WWOX and Its Binding Proteins in Neurodegeneration
Abstract
1. Brain Protein Aggregation Starts from Middle Age
2. Spread of Pathogenic Tau and Aβ in the Brain
3. Protein Instability and Aggregation and Induced Chronic Brain Inflammation with Age
4. TGF-β Induces Intracellular Protein Aggregation
5. Protein Aggregates of TRAPPC6A, TIAF1, and SH3GLB2 in the Middle-Aged Hippocampi
6. WWOX in Neural Diseases
6.1. WWOX-Interacting Partners for AD
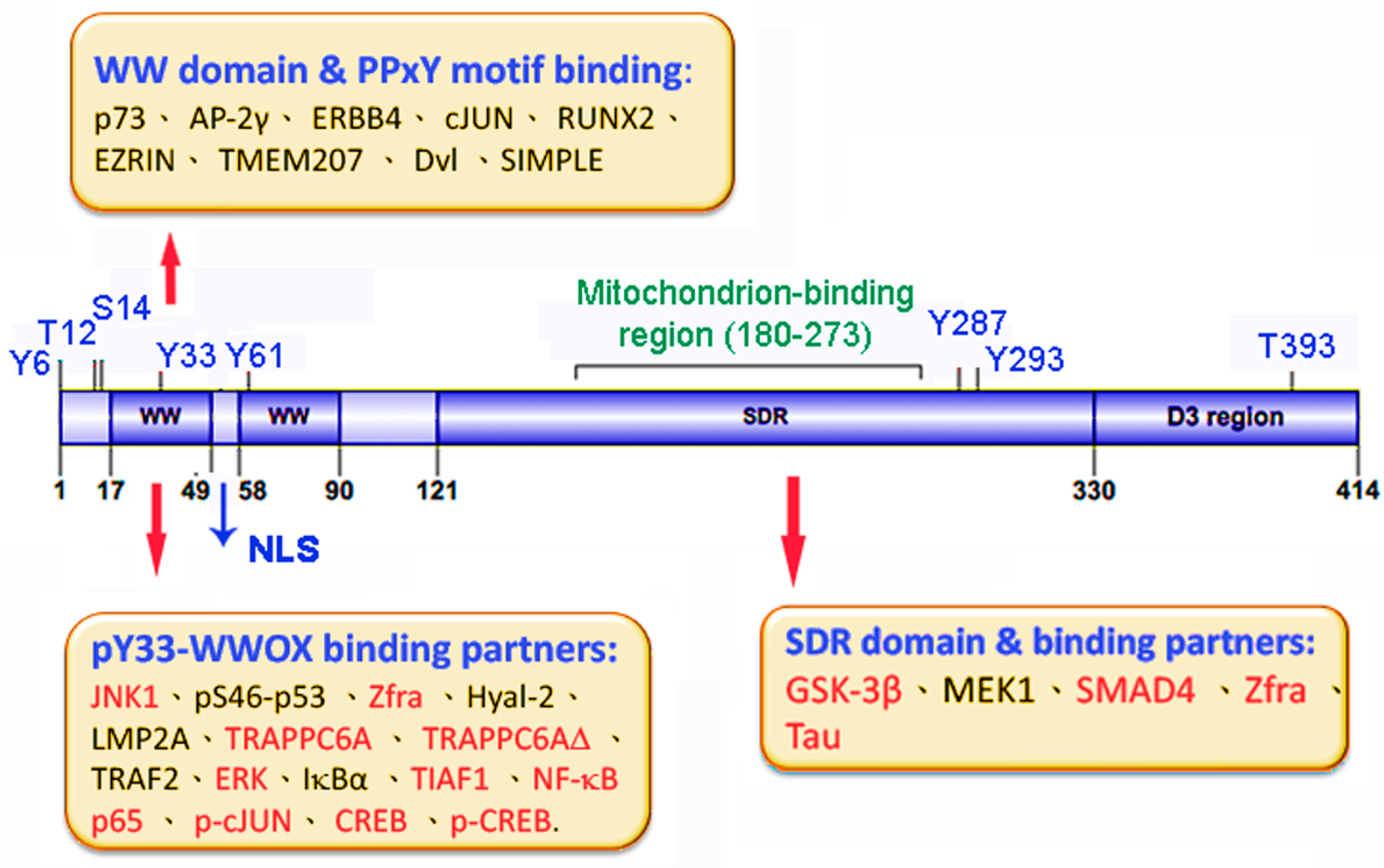
6.2. WWOX Loss or Dysfunction Accelerates Cell Migration: Potential Role in Neuronal Heterotopia
6.3. WWOX/Hyal-2/Smad4 Signaling in Traumatic Brain Injury
6.4. WWOX Binds Transcription Factors for Relocating to the Nucleus during Neuronal Damage
7. WWOX in Alzheimer’s Disease
7.1. WWOX in Neural Development and Neural Diseases
7.2. WWOX Downregulation and Induction of Protein Aggregation Cascade
7.3. pS14-WWOX Promotes AD Progression
7.4. Binding of Endogenous WWOX with Intracellular Partner Proteins In Vivo
8. p53 and WWOX in AD
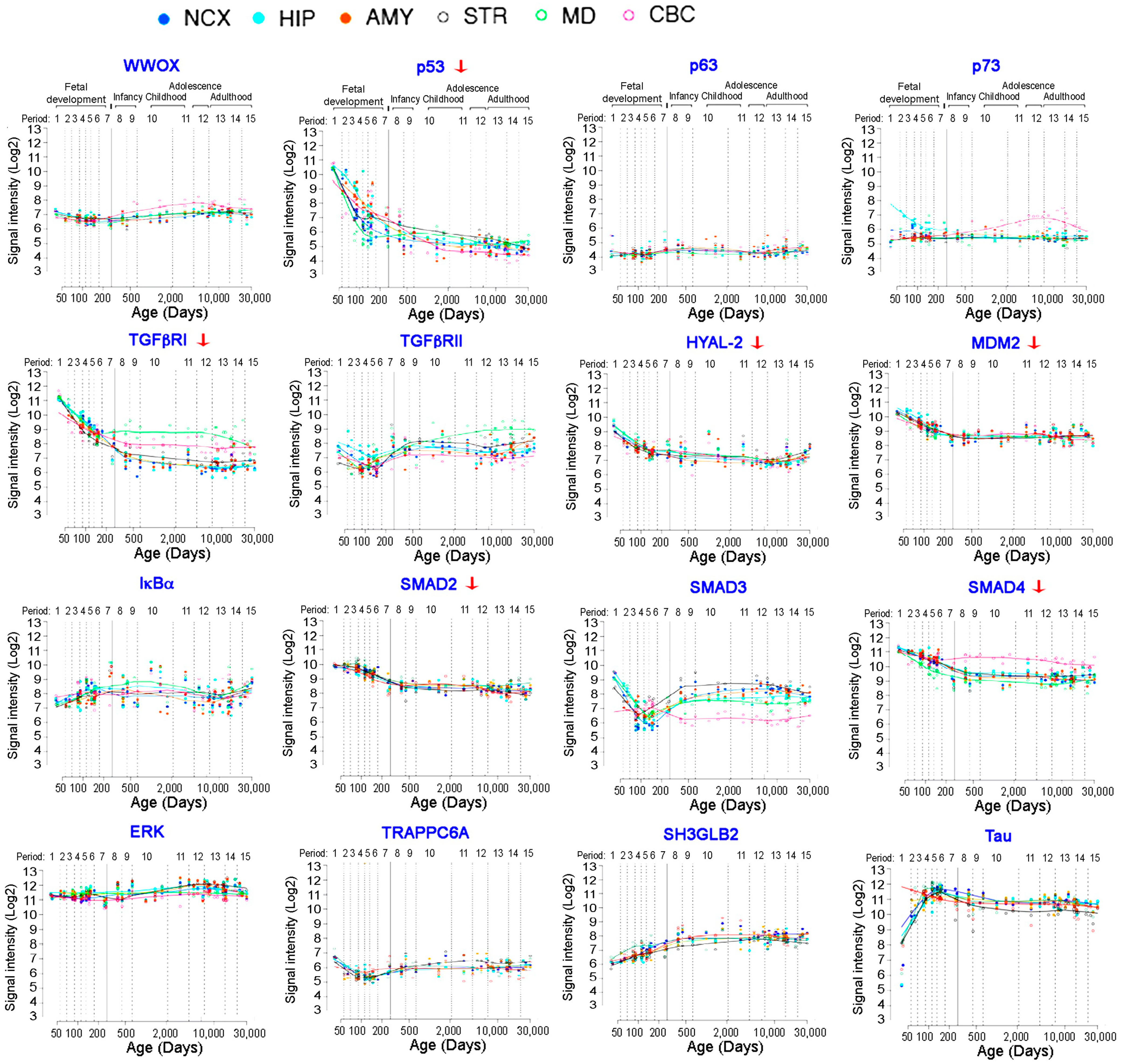
8.1. Expression of p53 and WWOX and Their Binding Partners in the Human Brains
8.2. p53 and WWOX Physical Binding and Induction of Apoptosis
8.3. Estrogen in the p53/WWOX Signaling
8.4. TIAF1, p53, and WWOX Triad in Apoptosis
8.5. Extracellular Matrix-Mediated TIAF1 Aggregation and Regulation of SMAD Promoter
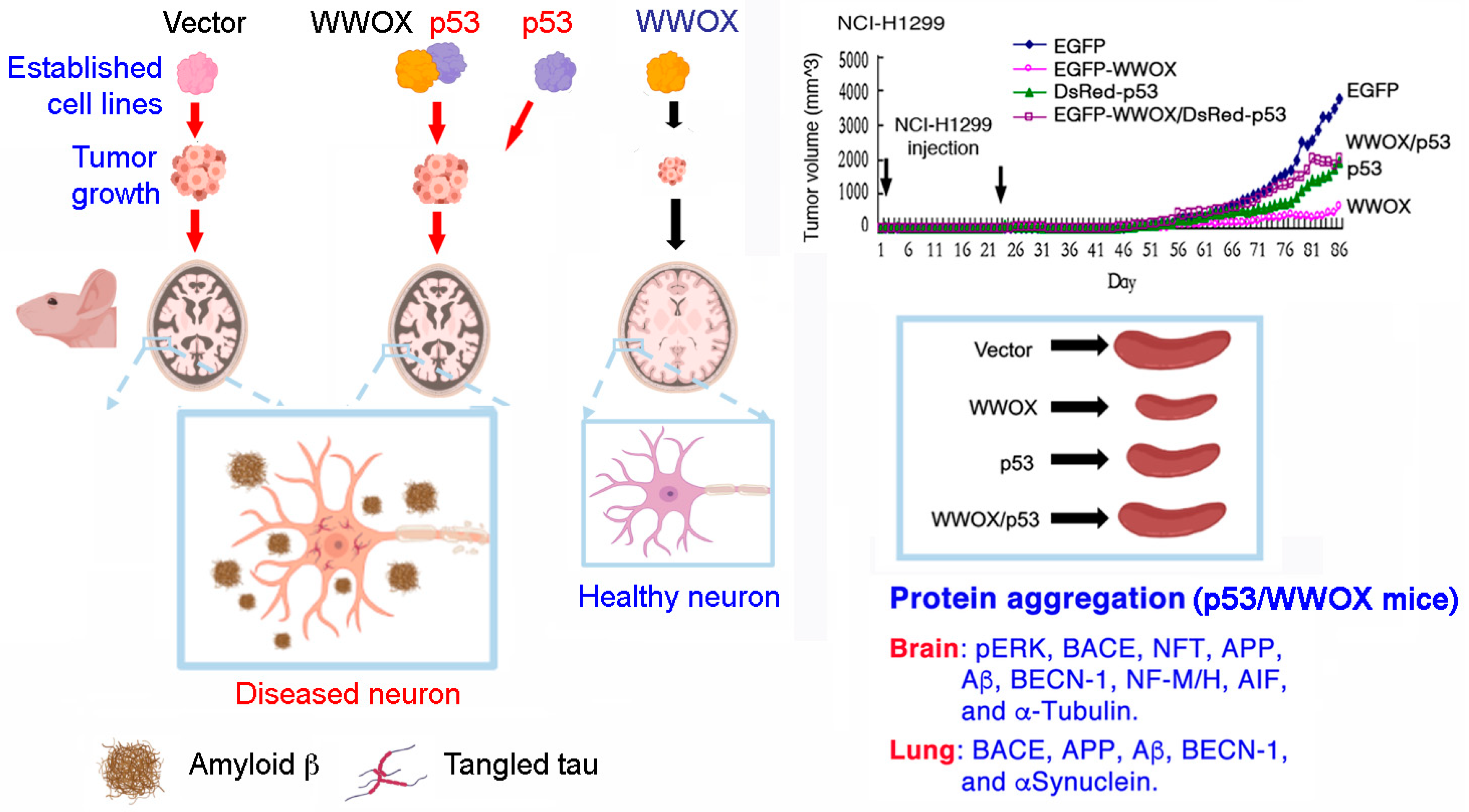
9. p53 and WWOX Functional Antagonism In Vivo
10. Concluding Remarks
10.1. pS14-WWOX Is Linked to AD Progression
10.2. Zfra and Zfra-Activated Z Cells for AD Therapy
10.3. Manipulating the Binding Strength between WWOX and Binding Partner Proteins to Limit Disease Progression
11. Conclusions and Perspectives for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, J.; Holtzman, D.M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.; Perry, G. A multilevel view of the development of Alzheimer’s disease. Neuroscience 2021, 457, 283–293. [Google Scholar] [CrossRef]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A long journey into aging, brain aging, and Alzheimer’s disease following the oxidative stress tracks. J. Alzheimer’s Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Chang, N.-S.; Chang, J.-Y.; Chang, N.-S. WWOX Dysfunction Induces Sequential Aggregation of TRAPPC6AΔ, TIAF1, Tau and Amyloid β, and Causes Apoptosis. Cell Death Discov. 2015, 1, 15003. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Lin, S.R.; Chang, J.Y.; Schultz, L.; Heath, J.; Hsu, L.J.; Kuo, Y.M.; Hong, Q.; Chiang, M.F.; Gong, C.X.; et al. TGF-Beta Induces TIAF1 Self-Aggregation via Type II Receptor-Independent Signaling that Leads to Generation of Amyloid Beta Plaques in Alzheimer’s Disease. Cell Death Dis. 2010, 1, e110. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-Y.; Lee, M.-H.; Lin, S.-R.; Yang, L.-Y.; Sun, S.; Sze, C.-I.; Hong, Q.; Lin, Y.-S.; Chou, Y.-T.; Hsu, L.-J.; et al. Trafficking Protein Particle Complex 6A Delta (TRAPPC6AΔ) is an Extracellular Plaque-Forming Protein in the Brain. Oncotarget 2015, 6, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Ho, P.-C.; Lee, I.-T.; Chen, Y.-A.; Chu, C.-H.; Teng, C.-C.; Wu, S.-N.; Sze, C.-I.; Chiang, M.-F.; Chang, N.-S. WWOX Phosphorylation, Signaling, and Role in Neurodegeneration. Front. Neurosci. 2018, 12, 563. [Google Scholar] [CrossRef] [PubMed]
- Bok, E.; Leem, E.; Lee, B.R.; Lee, J.M.; Yoo, C.J.; Lee, E.M.; Kim, J. Role of the Lipid Membrane and Membrane Proteins in tau Pathology. Front. Cell Dev. Biol. 2021, 9, 653815. [Google Scholar] [CrossRef] [PubMed]
- Forloni, G.; Balducci, C. Alzheimer’s disease, oligomers, and inflammation. J. Alzheimer’s Dis. 2018, 62, 1261–1276. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. Neurodegeneration. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 2015, 349, 1255555. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Trojanowski, J.Q.; Lee, V.M.-Y. Protein Transmission in Neurodegenerative Disease. Nat. Rev. Neurol. 2020, 16, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, S.; Hyman, B.T. Tau prion-like propagation: State of the art and current challenges. Adv. Exp. Med. Biol. 2019, 1184, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Peraza, F.; Nogueras-Ortiz, C.; Volpert, O.; Liu, D.; Goetzl, E.; Mattson, M.; Greig, N.; Eitan, E.; Kapogiannis, D. Neuronal and Astrocytic Extracellular Vesicle Biomarkers in Blood Reflect Brain Pathology in Mouse Models of Alzheimer’s Disease. Cells 2021, 10, 993. [Google Scholar] [CrossRef] [PubMed]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Body Fluid Biomarkers for Alzheimer’s Disease-An Up-to-Date Over-View. Biomedicines 2020, 8, 421. [Google Scholar] [CrossRef] [PubMed]
- Tracy, T.; Claiborn, K.C.; Gan, L. Regulation of Tau Homeostasis and Toxicity by Acetylation. Adv. Exp. Med. Biol. 2019, 1184, 47–55. [Google Scholar] [CrossRef] [PubMed]
- David, D.C. Aging and the Aggregating Proteome. Front. Genet. 2012, 3, 247. [Google Scholar] [CrossRef] [PubMed]
- Groh, N.; Bühler, A.; Huang, C.; Li, K.W.; van Nierop, P.; Smit, A.B.; Fändrich, M.; Baumann, F.; David, D.C. Age-Dependent Protein Aggregation Initiates Amyloid-Beta Aggregation. Front. Aging Neurosci. 2017, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.A.; Muraleva, N.A.; Korbolina, E.E.; Kiseleva, E.; Maksimova, K.Y.; Kolosova, N.G. Amyloid Accumulation is a Late Event in Sporadic Alzheimer’s Disease-Like Pathology in Nontransgenic rats. Oncotarget 2015, 6, 1396–1413. [Google Scholar] [CrossRef] [PubMed]
- Ayyadevara, S.; Balasubramaniam, M.; Gao, Y.; Yu, L.; Alla, R.; Reis, R.S. Proteins in Aggregates Functionally Impact Multiple Neurodegenerative Disease Models by Forming Proteasome-Blocking Complexes. Aging Cell 2014, 14, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, N.; De Sanctis, J.B.; Hajdúch, M.; Das, V. Tau secretion and propagation: Perspectives for potential preventive interventions in Alzheimer’s disease and other tauopathies. Exp. Neurol. 2021, 343, 113756. [Google Scholar] [CrossRef]
- Tominaga, K.; Suzuki, H.I. TGF-Beta Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Caveolae, CD109, and Endothelial Cells as Targets for Treating Alzheimer’s Disease. Alzheimers Dement. 2020, 6, e12066. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.R.; Chai, Y.L.; Lee, J.H.; Howlett, D.; Attems, J.; Ballard, C.G.; Aarsland, D.; Francis, P.T.; Chen, C.P.; Lai, M.K. Increased transforming growth factor beta2 in the neocortex of Alzheimer’s disease and dementia with Lewy bodies is correlated with disease severity and soluble Abeta42 load. J. Alzheimer’s Dis. 2017, 56, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.; Anusuyadevi, M.; Aigner, K.M.; Unger, M.S.; Kniewallner, K.M.; de Sousa, D.M.B.; Altendorfer, B.; Mrowetz, H.; Bogdahn, U.; Aigner, L. TGF-Beta Signaling: A Therapeutic Target to Reinstate Regenerative Plasticity in Vascular Dementia? Aging Dis. 2020, 11, 828–850. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Cruz, L.; Cabrera, D. Transforming growth factor beta type I role in neurodegeneration: Implications for Alzheimers disease. Curr. Protein Pept. Sci. 2018, 19, 1180–1188. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Chiang, M.-F.; Lin, S.-R.; Lee, M.-H.; He, H.; Chou, P.-Y.; Chen, S.-J.; Chen, Y.-A.; Yang, L.-Y.; Lai, F.-J.; et al. TIAF1 Self-Aggregation in Peritumor Capsule Formation, Spontaneous Activation of SMAD-Responsive Promoter in p53-Deficient Environment, and Cell Death. Cell Death Dis. 2012, 3, e302. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Hsu, L.J.; Chou, P.Y.; Chou, Y.T.; Lu, C.Y.; Chen, Y.A.; Chang, N.S. Self-Aggregating TIAF1 in Lung Cancer Progression. Transl. Respir. Med. 2013, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Shih, Y.H.; Lin, S.R.; Chang, J.Y.; Lin, Y.H.; Sze, C.I.; Kuo, Y.M.; Chang, N.S. Zfra Restores Memory Deficits in Alzheimer’s Disease Triple-Transgenic Mice by Blocking Aggregation of TRAPPC6AΔ, SH3GLB2, Tau, and Amyloid β, and Inflammatory NF-κB Activation. Alzheimers Dement. 2017, 3, 189–204. [Google Scholar] [CrossRef]
- Sacher, M.; Kim, Y.G.; Lavie, A.; Oh, B.H.; Segev, N. The TRAPP Complex: Insights into Its Architecture and Function. Traffic 2008, 9, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Mohamoud, H.S.; Ahmed, S.; Jelani, M.; Alrayes, N.; Childs, K.; Vadgama, N.; Almramhi, M.M.; Al-Aama, J.Y.; Goodbourn, S.; Nasir, J. A Missense Mutation in TRAPPC6A Leads to Build-Up of the Protein, in Patients with a Neurodevelopmental Syndrome and Dysmorphic Features. Sci. Rep. 2018, 8, 2053. [Google Scholar] [CrossRef]
- Chang, N.-S.; Pratt, N.; Heath, J.; Schultz, L.; Sleve, D.; Carey, G.B.; Zevotek, N. Hyaluronidase Induction of a WW Domain-containing Oxidoreductase that Enhances Tumor Necrosis Factor Cytotoxicity. J. Biol. Chem. 2001, 276, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-S.; Hsu, L.-J.; Lin, Y.-S.; Lai, F.-J.; Sheu, H.-M. WW Domain-Containing Oxidoreductase: A Candidate Tumor Suppressor. Trends Mol. Med. 2007, 13, 12–22. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Joy-Dodson, E.; Schueler-Furman, O.; Aqeilan, R.I. Pleiotropic Functions of Tumor Suppressor WWOX in Normal and Cancer Cells. J. Biol. Chem. 2015, 290, 30728–30735. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Aqeilan, R.I. The Tumor Suppressor WW Domain-Containing Oxidoreductase Modulates Cell Metabolism. Exp. Biol. Med. 2015, 240, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-S.; Chang, N.-S. Phosphorylation/De-Phosphorylation in Specific Sites of Tumor Suppressor WWOX and Control of Distinct Biological Events. Exp. Biol. Med. 2018, 243, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.J.; Chiang, M.F.; Sze, C.I.; Su, W.P.; Yap, Y.V.; Lee, I.T.; Kuo, H.L.; Chang, N.S. HYAL-2-WWOX-SMAD4 Signaling in Cell Death and Anticancer Response. Front. Cell Dev. Biol. 2016, 4, 141. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; He, R.Y.; Lin, H.P.; Hsu, L.J.; Lai, F.J.; Hong, Q.; Chen, S.J.; Chang, N.S. Signaling from Membrane Receptors to Tumor Suppressor WW Domain-Containing Oxidoreductase. Exp. Biol. Med. 2010, 235, 796–804. [Google Scholar] [CrossRef]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; Van Der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic Meta-Analysis of Diagnosed Alzheimer’s Disease Identifies New Risk Loci and Implicates Aβ, Tau, Immunity and Lipid Processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef]
- Bouteille, N.; Driouch, K.; Hage, P.E.; Sin, S.; Formstecher, E.; Camonis, J.; Lidereau, R.; Lallemand, F. Inhibition of the Wnt/Beta-catenin Pathway by the WWOX Tumor Suppressor Protein. Oncogene 2009, 28, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Saigo, C.; Kito, Y.; Takeuchi, T. Cancerous protein network that inhibits the tumor suppressor function of WW domain-containing oxidoreductase (WWOX) by aberrantly expressed molecules. Front. Oncol. 2018, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Sze, C.I.; Su, M.; Pugazhenthi, S.; Jambal, P.; Hsu, L.J.; Heath, J.; Schultz, L.; Chang, N.S. Downregulation of WOX1 Induces Tau Phosphorylation In Vitro: A Potential Role in Alzheimer’s Disease. J. Biol. Chem. 2004, 279, 30498–30506. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Lai, F.J.; Hsu, L.J.; Lo, C.P.; Cheng, C.L.; Lin, S.R.; Lee, M.H.; Chang, J.Y.; Subhan, D.; Tsai, M.S.; et al. Dramatic Coactivation of WWOX/WOX1 with CREB and NF-κB in Delayed Loss of Small Dorsal Root Ganglion Neurons upon Sciatic Nerve Transection in Rats. PLoS ONE 2009, 4, e7820. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Hsu, L.J.; Schultz, L.; Pratt, N.; Mattison, J.; Chang, N.S. Zfra Affects TNF-Mediated Cell Death by Interacting with Death Domain Protein TRADD and Negatively Regulates the Activation of NF-kappaB, JNK1, p53 and WOX1 during Stress Response. BMC Mol. Biol. 2007, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.Y.; Wu, S.Y.; Lai, H.C.; Chang, N.S.; Chang, F.H.; Tsai, M.H.; Su, I.J.; Chang, Y. WW Domain-Containing Oxidoreductase is Involved in Upregulation of Matrix Metalloproteinase 9 by Epstein-Barr Virus Latent Membrane Protein 2A. Biochem. Biophys. Res. Commun. 2013, 436, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Lahav, N.; Rotem-Bamberger, S.; Fahoum, J.; Dodson, E.; Kraus, Y.; Mousa, R.; Metanis, N.; Friedler, A.; Schueler-Furman, O. Phosphorylation of the WWOX Protein Regulates Its Interaction with p73. ChemBioChem 2020, 21, 1843–1851. [Google Scholar] [CrossRef]
- Aqeilan, R.I.; Pekarsky, Y.; Herrero, J.J.; Palamarchuk, A.; Letofsky, J.; Druck, T.; Trapasso, F.; Han, S.-Y.; Melino, G.; Huebner, K.; et al. Functional Association between Wwox Tumor Suppressor Protein and p73, a p53 Homolog. Proc. Natl. Acad. Sci. USA 2004, 101, 4401–4406. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Sie, Y.-D.; Liu, T.-Y.; Kuo, H.-L.; Chou, P.-Y.; Lee, K.-T.; Chen, P.-J.; Chen, S.-T.; Chang, N.-S. Normal Cells Repel WWOX-Negative or -Dysfunctional Cancer Cells via WWOX Cell Surface Epitope 286-299. Commun. Biol. 2021, 4, 753. [Google Scholar] [CrossRef]
- Chang, N.S.; Schultz, L.; Hsu, L.J.; Lewis, J.; Su, M.; Sze, C.I. 17beta-Estradiol Upregulates and Activates WOX1/WWOXv1 and WOX2/WWOXv2 In Vitro: Potential Role in Cancerous Progression of Breast and Prostate to a Premetastatic State In Vivo. Oncogene 2005, 24, 714–723. [Google Scholar] [CrossRef]
- Wang, H.Y.; Juo, L.I.; Lin, Y.T.; Hsiao, M.; Lin, J.T.; Tsai, C.H.; Tzeng, Y.H.; Chuang, Y.C.; Chang, N.S.; Yang, C.N.; et al. WW Domain-Containing Oxidoreductase Promotes Neuronal Differentiation via Negative Regulation of Glycogen Synthase Kinase 3β. Cell Death Differ. 2012, 19, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.J.; Hong, Q.Y.; Chen, S.T.; Kuo, H.L.; Schultz, L.; Heath, J.; Lin, S.R.; Lee, M.H.; Li, D.Z.; Li, Z.L.; et al. Hyaluronan Activates Hyal-2/WWOX/Smad4 Signaling and Causes Bubbling Cell Death when the Signaling Complex is Overexpressed. Oncotarget 2017, 8, 19137–19155. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.J.; Schultz, L.; Hong, Q.; Van More, K.; Heath, J.; Li, M.Y.; Lai, F.J.; Lin, S.R.; Lee, M.H.; Lo, C.P.; et al. Transforming Growth Factor Beta1 Signaling via Interaction with Cell Surface Hyal-2 and Recruitment of WWOX/WOX1. J. Biol. Chem. 2009, 284, 16049–16059. [Google Scholar] [CrossRef]
- Mahajan, N.P.; Whang, Y.E.; Mohler, J.L.; Earp, H.S. Activated Tyrosine Kinase Ack1 Promotes Prostate Tumorigenesis: Role of Ack1 in Polyubiquitination of Tumor Suppressor Wwox. Cancer Res. 2005, 65, 10514–10523. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-S.; Doherty, J.; Ensign, A. JNK1 Physically Interacts with WW Domain-containing Oxidoreductase (WOX1) and Inhibits WOX1-Mediated Apoptosis. J. Biol. Chem. 2003, 278, 9195–9202. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Su, W.P.; Lin, H.P.; Kuo, H.L.; Wei, H.L.; Chang, N.S. Role of WW Domain-Containing Oxidoreductase WWOX in Driving T Cell Acute Lymphoblastic Leukemia Maturation. J. Biol. Chem. 2016, 291, 17319–17331. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-S.; Hsu, L.-J.; Chang, N.-S. Functional Role of WW Domain-Containing Proteins in Tumor Biology and Diseases: Insight into the Role in Ubiquitin-Proteasome System. FASEB BioAdv. 2020, 2, 234–253. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Lai, F.J.; Chen, Y.A.; Sie, Y.D.; Kuo, H.L.; Su, W.P.; Wu, C.Y.; Liu, T.Y.; Wen, K.Y.; Hsu, L.J.; et al. Strategies by which WWOX-Deficient Metastatic Cancer Cells Utilize to Survive via Dodging, Compromising, and Causing Damage to WWOX-Positive Normal Microenvironment. Cell Death Discov. 2019, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-P.; Wang, W.-J.; Chang, J.-Y.; Ho, P.-C.; Liu, T.-Y.; Wen, K.-Y.; Kuo, H.-L.; Chen, Y.-J.; Huang, S.-S.; Subhan, D.; et al. Therapeutic Zfra4-10 or WWOX7-21 Peptide Induces Complex Formation of WWOX with Selective Protein Targets in Organs that Leads to Cancer Suppression and Spleen Cytotoxic Memory Z Cell Activation In Vivo. Cancers 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Ho, P.C.; Nagarajan, G.; Chen, Y.A.; Kuo, H.L.; Subhan, D.; Su, W.P.; Chang, J.Y.; Lu, C.Y.; Chang, K.T.; et al. WWOX Possesses N-Terminal Cell Surface-Exposed Epitopes WWOX7-21 and WWOX7-11 for Signaling Cancer Growth Suppression and Prevention In Vivo. Cancers 2019, 11, 1818. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B. Neuronal Migration Disorders. Radiol. Technol. 2018, 89, 279–295. [Google Scholar]
- Verrotti, A.; Spalice, A.; Ursitti, F.; Papetti, L.; Mariani, R.; Castronovo, A.; Mastrangelo, M.; Iannetti, P. New Trends in Neuronal Migration Disorders. Eur. J. Paediatr. Neurol. 2010, 14, 1–12. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Chou, Y.-T.; Lai, F.-J.; Jan, M.-S.; Chang, T.-H.; Jou, I.-M.; Chen, P.-S.; Lo, J.-Y.; Huang, S.-S.; Chang, N.-S.; et al. Wwox Deficiency Leads to Neurodevelopmental and Degenerative Neuropathies and Glycogen Synthase Kinase 3β-Mediated Epileptic Seizure Activity in Mice. Acta Neuropathol. Commun. 2020, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, C.M.; Ferguson, B.W.; Abba, M.C. WWOX at the Crossroads of Cancer, Metabolic Syndrome Related Traits and CNS Pathologies. Biochim. Biophys. Acta Bioenergy 2014, 1846, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Ehaideb, S.N.; Al-Bu Ali, M.J.; Al-Obaid, J.J.; Aljassim, K.M.; Alfadhel, M. Novel Homozygous Mutation in the WWOX Gene Causes Seizures and Global Developmental Delay: Report and Review. Transl. Neurosci. 2018, 9, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, M.; Baldassari, S.; Tochigi, Y.; Kośla, K.; Buffelli, F.; Torella, A.; Severino, M.; Paladini, D.; Mandarà, L.; Riva, A.; et al. Loss of Wwox Perturbs Neuronal Migration and Impairs Early Cortical Development. Front. Neurosci. 2020, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Lin, P.-W.; Lin, H.-P.; Huang, S.-S.; Lai, F.-J.; Sheu, H.-M.; Hsu, L.-J.; Chang, N.-S. UV Irradiation/Cold Shock-Mediated Apoptosis is Switched to Bubbling Cell Death at Low Temperatures. Oncotarget 2015, 6, 8007–8018. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.L.; Ho, P.C.; Huang, S.S.; Chang, N.S. Chasing the Signaling Run by Trimolecular Time-Lapse FRET Microscopy. Cell Death Discov. 2018, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-T.; Chuang, J.-I.; Cheng, C.-L.; Hsu, L.-J.; Chang, N.-S. Light-Induced Retinal Damage Involves Tyrosine 33 Phosphorylation, Mitochondrial and Nuclear Translocation of WW Domain-Containing Oxidoreductase In Vivo. Neuroscience 2005, 130, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Aderca, I.; Moser, C.D.; Veerasamy, M.; Bani-Hani, A.H.; Bonilla-Guerrero, R.; Ahmed, K.; Shire, A.; Cazanave, S.C.; Montoya, D.P.; Mettler, T.A.; et al. The JNK Inhibitor SP600129 Enhances Apoptosis of HCC Cells Induced by the Tumor Suppressor WWOX. J. Hepatol. 2008, 49, 373–383. [Google Scholar] [CrossRef][Green Version]
- Sze, C.-I.; Wen, K.-Y.; Chang, N.-S. WWOX is a Risk Factor for Alzheimer’s Disease: How and Why? Proc. Singapore Natl. Acad. Sci. 2020, 14, 31–45. [Google Scholar] [CrossRef]
- Teng, C.C.; Yang, Y.T.; Chen, Y.C.; Kuo, Y.M.; Sze, C.I. Role of WWOX/WOX1 in Alzheimer’s Disease Pathology and in Cell Death Signaling. Front. Biosci. 2013, 5, 72–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aldaz, C.M.; Hussain, T. WWOX Loss of Function in Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 8922. [Google Scholar] [CrossRef] [PubMed]
- Repudi, S.; Steinberg, D.J.; Elazar, N.; Breton, V.L.; Aquilino, M.S.; Saleem, A.; Abu-Swai, S.; Vainshtein, A.; Eshed-Eisenbach, Y.; Vijayaragavan, B.; et al. Neuronal Deletion of Wwox, Associated with WOREE Syndrome, Causes Epilepsy and Myelin Defects. Brain 2021. [Google Scholar] [CrossRef] [PubMed]
- Banne, E.; Abudiab, B.; Abu-Swai, S.; Repudi, S.; Steinberg, D.; Shatleh, D.; Alshammery, S.; Lisowski, L.; Gold, W.; Carlen, P.; et al. Neurological Disorders Associated with WWOX Germline Mutations—A Comprehensive Overview. Cells 2021, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.S. Editorial: Downregulation of WWOX and Inhibitory GABAergic Interneurons Correlates with Brain Inflammation during Progression of Alzheimer’s Disease. EC Neurol. 2021, 13.1, 8–11. [Google Scholar]
- Chang, N.S. TRAPPC6AΔ is a Potential Marker for the Progression of Alzheimer’s Disease from Middle Age. EC Neurol. 2021, 13, 23–27. [Google Scholar]
- Chou, P.Y.; Lin, S.R.; Lee, M.H.; Schultz, L.; Sze, C.I.; Chang, N.S. A p53/TIAF1/WWOX Triad Exerts Cancer Suppression but May Cause Brain Protein Aggregation due to p53/WWOX Functional Antagonism. Cell Commun. Signal 2019, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Chuang, J.I.; Wang, J.P.; Tsai, M.S.; Li, H.; Chang, N.S. Expression of WW Domain-Containing Oxidoreductase WOX1 in the Developing Murine Nervous System. Neuroscience 2004, 124, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Van Bergen, N.J.; Guo, Y.; Al-Deri, N.; Lipatova, Z.; Stanga, D.; Zhao, S.; Murtazina, R.; Gyurkovska, V.; Pehlivan, D.; Mitani, T.; et al. Deficiencies in Vesicular Transport Mediated by TRAPPC4 are Associated with Severe Syndromic Intellectual Disability. Brain 2020, 143, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, B.; DeGruttola, A.; Zhu, Y.; Lin, Y.; Zhang, Y.; Mo, X.; Hu, W. Emerging Role of NIK/IKK2-Binding Protein (NIBP)/Trafficking Protein Particle Complex 9 (TRAPPC9) in Nervous System Diseases. Transl. Res. 2020, 224, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.S.; Doherty, J.; Ensign, A.; Schultz, L.; Hsu, L.J.; Hong, Q.Y. WOX1 is Essential for Tumor Necrosis Factor-, UV Light-, Staurosporine-, and p53-Mediated Cell Death, and Its Tyrosine 33-Phosphorylated Form Binds and Stabilizes Serine 46-Phosphorylated p53. J. Biol. Chem. 2005, 280, 43100–43108. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Su, W.P.; Wang, W.J.; Lin, S.R.; Lu, C.Y.; Chen, Y.A.; Chang, J.Y.; Huang, S.S.; Chou, P.Y.; Ye, S.R.; et al. Zfra Activates Memory Hyal-2+CD3-CD19-Spleen Cells to Block Cancer Growth, Stemness, and Metastasis In Vivo. Oncotarget 2015, 6, 3737–3751. [Google Scholar] [CrossRef]
- Abate, G.; Frisoni, G.B.; Bourdon, J.C.; Piccirella, S.; Memo, M.; Uberti, D. The Pleiotropic Role of p53 in Functional/Dysfunctional Neurons: Focus on Pathogenesis and Diagnosis of Alzheimer’s Disease. Alzheimers Res. Ther. 2020, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Turnquist, C.; Horikawa, I.; Harris, C.C. Targeting Cellular Senescence in Cancer and Aging: Roles of p53 and Its Isoforms. Carcinogenesis 2020, 41, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.M.; Ghag, G.; Puangmalai, N.; Montalbano, M.; Bhatt, N.; Kayed, R. P53 Aggregation, Interactions with Tau, and Impaired DNA Damage Response in Alzheimer’s Disease. Acta Neuropathol. Commun. 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Kawasawa, Y.I.; Cheng, F.; Zhu, Y.; Xu, X.; Li, M.; Sousa, A.M.M.; Pletikos, M.; Meyer, K.A.; Sedmak, G.; et al. Spatio-Temporal Transcriptome of the Human Brain. Nat. Cell Biol. 2011, 478, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Pletikos, M.; Sousa, A.M.; Sedmak, G.; Meyer, K.A.; Zhu, Y.; Cheng, F.; Li, M.; Kawasawa, Y.I.; Sestan, N. Temporal Specification and Bilaterality of Human Neocortical Topographic Gene Expression. Neuron 2014, 81, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, S.K.; Aqeilan, R.I. Decoding the Link between WWOX and p53 in Aggressive Breast Cancer. Cell Cycle 2019, 18, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, M.; Salah, Z.; Herbel, C.; Hofmann, T.G.; Aqeilan, R.I. WWOX, the Common Fragile Site FRA16D Gene Product, Regulates ATM Activation and the DNA Damage Response. Proc. Natl. Acad. Sci. USA 2014, 111, E4716–E4725. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Chen, S.H.; Chen, S.J.; Chou, P.Y.; Huang, C.C.; Chang, N.S. WW Domain-Containing Oxidoreductase is a Potential Receptor for Sex Steroid Hormones. In Sex Hormones; Raghvendra, D., Ed.; InTech: Rijeka, Croatia, 2012; Chapter 16; pp. 333–351. [Google Scholar]
- Schultz, L.; Khera, S.; Sleve, D.; Heath, J.; Chang, N.S. TIAF1 and p53 Functionally Interact in Mediating Apoptosis and Silencing of TIAF1 Abolishes Nuclear Translocation of Serine 15-Phosphorylated p53. DNA Cell Biol. 2004, 23, 67–74. [Google Scholar] [CrossRef]
- Lee, M.H.; Lin, S.R.; Chang, N.S. TGF-β1-Induced TIAF1 Self-Association Leads to Apoptosis. In New Trends in Alzheimer & Parkinson Related Diseases: ADPD 2009; Fisher, A., Hanin, I., Eds.; Medimond International Proceedings: Bologna, Italy, 2009; pp. 149–155. [Google Scholar]
- Lanni, C.; Masi, M.; Racchi, M.; Govoni, S. Cancer and Alzheimer’s Disease Inverse Relationship: An Age-Associated Diverging Derailment of Shared Pathways. Mol. Psychiatry 2021, 26, 280–295. [Google Scholar] [CrossRef]
- Seo, J.; Park, M. Molecular Crosstalk between Cancer and Neurodegenerative Diseases. Cell. Mol. Life Sci. 2020, 77, 2659–2680. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lan, Y.Y.; Hsiao, J.R.; Chang, N.S. Strategies of Oncogenic Microbes to Deal with WW Domain-Containing Oxidoreductase. Exp. Biol. Med. 2015, 240, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.-J.; Schultz, L.; Mattison, J.; Lin, Y.-S.; Chang, N.-S. Cloning and Characterization of a Small-Size Peptide Zfra that Regulates the Cytotoxic Function of Tumor Necrosis Factor by Interacting with JNK1. Biochem. Biophys. Res. Commun. 2005, 327, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-T.; Liu, C.-C.; Chen, S.-T.; Yap, Y.V.; Chang, N.-S.; Sze, C.-I. WW Domain-Containing Oxidoreductase in Neuronal Injury and Neurological Diseases. Oncotarget 2014, 5, 11792–11799. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-P.; Hsu, L.-J.; Li, M.-Y.; Hsu, S.-Y.; Chuang, J.-I.; Tsai, M.-S.; Lin, S.-R.; Chang, N.-S.; Chen, S.-T. MPP+-Induced Neuronal Death in Rats Involves Tyrosine 33 Phosphorylation of WW Domain-Containing Oxidoreductase WOX1. Eur. J. Neurosci. 2008, 27, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA Approval for Biogen’s Aducanumab Sparks Alzheimer Disease Firestorm. Nat. Rev. Drug Discov. 2021, 20, 496. [Google Scholar] [CrossRef] [PubMed]
| Outstanding Questions | Suggested Future Research Directions |
|---|---|
| 1. WWOX is potent in blocking inflammation and preventing protein aggregation [28,74,79]. Is there a feasible approach to use autologous normal cells for stably expressing WWOX so as to suppress inflammation and AD progression? |
|
| 2. Zfra effectively blocks cancer growth and inhibits AD progression by blocking chronic systemic inflammation [74]. However, Zfra inactivates WWOX by degradation via an unknown proteolytic mechanism. Can WWOX be replaced by exogenous Zfra peptide in mitigating AD progression? |
|
| 3. Chronic inflammation induces protein aggregation in the lung. Do lung protein aggregates accelerate the formation of protein polymerization and plaque formation in the brain? |
|
| 4. Activated Z cells suppress and prevent cancer growth [28]. Can autologous Z cell therapy be effective in preventing and blocking AD progression? |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-Y.; Lee, K.-T.; Sun, T.-Y.; Sze, C.-I.; Huang, S.-S.; Hsu, L.-J.; Chang, N.-S. WWOX and Its Binding Proteins in Neurodegeneration. Cells 2021, 10, 1781. https://doi.org/10.3390/cells10071781
Hsu C-Y, Lee K-T, Sun T-Y, Sze C-I, Huang S-S, Hsu L-J, Chang N-S. WWOX and Its Binding Proteins in Neurodegeneration. Cells. 2021; 10(7):1781. https://doi.org/10.3390/cells10071781
Chicago/Turabian StyleHsu, Che-Yu, Kuan-Ting Lee, Tzu-Yu Sun, Chun-I. Sze, Shenq-Shyang Huang, Li-Jin Hsu, and Nan-Shan Chang. 2021. "WWOX and Its Binding Proteins in Neurodegeneration" Cells 10, no. 7: 1781. https://doi.org/10.3390/cells10071781
APA StyleHsu, C.-Y., Lee, K.-T., Sun, T.-Y., Sze, C.-I., Huang, S.-S., Hsu, L.-J., & Chang, N.-S. (2021). WWOX and Its Binding Proteins in Neurodegeneration. Cells, 10(7), 1781. https://doi.org/10.3390/cells10071781






