Salivary Gland Tissue Engineering Approaches: State of the Art and Future Directions
Abstract
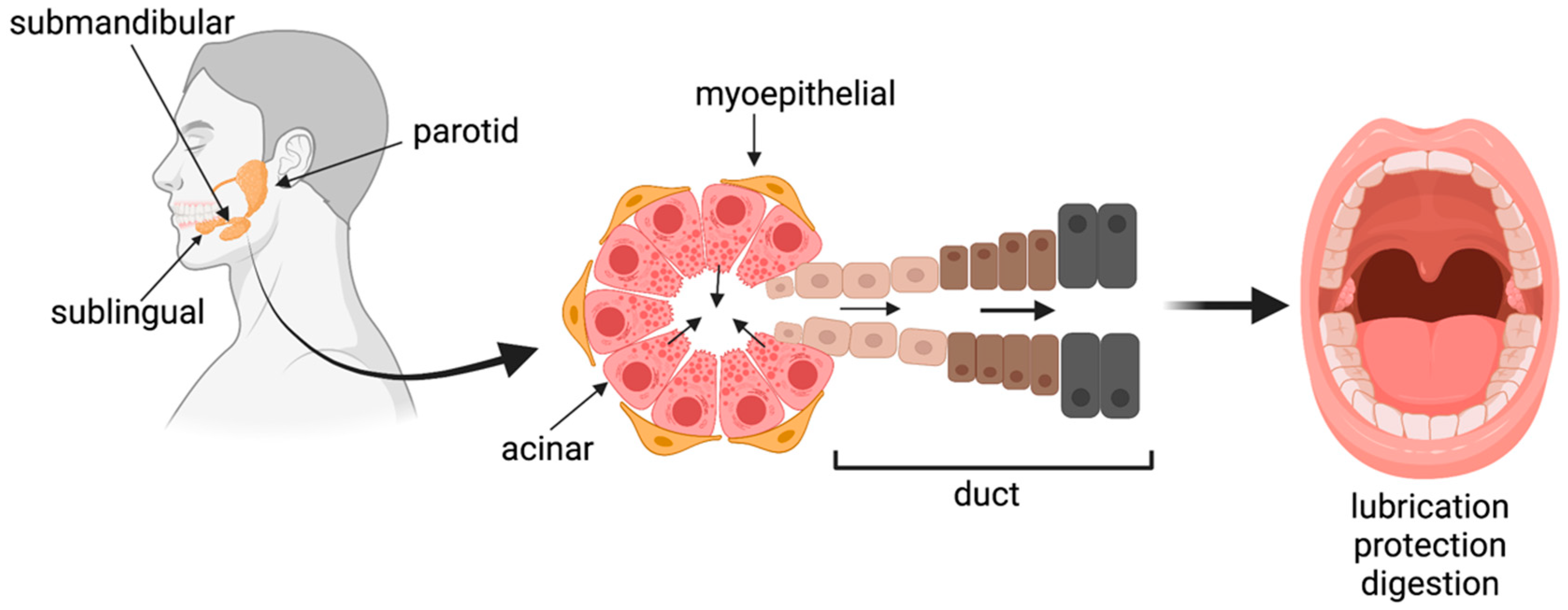
1. Introduction
2. Materials and Methods
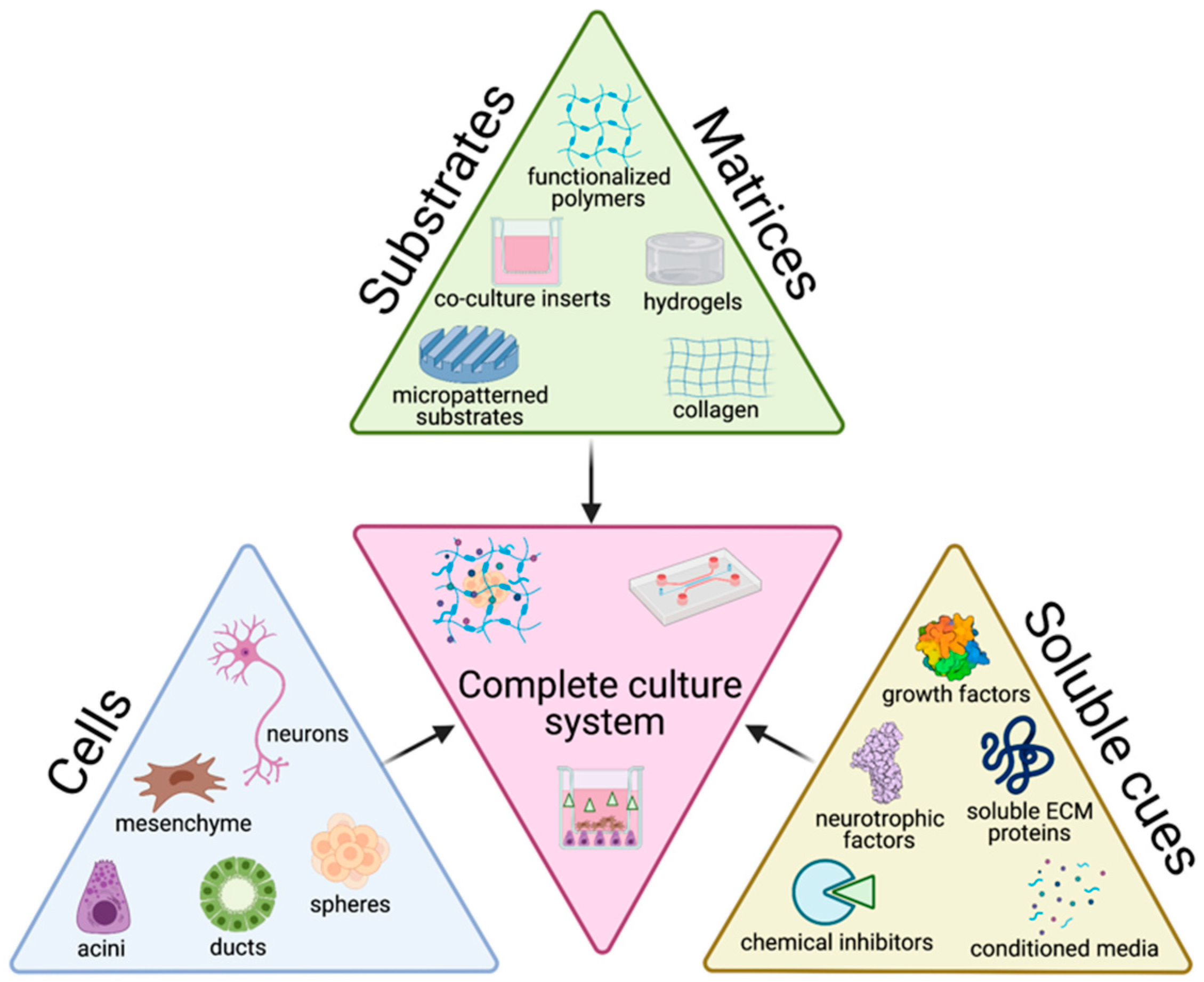
3. Trends in In Vitro Salivary Gland Tissue Culture
3.1. Cells
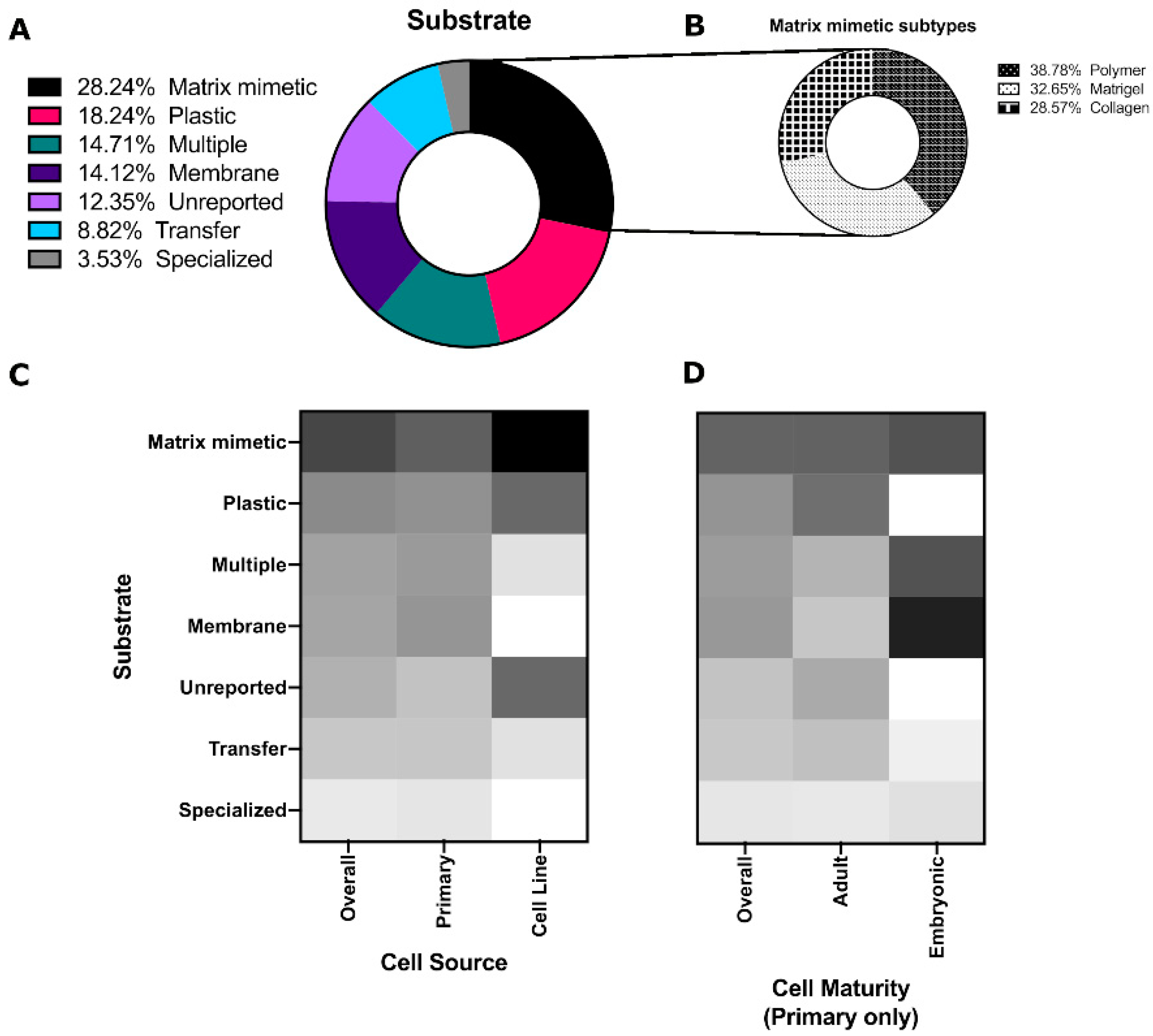
3.2. Matrices/Substrates
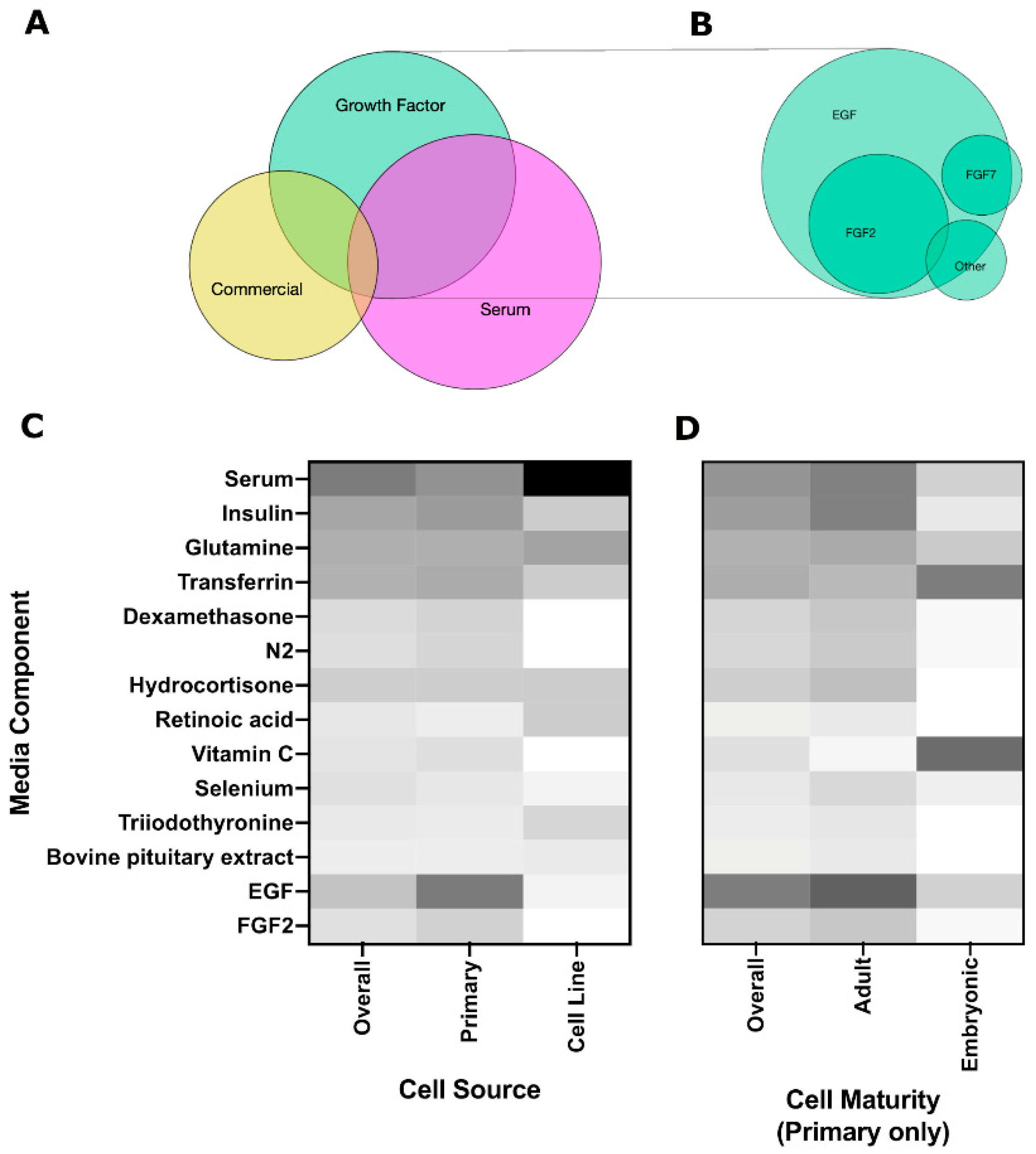
3.3. Soluble Cues
3.3.1. Growth Factors
3.3.2. Chemical Inhibitors
ROCK Inhibitor
EGFR Inhibitors
TGFβR Inhibitors
3.3.3. Neurotrophic Factors
3.3.4. Conditioned Media
3.3.5. Soluble ECM Proteins
4. Opportunities for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Whelton, H. Introduction: Anatomy and physiology of salivary glands. In Saliva and Oral Health, 4th ed.; Dawes, C., Edgar, W.M., O’Mullane, D.M., Eds.; Stephen Hancock Limited: Oxford, UK, 2012; pp. 1–36. [Google Scholar]
- Rocchi, C.; Emmerson, E. Mouth-Watering Results: Clinical Need, Current Approaches, and Future Directions for Salivary Gland Regeneration. Trends Mol. Med. 2020, 26, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Varga, G. Physiology of the salivary glands. Surgery (Oxf.) 2012, 30, 578–583. [Google Scholar] [CrossRef][Green Version]
- Porcheri, C.; Mitsiadis, T.A. Physiology, Pathology and Regeneration of Salivary Glands. Cells 2019, 8, 976. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Aitken-Saavedra, J.P. Xerostomia: An Update of Causes and Treatments. Salivary Gland. New Approaches Diagn. Treat. 2019, 15–37. [Google Scholar] [CrossRef]
- Wolff, A.; Joshi, R.K.; Ekström, J.; Aframian, D.; Pedersen, A.M.L.; Proctor, G.; Narayana, N.; Villa, A.; Sia, Y.W.; Aliko, A.; et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia, and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs R&D 2017, 17, 1–28. [Google Scholar] [CrossRef]
- Eisbruch, A. Reducing Xerostomia by IMRT: What May, and May Not, Be Achieved. J. Clin. Oncol. 2007, 25, 4863–4864. [Google Scholar] [CrossRef]
- Rades, D.; Fehlauer, F.; Bajrovic, A.; Mahlmann, B.; Richter, E.; Alberti, W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother. Oncol. 2004, 70, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Dijkema, T.; Terhaard, C.H.J.; Roesink, J.M.; Raaijmakers, C.P.J.; van den Keijbus, P.A.; Brand, H.S.; Veerman, E.C. MUC5B levels in submandibular gland saliva of patients treated with radiotherapy for head-and-neck cancer: A pilot study. Radiat. Oncol. 2012, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Eisbruch, A. IMRT for head and neck cancer: Reducing xerostomia and dysphagia. J. Radiat. Res. 2016, 57, i69–i75. [Google Scholar] [CrossRef]
- Quissell, D.O.; Redman, R.S.; Mark, M.R. Short-term primary culture of acinar-intercalated duct complexes from rat submandibular glands. Vitr. Cell. Dev. Biol. 1986, 22, 469–480. [Google Scholar] [CrossRef]
- Yeh, C.-K.; Mertz, P.M.; Oliver, C.; Baum, B.J.; Kousvelari, E.E. Cellular characteristics of long-term cultured rat parotid acinar cells. Vitr. Cell. Dev. Biol. 1991, 27, 707–712. [Google Scholar] [CrossRef]
- Pin, C.; Rukstalis, J.M.; Johnson, C.; Konieczny, S.F. The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J. Cell Biol. 2001, 155, 519–530. [Google Scholar] [CrossRef]
- Shubin, A.D.; Felong, T.J.; Schutrum, B.E.; Joe, D.S.; Ovitt, C.E.; Benoit, D.S. Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater. 2017, 50, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.D.; Sharipol, A.; Felong, T.J.; Weng, P.-L.; Schutrum, B.E.; Joe, D.S.; Aure, M.H.; Benoit, D.S.; Ovitt, C.E. Stress or injury induces cellular plasticity in salivary gland acinar cells. Cell Tissue Res. 2020, 380, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Manzella, K.; Baker, O. Current cell models for bioengineering a salivary gland: A mini-review of emerging technologies. Oral Dis. 2012, 19, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Capes-Davis, A.; Theodosopoulos, G.; Atkin, I.; Drexler, H.G.; Kohara, A.; MacLeod, R.A.; Masters, J.R.; Nakamura, Y.; Reid, Y.A.; Reddel, R.; et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int. J. Cancer 2010, 127, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Elkashty, O.; Ramamoorthi, M.; Trinh, N.; Liu, Y.; Sunavala-Dossabhoy, G.; Pranzatelli, T.; Michael, D.G.; Chivasso, C.; Perret, J.; et al. Cross-contamination of the human salivary gland HSG cell line with HeLa cells: A STR analysis study. Oral Dis. 2018, 24, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Ogawa, M.; Hojo, H.; Kawashima, Y.; Mabuchi, Y.; Hata, K.; Nakamura, S.; Yasuhara, R.; Takamatsu, K.; Irié, T.; et al. Generation of orthotopically functional salivary gland from embryonic stem cells. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, C.L.; Monroe, M.; Hunt, J.; Buchmann, L.; Baker, O.J. Comparing human and mouse salivary glands: A practice guide for salivary researchers. Oral Dis. 2019, 25, 403–415. [Google Scholar] [CrossRef]
- Hosseini, Z.F.; Nelson, D.; Moskwa, N.; Sfakis, L.M.; Castracane, J.; Larsen, M. FGF2-dependent mesenchyme and laminin-111 are niche factors in salivary gland organoids. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.; Lombaert, I.M.A.; Patel, V.N.; Kibbey, S.E.; Pradhan-Bhatt, S.; Witt, R.L.; Hoffman, M.P. Neurturin-containing laminin matrices support innervated branching epithelium from adult epithelial salispheres. Biomaterials 2019, 216, 119245. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, H.; Matsumoto, T.; Sakai, T.; Yamaguchi, S.; An, S.H.; Abe, M.; Wakisaka, S.; Lee, K.Y.; Egusa, H.; Imazato, S. Hydrogel-based biomimetic environment for in vitro modulation of branching morphogenesis. Biomaterials 2011, 32, 6754–6763. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Hsiao, Y.-C.; Young, T.-H.; Yang, T.-L. Maintenance of the spheroid organization and properties of glandular progenitor cells by fabricated chitosan based biomaterials. Biomater. Sci. 2018, 6, 1445–1456. [Google Scholar] [CrossRef]
- Pringle, S.; Maimets, M.; Van Der Zwaag, M.; Stokman, M.A.; Van Gosliga, D.; Zwart, E.; Witjes, M.; de Haan, G.; Van Os, R.; Coppes, R.P. Human Salivary Gland Stem Cells Functionally Restore Radiation Damaged Salivary Glands. Stem Cells 2016, 34, 640–652. [Google Scholar] [CrossRef]
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Faber, H.; Stokman, M.A.; Kok, T.; Visser, W.H.; Kampinga, H.; de Haan, G.; Coppes, R.P. Rescue of Salivary Gland Function after Stem Cell Transplantation in Irradiated Glands. PLoS ONE 2008, 3, e2063. [Google Scholar] [CrossRef] [PubMed]
- Shubin, A.D.; Felong, T.J.; Graunke, D.; Ovitt, C.E.; Benoit, D.S. Development of Poly(Ethylene Glycol) Hydrogels for Salivary Gland Tissue Engineering Applications. Tissue Eng. Part A 2015, 21, 1733–1751. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.J.; Lilliu, M.A.; Abu Elghanam, G.; Nguyen, T.; Liu, Y.; Lee, J.C.; Presley, J.F.; Zeitouni, A.; El-Hakim, M.; Tran, S.D. Cell culture of differentiated human salivary epithelial cells in a serum-free and scalable suspension system: The salivary functional units model. J. Tissue Eng. Regen. Med. 2019, 13, 1559–1570. [Google Scholar] [CrossRef]
- Song, Y.; Uchida, H.; Sharipol, A.; Piraino, L.; Mereness, J.A.; Ingalls, M.H.; Rebhahn, J.; Newlands, S.D.; DeLouise, L.A.; Ovitt, C.E.; et al. Development of a functional salivary gland tissue chip with potential for high-content drug screening. Commun. Biol. 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Chen, C.-N.; Chen, Y.-T.; Yang, T.-L. Controlling branching structure formation of the salivary gland by the degree of chitosan deacetylation. Acta Biomater. 2013, 9, 8214–8223. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-L.; Hsiao, Y.-C. Chitosan facilitates structure formation of the salivary gland by regulating the basement membrane components. Biomaterials 2015, 66, 29–40. [Google Scholar] [CrossRef]
- Kim, J.M.; Choi, S.; Lee, S.W.; Park, K. Voltage-dependent Ca2+ channels promote branching morphogenesis of salivary glands by patterning differential growth. Sci. Rep. 2018, 8, 7566. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; van der Zwaag, M.; Stokman, M.A.; van Os, R.; Coppes, R.P. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother. Oncol. 2009, 92, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, L.S.; Baanstra, M.; Faber, H.; Rocchi, C.; Zwart, E.; de Haan, G.; van Os, R.; Coppes, R.P. Purification and Ex Vivo Expansion of Fully Functional Salivary Gland Stem Cells. Stem Cell Rep. 2014, 3, 957–964. [Google Scholar] [CrossRef]
- Patel, V.N.; Lombaert, I.M.; Cowherd, S.N.; Shworak, N.W.; Xu, Y.; Liu, J.; Hoffman, M.P. Hs3st3-Modified Heparan Sulfate Controls KIT+ Progenitor Expansion by Regulating 3-O-Sulfotransferases. Dev. Cell 2014, 29, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.P.; Patel, V.N.; Liu, S.; Harrington, D.A.; Hoffman, M.P.; Jia, X.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S. Primary Salivary Human Stem/Progenitor Cells Undergo Microenvironment-Driven Acinar-Like Differentiation in Hyaluronate Hydrogel Culture. Stem Cells Transl. Med. 2016, 6, 110–120. [Google Scholar] [CrossRef]
- Baker, O.J.; Sommakia, S.; Internationals, O. Neurons Self-Organize Around Salivary Epithelial Cells in Novel Co-Culture Model. J. Stem Cell Regen. Biol. 2016, 2, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwon, H.R.; Nelson, D.; DeSantis, K.A.; Morrissey, J.M.; Larsen, M. Endothelial cell regulation of salivary gland epithelial patterning. Development 2017, 144, 211–220. [Google Scholar] [CrossRef]
- Teti, A. Regulation of cellular functions by extracellular matrix. J. Am. Soc. Nephrol. 1992, 2, S83. [Google Scholar] [CrossRef]
- Sequeira, S.J.; Larsen, M.; Devine, T. Extracellular Matrix and Growth Factors in Salivary Gland Development. Salivary Gland. 2010, 14, 48–77. [Google Scholar]
- Lee, S.-W.; Ryu, J.H.; Do, M.J.; Namkoong, E.; Lee, H.; Park, K. NiCHE Platform: Nature-Inspired Catechol-Conjugated Hyaluronic Acid Environment Platform for Salivary Gland Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 4285–4294. [Google Scholar] [CrossRef] [PubMed]
- Maria, O.M.; Zeitouni, A.; Gologan, O.; Tran, S.D. Matrigel Improves Functional Properties of Primary Human Salivary Gland Cells. Tissue Eng. Part A 2011, 17, 1229–1238. [Google Scholar] [CrossRef]
- Maria, O.M.; Liu, Y.; El-Hakim, M.; Zeitouni, A.; Tran, S.D. The role of human fibronectin- or placenta basement membrane extract-based gels in favouring the formation of polarized salivary acinar-like structures. J. Tissue Eng. Regen. Med. 2017, 11, 2643–2657. [Google Scholar] [CrossRef]
- Soscia, D.A.; Sequeira, S.J.; Schramm, R.A.; Jayarathanam, K.; Cantara, S.I.; Larsen, M.; Castracane, J. Salivary gland cell differentiation and organization on micropatterned PLGA nanofiber craters. Biomaterials 2013, 34, 6773–6784. [Google Scholar] [CrossRef]
- Sfakis, L.; Kamaldinov, T.; Khmaladze, A.; Hosseini, Z.F.; Nelson, D.A.; Larsen, M.; Castracane, J. Mesenchymal Cells Affect Salivary Epithelial Cell Morphology on PGS/PLGA Core/Shell Nanofibers. Int. J. Mol. Sci. 2018, 19, 1031. [Google Scholar] [CrossRef]
- Bécavin, T.; Kökten, T.; Huck, O.; Messaddeq, N.; Lesot, H.; Deveaux, E.; Benkirane-Jessel, N.; Laetitia, K.; Kuchler-Bopp, S. Well-organized spheroids as a new platform to examine cell interaction and behaviour during organ development. Cell Tissue Res. 2016, 366, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Burghartz, M.; Lennartz, S.; Schweinlin, M.; Hagen, R.; Kleinsasser, N.; Hackenberg, S.; Steußloff, G.; Scherzad, A.; Radeloff, K.; Ginzkey, C.; et al. Development of Human Salivary Gland-Like Tissue In Vitro. Tissue Eng. Part A 2018, 24, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Zhang, S.; Li, Y.; Zhang, X.; Hu, W.; Feng, Y.; Xiong, J.; Zhang, Y.; Wei, S. Generation of functional salivary gland tissue from human submandibular gland stem/progenitor cells. Stem Cell Res. Ther. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Janebodin, K.; Buranaphatthana, W.; Ieronimakis, N.; Hays, A.L.; Reyes, M. An In Vitro Culture System for Long-Term Expansion of Epithelial and Mesenchymal Salivary Gland Cells: Role of TGF-β1 in Salivary Gland Epithelial and Mesenchymal Differentiation. BioMed Res. Int. 2013, 2013, 1–20. [Google Scholar] [CrossRef]
- Nagai, K.; Arai, H.; Okudera, M.; Yamamura, T.; Oki, H.; Komiyama, K. Epiregulin is critical for the acinar cell regeneration of the submandibular gland in a mouse duct ligation model. J. Oral Pathol. Med. 2013, 43, 378–387. [Google Scholar] [CrossRef]
- Mitsui, R.; Fujita-Yoshigaki, J.; Narita, T.; Matsuki-Fukushima, M.; Satoh, K.; Qi, B.; Guo, M.-Y.; Katsumata-Kato, O.; Sugiya, H. Maintenance of paracellular barrier function by insulin-like growth factor-I in submandibular gland cells. Arch. Oral Biol. 2010, 55, 963–969. [Google Scholar] [CrossRef]
- Han, C.; An, G.H.; Woo, D.-H.; Kim, J.-H.; Park, H.-K. Rho-associated kinase inhibitor enhances the culture condition of isolated mouse salivary gland cells in vitro. Tissue Cell 2018, 54, 20–25. [Google Scholar] [CrossRef]
- Chou, Y.-S.; Young, T.-H.; Lou, P.-J. Effects of biomaterial-derived fibroblast conditioned medium on the α-amylase expression of parotid gland acinar cells. Acta Biomater. 2015, 27, 214–223. [Google Scholar] [CrossRef]
- Maimets, M.; Rocchi, C.; Bron, R.; Pringle, S.; Kuipers, J.; Giepmans, B.N.; Vries, R.G.; Clevers, H.; de Haan, G.; van Os, R.; et al. Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Rep. 2016, 6, 150–162. [Google Scholar] [CrossRef]
- Maruyama, C.; Leigh, N.; Nelson, J.; McCall, A.; Mellas, R.; Lei, P.; Andreadis, S.; Baker, O. Stem Cell–Soluble Signals Enhance Multilumen Formation in SMG Cell Clusters. J. Dent. Res. 2015, 94, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Fujita-Yoshigaki, J. Analysis of Changes in the Expression Pattern of Claudins Using Salivary Acinar Cells in Primary Culture. In Claudins: Methods and Protocols; Humana Press: New York, NY, USA, 2011; pp. 245–258. [Google Scholar]
- Lombaert, I.M.; Abrams, S.R.; Li, L.; Eswarakumar, J.; Sethi, A.J.; Witt, R.L.; Hoffman, M.P. Combined KIT and FGFR2b Signaling Regulates Epithelial Progenitor Expansion during Organogenesis. Stem Cell Rep. 2013, 1, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, Z.; Myers, C.; Heim, V.M.; Lathrop, C.A.; Rebustini, I.T.; Stewart, J.S.; Larsen, M.; Hoffman, M.P. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development 2005, 132, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.M.; Lombaert, I.M.A.; Reed, X.; Vitale-Cross, L.; Gutkind, J.S.; Hoffman, M.P. Parasympathetic Innervation Maintains Epithelial Progenitor Cells During Salivary Organogenesis. Science 2010, 329, 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Seto, M.; Noma, K. Rho Kinase (ROCK) Inhibitors. J. Cardiovasc. Pharmacol. 2007, 50, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Daley, W.P.; Kohn, J.M.; Larsen, M. A focal adhesion protein-based mechanochemical checkpoint regulates cleft progression during branching morphogenesis. Dev. Dyn. 2011, 240, 2069–2083. [Google Scholar] [CrossRef][Green Version]
- Daley, W.P.; Gervais, E.M.; Centanni, S.W.; Gulfo, K.M.; Nelson, D.; Larsen, M. ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development 2012, 139, 411–422. [Google Scholar] [CrossRef]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.-I.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tristan, C.A.; Chen, L.; Jovanovic, V.M.; Malley, C.; Chu, P.-H.; Ryu, S.; Deng, T.; Ormanoglu, P.; Tao, D.; et al. A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat. Methods 2021, 18, 528–541. [Google Scholar] [CrossRef]
- Zhang, L.; Valdez, J.M.; Zhang, B.; Wei, L.; Chang, J.; Xin, L. ROCK Inhibitor Y-27632 Suppresses Dissociation-Induced Apoptosis of Murine Prostate Stem/Progenitor Cells and Increases Their Cloning Efficiency. PLoS ONE 2011, 6, e18271. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Chen, Q.; Li, N.; Xiang, H.; Pan, Y.; Qu, Y.; Shang, D.; Go, V.L.W.; Xue, J.; Sun, Y.; et al. Serotonin-RhoA/ROCK axis promotes acinar-to-ductal metaplasia in caerulein-induced chronic pancreatitis. Biomed. Pharmacother. 2020, 125, 109999. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y. Establishment of long-term serum-free culture for lacrimal gland stem cells aiming at lacrimal gland repair. Stem Cell Res. Ther. 2020, 11, 13–20. [Google Scholar] [CrossRef]
- Koslow, M.; O’Keefe, K.J.; Hosseini, Z.F.; Nelson, D.A.; Larsen, M. ROCK inhibitor increases proacinar cells in adult salivary gland organoids. Stem Cell Res. 2019, 41, 101608. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.-Z.; Harris, R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016, 96, 1025–1069. [Google Scholar] [CrossRef]
- Gresik, E.W.; Kashimata, M.; Kadoya, Y.; Mathews, R.; Minami, N.; Yamashina, S. Expression of epidermal growth factor receptor in fetal mouse submandibular gland detected by a biotinyltyramide-based catalyzed signal amplification method. J. Histochem. Cytochem. 1997, 45, 1651–1657. [Google Scholar] [CrossRef]
- Mattingly, A.; Finley, J.K.; Knox, S.M. Salivary gland development and disease. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 573–590. [Google Scholar] [CrossRef]
- Means, A.L.; Meszoely, I.M.; Suzuki, K.; Miyamoto, Y.; Rustgi, A.K.; Coffey, R.J.; Wright, C.V.E.; Stoffers, D.A.; Leach, S.D. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 2005, 132, 3767–3776. [Google Scholar] [CrossRef]
- Jaskoll, T.; Melnick, M. Embryonic Salivary Gland Branching Morphogenesis. In Branching Morphogenesis; Davies, J.A., Ed.; Landes Bioscience: Georgetown, TX, USA, 2007; pp. 160–175. [Google Scholar]
- Woods, L.T.; Camden, J.M.; El-Sayed, F.G.; Khalafalla, M.G.; Petris, M.J.; Erb, L.; Weisman, G.A. Increased Expression of TGF-β Signaling Components in a Mouse Model of Fibrosis Induced by Submandibular Gland Duct Ligation. PLoS ONE 2015, 10, e0123641. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.B.; Zheng, C.; Swaim, W.D.; Cho, A.; Nagineni, C.N.; Eckhaus, M.A.; Flanders, K.C.; Ambudkar, I.S.; Baum, B.J.; Kulkarni, A.B. Conditional overexpression of TGF-β1 disrupts mouse salivary gland development and function. Lab. Investig. 2010, 90, 543–555. [Google Scholar] [CrossRef]
- Suzuki, D.; Pinto, F.; Senoo, M. Inhibition of TGF-β signaling supports high proliferative potential of diverse p63+ mouse epithelial progenitor cells in vitro. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Hoffman, M.P. Interactions between developing nerves and salivary glands. Organogenesis 2013, 9, 199–205. [Google Scholar] [CrossRef]
- Nakao, A.; Inaba, T.; Murakami-Sekimata, A.; Nogawa, H. Morphogenesis and Mucus Production of Epithelial Tissues of Three Major Salivary Glands of Embryonic Mouse in 3D Culture. Zool. Sci. 2017, 34, 475. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.M.; Lombaert, I.M.A.; Haddox, C.; Abrams, S.R.; Cotrim, A.P.; Wilson, A.J.; Hoffman, M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nedvetsky, P.; Emmerson, E.; Finley, J.K.; Ettinger, A.; Cruz-Pacheco, N.; Prochazka, J.; Haddox, C.; Northrup, E.; Hodges, C.; Mostov, K.E.; et al. Parasympathetic Innervation Regulates Tubulogenesis in the Developing Salivary Gland. Dev. Cell 2014, 30, 449–462. [Google Scholar] [CrossRef]
- Xiao, N.; Lin, Y.; Cao, H.; Sirjani, D.; Giaccia, A.J.; Koong, A.; Kong, C.S.; Diehn, M.; Le, Q.-T. Neurotrophic factor GDNF promotes survival of salivary stem cells. J. Clin. Investig. 2014, 124, 3364–3377. [Google Scholar] [CrossRef]
- Suzuki, A.; Ogata, K.; Iwata, J. Cell signaling regulation in salivary gland development. Cell. Mol. Life Sci. 2021, 78, 3299–3315. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-S.; Lin, Y.-C.; Young, T.-H.; Lou, P.-J. Effects of fibroblasts on the function of acinar cells from the same human parotid gland. Head Neck 2016, 38, E279–E286. [Google Scholar] [CrossRef]
- Farahat, M.; Kazi, G.A.S.; Taketa, H.; Hara, E.S.; Oshima, M.; Kuboki, T.; Matsumoto, T. Fibronectin-induced ductal formation in salivary gland self-organization model. Dev. Dyn. 2019, 248, 813–825. [Google Scholar] [CrossRef]
- Zhang, S.; Sui, Y.; Fu, X.; Feng, Y.; Luo, Z.; Zhang, Y.; Wei, S. Specific complexes derived from extracellular matrix facilitate generation of structural and drug-responsive human salivary gland microtissues through maintenance stem cell homeostasis. J. Tissue Eng. Regen. Med. 2019, 14, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Czerniecki, S.M.; Cruz, N.M.; Harder, J.L.; Menon, R.; Annis, J.; Otto, E.A.; Gulieva, R.E.; Islas, L.V.; Kim, Y.K.; Tran, L.M.; et al. High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell 2018, 22, 929–940. [Google Scholar] [CrossRef]
- Sidar, B.; Jenkins, B.R.; Huang, S.; Spence, J.R.; Walk, S.T.; Wilking, J.N. Long-term flow through human intestinal organoids with the gut organoid flow chip (GOFlowChip). Lab Chip 2019, 19, 3552–3562. [Google Scholar] [CrossRef] [PubMed]
- Chramiec, A.; Teles, D.; Yeager, K.; Marturano-Kruik, A.; Pak, J.; Chen, T.; Hao, L.; Wang, M.; Lock, R.; Tavakol, D.N.; et al. Integrated human organ-on-a-chip model for predictive studies of anti-tumor drug efficacy and cardiac safety. Lab Chip 2020, 20, 4357–4372. [Google Scholar] [CrossRef] [PubMed]
- Seiler, K.M.; Bajinting, A.; Alvarado, D.M.; Traore, M.A.; Binkley, M.M.; Goo, W.; Lanik, W.E.; Ou, J.; Ismail, U.; Iticovici, M.; et al. Patient-derived small intestinal myofibroblasts direct perfused, physiologically responsive capillary development in a microfluidic Gut-on-a-Chip Model. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]

| Cell Source | Cell Maturity | Cell Species | Gland Type | Media Type | Substrate |
|---|---|---|---|---|---|
| Primary Cell line Both | Adult Embryonic Both | Human Mouse Rat Other Multiple | Submandibular Parotid Minor Other Multiple | Growth Factor Serum Commercial | Matrix mimetic Plastic Transfer Specialized Multiple Membrane Unreported |
| Soluble Factor | Cells | Substrate/Matrix | Outcomes | |
|---|---|---|---|---|
| Fibroblast growth factor 10 (FGF10) | Human submandibular | Matrigel® | Increased Mist1, AQP5, α-amylase and α-SMA, decreased K5, promoted budding with no effect on organoid size, increased carbachol-induced calcium release | [49] |
| Epidermal growth factor receptor (EGFR) inhibitor | Mouse E16 submandibular | Nuclepore filter | EGFR inhibitor AG1478 retained epithelial cells and AQP5 | [22] |
| Transforming growth factor β receptor I (TGFβRI) inhibitor | Mouse submandibular | Matrigel® | Treatment with TGFβ and TGFβR-I inhibitor SB525334 increased expression of amylase-1, Aqp5, ZO-1, Occuldin, Fgf7 and Fgf10 but not collagen type I when cultured on Matrigel and increased the size of acinar clusters | [50] |
| Epiregulin | Mouse submandibular | Collagen-coated culture dish | Increased cell proliferation and levels of EGFR ligands epiregulin, HB-EGF, amphiregulin and TGFα | [51] |
| Insulin-like growth factor I (IGF-I) | SMIE cell line (rat submandibular) | Collagen-coated Transwell | Treatment with 100 ng/mL IGF-I maintained cell number, cell viability, tight junction expression and localization and paracellular barrier function | [52] |
| Rho-associated protein kinase (ROCK) inhibitor Y-27632 | Mouse submandibular | Matrigel® | Enhanced growth (cell numbers), survival (Live/Dead), proliferation (EdU), motility (scratch assay), maintained α-amylase expression and induced C-Met expression | [53] |
| Neurotrophin 4 (NT-4) | Human parotid | Cell culture plate | Highest levels of intracellular and secreted α-amylase at 1 ng/mL NT-4 | [54] |
| Neurturin (NRTN) | Mouse or human submandibular with E13 mesenchyme and neuronal cells | Laminin-111 hydrogel | Initiated branching, innervation and self-aggregation of spheres | [23] |
| Neureglin 1 (NRG1) | Human parotid | Cell culture plate | Promoted branching and retention of acinar-like cells in submandibular | [54] |
| Wnt and R-spondin conditioned media | Mouse submandibular | Matrigel® | Increased population doubling and sphere-forming efficiency | [55] |
| Mesenchymal stem cell (MSC) conditioned media | Mouse submandibular | Matrigel® or laminin-1 | Increased acinar-like structure and when combined with laminin-111, increased AQP5 and K14 and decreased in α-SMA compared to fresh submandibular gland | [56] |
| p38 MAPK inhibitor/Src inhibitor | Rat parotid | Collagen-coated dish or cell culture insert | Reduced cell stress | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piraino, L.R.; Benoit, D.S.W.; DeLouise, L.A. Salivary Gland Tissue Engineering Approaches: State of the Art and Future Directions. Cells 2021, 10, 1723. https://doi.org/10.3390/cells10071723
Piraino LR, Benoit DSW, DeLouise LA. Salivary Gland Tissue Engineering Approaches: State of the Art and Future Directions. Cells. 2021; 10(7):1723. https://doi.org/10.3390/cells10071723
Chicago/Turabian StylePiraino, Lindsay R., Danielle S. W. Benoit, and Lisa A. DeLouise. 2021. "Salivary Gland Tissue Engineering Approaches: State of the Art and Future Directions" Cells 10, no. 7: 1723. https://doi.org/10.3390/cells10071723
APA StylePiraino, L. R., Benoit, D. S. W., & DeLouise, L. A. (2021). Salivary Gland Tissue Engineering Approaches: State of the Art and Future Directions. Cells, 10(7), 1723. https://doi.org/10.3390/cells10071723






