E.; Adinolfi, E. P2X7 Variants in Oncogenesis. Cells 2021, 10, 189. [Google Scholar] [CrossRef]
- Glaser, T.; De Oliveira, S.L.B.; Cheffer, A.; Beco, R.; Martins, P.; Fornazari, M.; Lameu, C.; Costa Junior, H.M.; Coutinho-Silva, R.; Ulrich, H. Modulation of Mouse Embryonic Stem Cell Proliferation and Neural Differentiation by the P2X7 Receptor. PLoS ONE 2014, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, S.P.; Thompson, B.C.; Ward, P.S.; Thompson, C.B.; Ward, S.P.; Thompson, B.C. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2013, 21, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.S.; Chandel, N.S. Mitochondrial Metabolism and ROS Generation Are Essential for Kras-Mediated Tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffaghello, L.; Longo, V. Metabolic Alterations at the Crossroad of Aging and Oncogenesis. Int. Rev. Cell Mol. Biol. 2017, 332, 1–42. [Google Scholar] [PubMed]
- Shin, M.K.; Cheong, J.H. Mitochondria-Centric Bioenergetic Characteristics in Cancer Stem-like Cells. Arch. Pharm. Res. 2019, 42, 113–127. [Google Scholar] [CrossRef] [Green Version]
- De Francesco, E.M.; Sotgia, F.; Lisanti, M.P. Cancer Stem Cells (CSCs): Metabolic Strategies for Their Identification and Eradication. Biochem. J. 2018, 475, 1611–1634. [Google Scholar] [CrossRef] [Green Version]
- Turdo, A.; Veschi, V.; Gaggianesi, M.; Chinnici, A.; Bianca, P.; Todaro, M.; Stassi, G. Meeting the Challenge of Targeting Cancer Stem Cells. Front. Cell Dev. Biol. 2019, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Li, J.; Chen, S.; Cai, J.; Ban, Y.; Peng, Q.; Zhou, Y.; Zeng, Z.; Peng, S.; Li, X.; et al. Emerging Role of Lipid Metabolism Alterations in Cancer Stem Cells. J. Exp. Clin. Cancer Res. 2018, 37, 118. [Google Scholar] [CrossRef]
- Chen, Z.P.; Li, M.; Zhang, L.J.; He, J.Y.; Wu, L.; Xiao, Y.Y.; Duan, J.A.; Cai, T.; Li, W.D. Mitochondria-Targeted Drug Delivery System for Cancer Treatment. J. Drug Target. 2016, 24, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, J.; Wang, H.; Liu, C.B. Mitochondrial-Targeted Penetrating Peptide Delivery for Cancer Therapy. Expert Opin. Drug Deliv. 2018, 15, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Q.; Wang, G.H.; Huang, G.J.; Bian, X.W.; Qian, G.S.; Yu, S.C. Heterogeneity of Mitochondrial Membrane Potential: A Novel Tool to Isolate and Identify Cancer Stem Cells from a Tumor Mass? Stem Cell Rev. Rep. 2011, 7, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ito, K. Metabolism and the Control of Cell Fate Decisions and Stem Cell Renewal. Annu. Rev. Cell Dev Biol. 2016, 3, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Aulestia, F.J.; Néant, I.; Dong, J.; Haiech, J.; Kilhoffer, M.C.; Moreau, M.; Leclerc, C. Quiescence Status of Glioblastoma Stem-like Cells Involves Remodelling of Ca2+ signalling and Mitochondrial Shape. Sci. Rep. 2018, 8, 9731. [Google Scholar] [CrossRef]
- Liu, X.; Hajnóczky, G. Altered Fusion Dynamics Underlie Unique Morphological Changes in Mitochondria during Hypoxia-Reoxygenation Stress. Cell Death Differ. 2011, 18, 1561–1572. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.Y.; Ann, D.K. When Fats Commit Crimes: Fatty Acid Metabolism, Cancer Stemness and Therapeutic Resistance. Cancer Commun. 2018, 38, 47. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhou, Y.; Shingu, T.; Feng, L.; Chen, Z.; Ogasawara, M.; Keating, M.J.; Kondo, S.; Huang, P. Metabolic Alterations in Highly Tumorigenic Glioblastoma Cells: Preference for Hypoxia and High Dependency on Glycolysis. J. Biol. Chem. 2011, 286, 32843–32853. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Shen, Y.; Jin, F.; Miao, Y.; Qiu, X. Cancer Stem Cells in Small Cell Lung Cancer Cell Line H446: Higher Dependency on Oxidative Phosphorylation and Mitochondrial Substrate-Level Phosphorylation than Non-Stem Cancer Cells. PLoS ONE 2016, 11, e0154576. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.P.; Liao, J.; Tang, Z.J.; Wu, W.J.; Yang, J.; Zeng, Z.L.; Hu, Y.; Wang, P.; Ju, H.Q.; Xu, R.H.; et al. Metabolic Regulation of Cancer Cell Side Population by Glucose through Activation of the Akt Pathway. Cell Death Differ. 2014, 21, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Menendez, J.A.; Lupu, R. Fatty Acid Synthase and the Lipogenic Phenotype in Cancer Pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- Xu, S.; Chen, T.; Dong, L.; Li, T.; Xue, H.; Gao, B.; Ding, X.; Wang, H.; Li, H. Fatty Acid Synthase Promotes Breast Cancer Metastasis by Mediating Changes in Fatty Acid Metabolism. Oncol. Lett. 2021, 21, 27. [Google Scholar] [PubMed]
- Fhu, C.W.; Ali, A. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 2020, 25, 3935. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Miyazaki, H.; Vaidyan, L.K.; Kagawa, Y.; Ebrahimi, M.; Yamamoto, Y.; Ogata, M.; Katsuyama, Y.; Sadahiro, H.; Suzuki, M.; et al. Inhibition of Fatty Acid Synthase Decreases Expression of Stemness Markers in Glioma Stem Cells. PLoS ONE 2016, 11, e0147717. [Google Scholar] [CrossRef] [PubMed]
- Chiozzi, P.; Sarti, A.C.; Sanz, J.M.; Giuliani, A.L.; Adinolfi, E.; Vultaggio-Poma, V.; Falzoni, S.; Di Virgilio, F. Amyloid β-Dependent Mitochondrial Toxicity in Mouse Microglia Requires P2X7 Receptor Expression and Is Prevented by Nimodipine. Sci. Rep. 2019, 9, 6475. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H. Purinergic Receptors in Stem Cell Biology. Stem Cells Cancer Stem Cells 2013, 8, 267–274. [Google Scholar]
- Choi, H.Y.; Siddique, H.R.; Zheng, M.; Kou, Y.; Yeh, D.W.; Machida, T.; Chen, C.L.; Kumar, D.B.U.; Punj, V.; Winer, P.; et al. P53 Destabilizing Protein Skews Asymmetric Division and Enhances NOTCH Activation to Direct Self-Renewal of TICs. Nat. Commun. 2020, 11, 3084. [Google Scholar] [CrossRef]
- Ledderose, C.; Woehrle, T.; Ledderose, S.; Strasser, K.; Seist, R.; Bao, Y.; Zhang, J.; Junger, W.G. Cutting off the Power: Inhibition of Leukemia Cell Growth by Pausing Basal ATP Release and P2X Receptor Signaling? Purinergic Signal. 2016, 12, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, A.B.; Young, M.T.; Adinolfi, E.; Surprenant, A. Pseudoapoptosis Induced by Brief Activation of ATP-Gated P2X7 Receptors. J. Biol. Chem. 2005, 280, 33968–33976. [Google Scholar] [CrossRef] [Green Version]
- Amoroso, F.; Falzoni, S.; Adinolfi, E.; Ferrari, D.; Di Virgilio, F. The P2X7 Receptor Is a Key Modulator of Aerobic Glycolysis. Cell Death Dis. 2012, 3, e370. [Google Scholar] [CrossRef] [Green Version]
- Bourzac, J.F.; L’Ériger, K.; Larrivée, J.F.; Arguin, G.; Bilodeau, M.S.; Stankova, J.; Gendron, F.P. Glucose Transporter 2 Expression Is down Regulated Following P2X7 Activation in Enterocytes. J. Cell. Physiol. 2013, 228, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Giacovazzo, G.; Apolloni, S.; Coccurello, R. Loss of P2X7 Receptor Function Dampens Whole Body Energy Expenditure and Fatty Acid Oxidation. Purinergic Signal. 2018, 14, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, A.; Guiu-Jurado, E.; Porras, J.A.; Auguet, T. Molecular Pathways in Non-Alcoholic Fatty Liver Disease. Clin. Exp. Gastroenterol. 2014, 7, 221–239. [Google Scholar] [PubMed] [Green Version]
- Giacovazzo, G.; Fabbrizio, P.; Apolloni, S.; Coccurello, R.; Volonté, C. Stimulation of P2X7 Enhances Whole Body Energy Metabolism in Mice. Front. Cell. Neurosci. 2019, 13, 390. [Google Scholar] [CrossRef]
- De Marchi, E.; Pegoraro, A.; Adinolfi, E. P2X7 Receptor in Hematological Malignancies. Front. Cell Dev. Biol. 2021, 9, 5–11. [Google Scholar] [CrossRef]
- Salvestrini, V. Extracellular ATP Induces Apoptosis through P2X7R Activation in Acute Myeloid Leukemia Cells but Not in Normal Hematopoietic Stem Cells. Oncotarget 2017, 8, 5895–5908. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Wan, J.; Yang, X.; Zhang, X.; Huang, D.; Li, X.; Zou, Y.; Chen, C.; Yu, Z.; Xie, L.; et al. Bone Marrow Niche ATP Levels Determine Leukemia-Initiating Cell Activity via P2X7 in Leukemic Models. J. Clin. Investig. 2021, 131, 4. [Google Scholar] [CrossRef]
- Feng, W.; Yang, X.; Wang, L.; Wang, R.; Yang, F.; Wang, H.; Liu, X.; Ren, Q.; Zhang, Y.; Zhu, X.; et al. P2X7 Promotes the Progression of MLL-AF9-Induced Acute Myeloid Leukemia by Upregulation of Pbx3. Haematologica 2021, 106, 1278–1289. [Google Scholar] [CrossRef] [Green Version]
- Pegoraro, A.; Orioli, E.; De Marchi, E.; Salvestrini, V.; Milani, A.; Di Virgilio, F.; Curti, A.; Adinolfi, E. Differential Sensitivity of Acute Myeloid Leukemia Cells to Daunorubicin Depends on P2X7A versus P2X7B Receptor Expression. Cell Death Dis. 2020, 11, 10. [Google Scholar] [CrossRef]
- Koo, T.Y.; Lee, J.G.; Yan, J.J.; Jang, J.Y.; Ju, K.D.; Han, M.; Oh, K.H.; Ahn, C.; Yang, J. The P2X7 Receptor Antagonist, Oxidized Adenosine Triphosphate, Ameliorates Renal Ischemia-Reperfusion Injury by Expansion of Regulatory T Cells. Kidney Int. 2017, 92, 415–431. [Google Scholar] [CrossRef]
- Ferrazoli, E.G.; de Souza, H.D.N.; Nascimento, I.C.; Oliveira-Giacomelli, Á.; Schwindt, T.T.; Britto, L.R.; Ulrich, H. Brilliant Blue-G but Not Fenofibrate Treatment Reverts Hemiparkinsonian Behavior and Restores Dopamine Levels in an Animal Model of Parkinson’s Disease. Cell Transplant. 2017, 26, 669–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.; Bean, B.P. Inhibition of Neuronal Voltage-Gated Sodium Channels by Brilliant Blue, G. Mol. Pharmacol. 2011, 80, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, F.; Dahl, G. A Permeant Regulating Its Permeation Pore: Inhibition of Pannexin 1 Channels by ATP. AJP Cell Physiol. 2008, 296, C250–C255. [Google Scholar] [CrossRef]
- Michel, A.D.; Ng, S.-W.; Roman, S.; Clay, W.C.; Dean, D.K.; Walter, D.S. Mechanism of Action of Species-Selective P2X(7) Receptor Antagonists. Br. J. Pharmacol. 2009, 156, 1312–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponore, H.; Roberts, D.; Namovic, M.T.; Hsieh, G.; Zhu, Z.; Mikusa, J.P.; Hernandez, G.; Zong, C.; Gauvin, M.; Chandran, P.; et al. A-740003 [N-(1-{[(Cyanoimino)(5-Quinolinylamino) Methyl] Amino}-2,2-Dimethylpropyl)-2-(3,4-Dimethoxyphenyl)Acetamide], a Novel and Selective P2X7 Receptor Antagonist, Dose-Dependently Reduces Neuropathic Pain in the Rat. J. Pharmacol. Exp. Ther. 2006, 319, 1376–1385. [Google Scholar]
- Brisson, L.; Lopez-Charcas, O.; Jelassi, B.; Ternant, D.; Trovero, F.; Besson, P. P2X7 Receptor Promotes Mouse Mammary Cancer Cell Invasiveness and Tumour Progression, and Is a Target for Anticancer Treatment. Cancers 2020, 12, 2342. [Google Scholar] [CrossRef]
- Jelassi, B.; Chantôme, A.; Alcaraz-Pérez, F.; Baroja-Mazo, A.; Cayuela, M.L.; Pelegrin, P.; Surprenant, A.; Roger, S. P2X7 Receptor Activation Enhances SK3 Channels- and Cystein Cathepsin-Dependent Cancer Cells Invasiveness. Oncogene 2011, 30, 2108–2122. [Google Scholar] [CrossRef] [Green Version]
- Adinolfi, E.; Raffaghello, L.; Giuliani, A.L.; Cavazzini, L.; Capece, M.; Chiozzi, P.; Bianchi, G.; Kroemer, G.; Pistoia, V.; Di Virgilio, F. Expression of P2X7 Receptor Increases in Vivo Tumor Growth. Cancer Res. 2012, 72, 2957–2969. [Google Scholar] [CrossRef] [Green Version]
- Adinolfi, E.; Capece, M.; Franceschini, A.; Falzoni, S.; L.Giuliani, A.; Rotondo, A.; Sarti, A.C.; Bonora, M.; Syberg, S.; Corigliano, D.; et al. Accelerated Tumor Progression in Mice Lacking the ATP Receptor P2X7. Cancer Res. 2015, 75, 635–644. [Google Scholar] [CrossRef] [Green Version]
- De Marchi, E.; Orioli, E.; Pegoraro, A.; Sangaletti, S.; Portararo, P.; Curti, A.; Colombo, M.P.; Di Virgilio, F.; Adinolfi, E. The P2X7 Receptor Modulates Immune Cells Infiltration, Ectonucleotidases Expression and Extracellular ATP Levels in the Tumor Microenvironment. Oncogene 2019, 38, 3636–3650. [Google Scholar] [CrossRef]
- Bergamin, L.S.; Capece, M.; Salaro, E.; Sarti, A.C.; Falzoni, S.; Stéfani, M.; Pereira, L.; Bastiani, M.A. De Role of the P2X7 Receptor in in Vitro and in Vivo Glioma Tumor Growth. Oncotarget 2019, 10, 4840–4856. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Luo, S.; Zhang, Y.; Jia, L.; Yang, C.; Peng, X.; Zhao, R. Production, Characterization, and Application of a Monoclonal Antibody Specific for the Extracellular Domain of Human P2X7R. Appl. Microbiol. Biotechnol. 2020, 104, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.K.; Seneviratne, S.; Drummond, K.J.; Williams, D.A.; O’Brien, T.J.; Monif, M. P2X7 Receptor Antagonism Inhibits Tumour Growth in Human High-Grade Gliomas. Purinergic Signal. 2020, 16, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Beamer, E.; Fischer, W.; Engel, T. The ATP-Gated P2X7 Receptor as a Target for the Treatment of Drug-Resistant Epilepsy. Front. Neurosci. 2017, 11, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, S.; Jelassi, B.; Couillin, I.; Pelegrin, P.; Besson, P.; Jiang, L. Understanding the Roles of the P2X7 Receptor in Solid Tumour Progression and Therapeutic Perspectives. Biochim. Biophys. Acta 2015, 1848, 2584–2602. [Google Scholar] [CrossRef] [Green Version]
- Eser, A.; Colombel, J.-F.; Rutgeerts, P.; Vermeire, S.; Vogelsang, H.; Braddock, M.; Persson, T.; Reinisch, W. Safety and Efficacy of an Oral Inhibitor of the Purinergic Receptor P2X7 in Adult Patients with Moderately to Severely Active Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Hira, S.K.; Srivastava, D.N.; Singh, V.K.; Gupta, U.; Singh, R.; Singh, R.A.; Manna, P.P. ATP-Decorated Mesoporous Silica for Biomineralization of Calcium Carbonate and P2 Purinergic Receptor-Mediated Antitumor Activity against Aggressive Lymphoma. ACS Appl. Mater. Interfaces 2018, 10, 6917–6929. [Google Scholar] [CrossRef]
- Michaud, M.; Martins, I.; Sukkurwala, A.Q.; Adjemian, S.; Ma, Y.; Pellegatti, P.; Shen, S.; Kepp, O.; Scoazec, M.; Mignot, G.; et al. Autophagy-Dependent Anticancer Immune Responses Induced by Chemotherapeutic Agents in Mice. Science 2011, 334, 1573–1577. [Google Scholar] [CrossRef]
- Lecciso, M.; Ocadlikova, D.; Sangaletti, S.; Trabanelli, S.; De Marchi, E.; Orioli, E.; Pegoraro, A.; Portararo, P.; Jandus, C.; Bontadini, A.; et al. ATP Release from Chemotherapy-Treated Dying Leukemia Cells Elicits an Immune Suppressive Effect by Increasing Regulatory T Cells and Tolerogenic Dendritic Cells. Front. Immunol. 2017, 8, 1918. [Google Scholar] [CrossRef]
- Munerati, M.; Cortesi, R.; Ferrari, D.; Di Virgilio, F.; Nastruzzi, C. Macrophages Loaded with Doxorubicin by ATP-Mediated Permeabilization: Potential Carriers for Antitumor Therapy. BBA Mol. Cell Res. 1994, 1224, 269–276. [Google Scholar] [CrossRef]
- Gilbert, S.M.; Oliphant, C.J.; Hassan, S.; Peille, A.; Bronsert, P.P.; Falzoni, S.; Di Virgilio, F.; McNulty, S.; Lara, R.; Al, S.H.; et al. ATP in the Tumour Microenvironment Drives Expression of NfP2X 7, a Key Mediator of Cancer Cell Survival. Oncogene 2019, 38, 194–208. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, S.M.; Gidley, B.A.; Glazer, S.; Barden, J.A.; Glazer, A.; Teh, L.C.; King, J. A Phase I Clinical Trial Demonstrates That NfP2X7-Targeted Antibodies Provide a Novel, Safe and Tolerable Topical Therapy for Basal Cell Carcinoma. Br. J. Dermatol. 2017, 177, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Kim, Y.C. P2X7 Receptor Antagonists: A Patent Review (2010–2015). Expert Opin. Ther. Pat. 2016, 27, 257–267. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. The Potential of P2X7 Receptors as a Therapeutic Target, Including Inflammation and Tumour Progression. Purinergic Signal. 2018, 14, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drill, M.; Jones, N.C.; Hunn, M.; O’Brien, T.J.; Monif, M. Antagonism of the ATP-Gated P2X7 Receptor: A Potential Therapeutic Strategy for Cancer. Purinergic Signal. 2021, 17, 215–227. [Google Scholar] [CrossRef]
- Schwarz, N.; Drouot, L.; Nicke, A.; Fliegert, R.; Boyer, O.; Guse, A.H.; Haag, F.; Adriouch, S.; Koch-Nolte, F. Alternative Splicing of the N-Terminal Cytosolic and Transmembrane Domains of P2X7 Controls Gating of the Ion Channel by ADP-Ribosylation. PLoS ONE 2012, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, A.; Michalski, K.; Mikhelzon, P.; Kawate, T. The P2X7 Receptor Forms a Dye-Permeable Pore Independent of Its Intracellular Domain but Dependent on Membrane Lipid Composition. Elife 2017, 6, e31186. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.E.; Shridar, M.; Smith, P.; Murrell-Lagnado, R.D. Plasma Membrane Cholesterol as a Regulator of Human and Rodent P2X7 Receptor Activation and Sensitization. J. Biol. Chem. 2014, 289, 31983–31994. [Google Scholar] [CrossRef] [Green Version]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X Receptor Properties and Their Pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; von Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y Receptor Pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef]
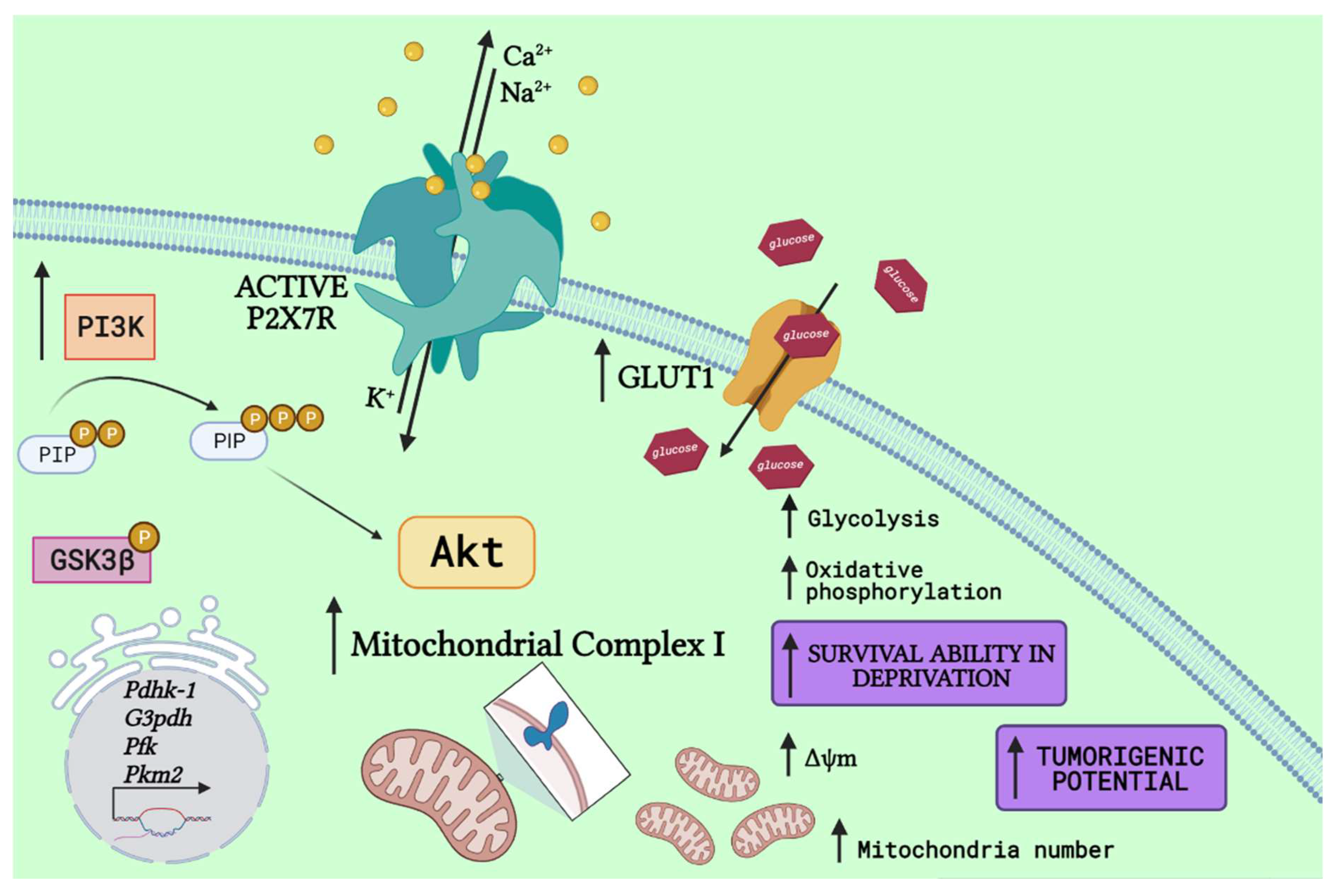

| Metabolic Target | P2X7 Receptor Effect | Related Cellular Events | Available Evidence |
|---|---|---|---|
| Complex I protein | Protein levels: Upregulated on P2X7R-expressing cells [18] | Increased mitochondrial potential, increased respiration | HEK 293 cells overexpressing P2X7R vs. wild-type; N13 microglia cells sufficient vs. deficient for P2X7R |
| Complex II protein | Protein levels: Downregulated on P2X7R-expressing cells [18] | Not reported | HEK 293 cells overexpressing P2X7R vs. wild-type |
| GLUT1 | Protein levels: Upregulated on P2X7R-expressing cells [72] | Growth in absence of serum or low glucose; increased cellular ATP content | HEK 293 cells overexpressing P2X7R vs. mock-transfected cells; human neuroblastoma cells |
| GLUT2 | Protein levels on cell surface: Downregulated by P2X7R activation [73] | Reduced glucose transport | Pharmacologically activated (BzATP 100 µM) intestinal epithelial cells (IEC)-6 and Caco-2 cells |
| G3PDH | mRNA expression levels: Upregulated on P2X7R-expressing cells [72] | Growth in absence of serum or low glucose; increased cellular ATP content | HEK 293 cells overexpressing P2X7R vs. mock-transfected cells; human neuroblastoma cells |
| PFK | Protein levels: Upregulated on P2X7R-expressing cells [72] | Growth in absence of serum or low glucose; increased cellular ATP content | HEK 293 cells overexpressing P2X7R vs. mock-transfected cells, in low glucose conditions; human neuroblastoma cells |
| PKM2 | Protein levels: Upregulated on P2X7R-expressing cells [72] | Growth in absence of serum or low glucose; increased cellular ATP content; increased glycolysis | HEK 293 cells overexpressing P2X7R vs. mock-transfected cells, in low glucose conditions; human neuroblastoma cells |
| PDHK1 | Protein levels: Upregulated on P2X7R-expressing cells [72] | Growth in absence of serum or low glucose; increased cellular ATP content; increased glycolysis | HEK 293 cells overexpressing P2X7R vs. mock-transfected cells, in low glucose conditions; human neuroblastoma cells |
| PDH | Enzyme activity: Downregulated on P2X7R-expressing cells [72] | Growth in absence of serum or low glucose; increased cellular ATP content; increased glycolysis | HEK 293 cells overexpressing P2X7R vs. mock-transfected cells; human neuroblastoma cells |
| GSK3β | Phosphorylated protein levels: Upregulated (reduced enzyme activity) on P2X7R-expressing cells/upon P2X7R activation [20] | Increased glycogen stores; tumor cell survival | Human neuroblastoma cells silenced for P2X7 by shRNAs vs. scrambled control; neuroblastoma cells pharmacologically modulated by agonists (BzATP or ATP) or inhibited by AZ10606120 or A740003 |
| NADPH oxidase 2 | Protein levels on skeletal muscle: Upregulated upon P2X7R activation [76] | Increased metabolic rate, O2 consumption, decreased respiratory rate | P2X7 receptor systemic activation in mice (BzATP, 1 mg/kg) |
| ACC (acetyl-CoA carboxylase) | mRNA and protein levels: Downregulated in knockout mice [21] | Glucose intolerance, increased serum triglycerides and cholesterol levels | P2X7−/− mice vs. wild-type |
| FASN (fatty acid synthase) | mRNA and protein levels: Downregulated in knockout mice [21] | Glucose intolerance, increased serum triglycerides and cholesterol levels | P2X7−/− mice vs. wild-type |
| PHGDH | mRNA and protein levels: Downregulated in knockdown cells [79] | Lower serine levels; reduced migration, homing and self-renewal abilities | Leukemia initiating cells of mice with MLL-AF9 induced AML (Acute myeloid leukemia); P2X7 knockdown by shRNAs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo, I.L.A.; Arnaud-Sampaio, V.F.; Adinolfi, E.; Ulrich, H.; Lameu, C. Cancer Metabostemness and Metabolic Reprogramming via P2X7 Receptor. Cells 2021, 10, 1782. https://doi.org/10.3390/cells10071782
Rabelo ILA, Arnaud-Sampaio VF, Adinolfi E, Ulrich H, Lameu C. Cancer Metabostemness and Metabolic Reprogramming via P2X7 Receptor. Cells. 2021; 10(7):1782. https://doi.org/10.3390/cells10071782
Chicago/Turabian StyleRabelo, Izadora Lorrany Alves, Vanessa Fernandes Arnaud-Sampaio, Elena Adinolfi, Henning Ulrich, and Claudiana Lameu. 2021. "Cancer Metabostemness and Metabolic Reprogramming via P2X7 Receptor" Cells 10, no. 7: 1782. https://doi.org/10.3390/cells10071782
APA StyleRabelo, I. L. A., Arnaud-Sampaio, V. F., Adinolfi, E., Ulrich, H., & Lameu, C. (2021). Cancer Metabostemness and Metabolic Reprogramming via P2X7 Receptor. Cells, 10(7), 1782. https://doi.org/10.3390/cells10071782








