Murder on the Ovarian Express: A Tale of Non-Autonomous Cell Death in the Drosophila Ovary
Abstract
1. Introduction
2. Drosophila melanogaster, a Powerful System for Cell Biology Research
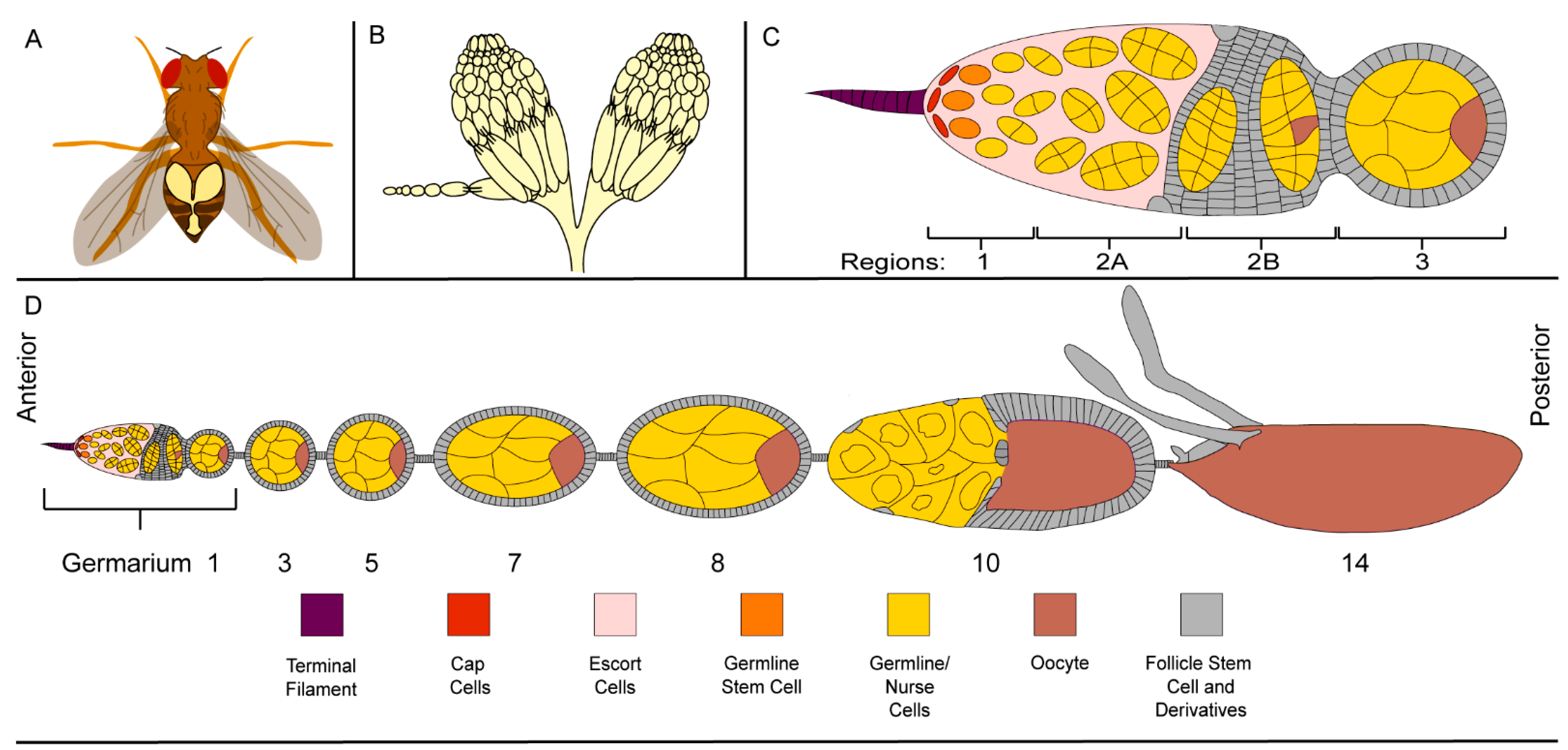
2.1. The Ovary, a Structurally Simple Tissue
2.2. Germline Development
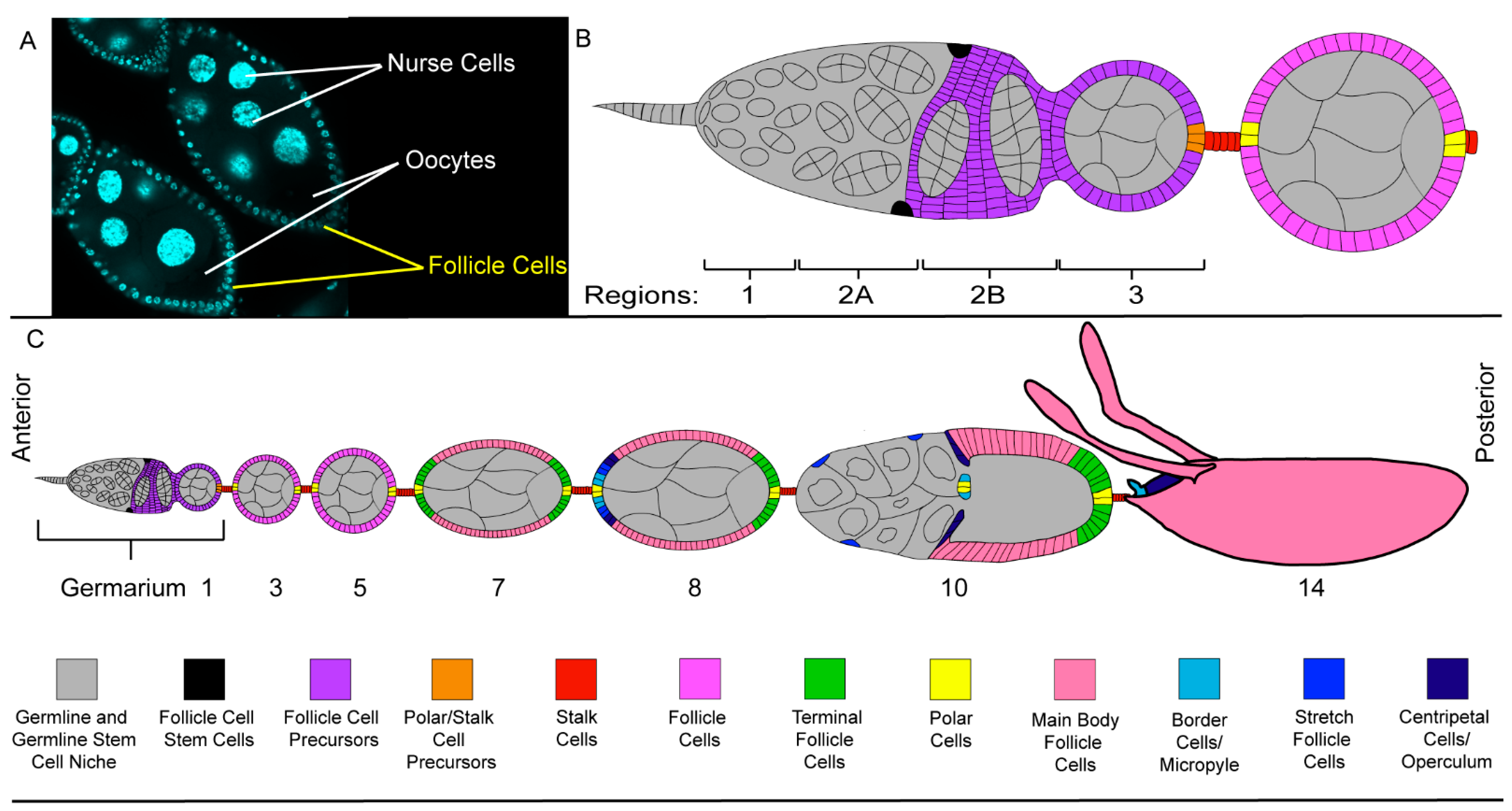
2.3. The Follicle Cell Layer
3. Development, Death, and Nonprofessional Phagocytes during Early and Mid Oogenesis
3.1. Cell Death during Mid-Oogenesis
3.2. Engulfment Machinery
3.3. Follicle Cell Genes Can Affect Germline Cell Death during Mid-Oogenesis
4. Non-Autonomous Developmental Death by Nonprofessional Phagocytes
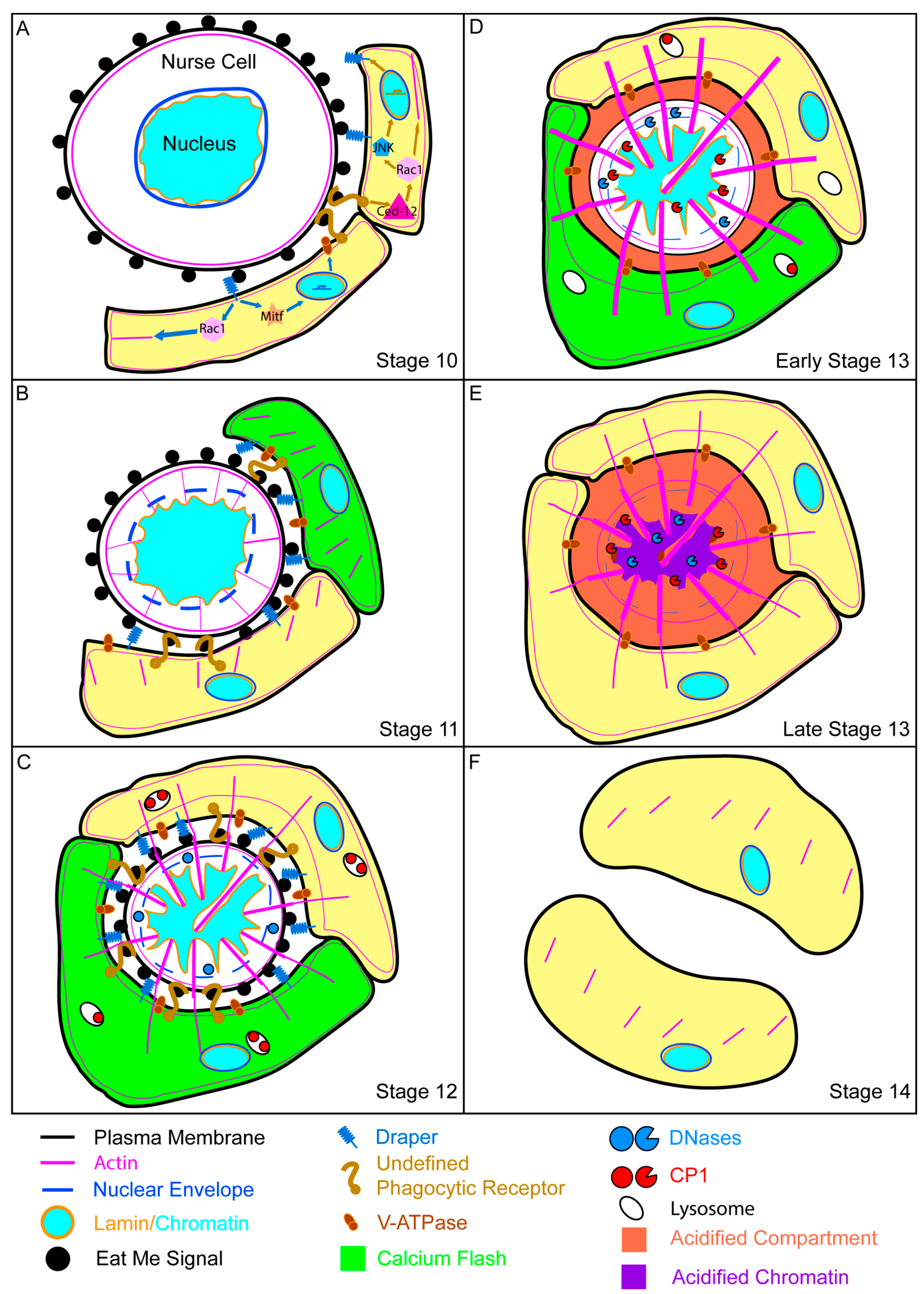
4.1. Morphology of Developmental Cell Death
4.2. Stretch Follicle Cells Are Required for the Phagocyte-Dependent Developmental Cell Death of Nurse Cells
4.3. Molecular Mechanisms of Nonprofessional Phagocytes during Nurse Cell Death
4.4. Collapse of the Nurse Cell Nucleus
4.5. Acidification and Nurse Cell Destruction
4.6. Two Nurse Cell Nuclei “Egg” Ceptions
4.7. Death and Clearance of the Follicle Cells
5. Nonprofessional Phagocytes and Phagoptosis beyond the Ovary
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sender, R.; Milo, R. The distribution of cellular turnover in the human body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nat. Immunol. 2015, 16, 907–917. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis: An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Kane, A.B. Redefining cell death. Am. J. Pathol. 1995, 146, 1–2. [Google Scholar]
- Lockshin, R.A.; Williams, C.M. Programmed cell death-II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J. Insect Physiol. 1964, 10, 643–649. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef]
- Peter, C.; Wesselborg, S.; Herrmann, M.; Lauber, K. Dangerous attraction: Phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis 2010, 15, 1007–1028. [Google Scholar] [CrossRef]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, Z.; Liu, D.; Gregory, S. Sterile inflammation in Drosophila. Mediat. Inflamm. 2015, 2015, 364286. [Google Scholar] [CrossRef]
- Chekeni, F.B.; Ravichandran, K.S. The role of nucleotides in apoptotic cell clearance: Implications for disease pathogenesis. J. Mol. Med. 2011, 89, 13–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, C.S.; Penberthy, K.K.; Wheeler, K.M.; Juncadella, I.J.; Vandenabeele, P.; Lysiak, J.J.; Ravichandran, K.S. Boosting Apoptotic Cell Clearance by Colonic Epithelial Cells Attenuates Inflammation In Vivo. Immunity 2016, 44, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Penberthy, K.K.; Lysiak, J.J.; Ravichandran, K.S. Rethinking Phagocytes: Clues from the Retina and Testes. Trends Cell Biol. 2018, 28, 317–327. [Google Scholar] [CrossRef]
- Nagata, S.; Hanayama, R.; Kawane, K. Autoimmunity and the Clearance of Dead Cells. Cell 2010, 140, 619–630. [Google Scholar] [CrossRef]
- Freeman, S.A.; Grinstein, S. Phagocytosis: How Macrophages Tune Their Non-professional Counterparts. Curr. Biol. 2016, 26, R1279–R1282. [Google Scholar] [CrossRef]
- Serizier, S.B.; McCall, K. Scrambled eggs: Apoptotic cell clearance by non-professional phagocytes in the Drosophila ovary. Front. Immunol. 2017, 8, 1642. [Google Scholar] [CrossRef]
- Burstyn-Cohen, T.; Lew, E.D.; Través, P.G.; Burrola, P.G.; Hash, J.C.; Lemke, G. Genetic Dissection of TAM Receptor-Ligand Interaction in Retinal Pigment Epithelial Cell Phagocytosis. Neuron 2012, 76, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Freeman, S.A. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front. Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Eaten alive! Cell death by primary phagocytosis: “Phagoptosis”. Trends Biochem. Sci. 2012, 37, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Hornik, T.C.; Vilalta, A.; Brown, G.C. Activated microglia cause reversible apoptosis of pheochromocytoma cells, inducing their cell death by phagocytosis. J. Cell Sci. 2016, 129, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014, 15, 209–216. [Google Scholar] [CrossRef]
- Métayer, L.E.; Vilalta, A.; Amos Burke, G.A.; Brown, G.C. Anti-CD47 antibodies induce phagocytosis of live, malignant B cells by macrophages via the Fc domain, resulting in cell death by phagoptosis. Oncotarget 2017, 8, 60892–60903. [Google Scholar] [CrossRef]
- Brown, G.C.; Vilalta, A.; Fricker, M. Phagoptosis-cell death by phagocytosis-plays central roles in physiology, host defense and pathology. Curr. Mol. Med. 2015, 15, 842–851. [Google Scholar] [CrossRef]
- Hakim-Mishnaevski, K.; Flint-Brodsly, N.; Shklyar, B.; Levy-Adam, F.; Kurant, E. Glial Phagocytic Receptors Promote Neuronal Loss in Adult Drosophila Brain. Cell Rep. 2019, 29, 1438–1448. [Google Scholar] [CrossRef]
- Johnsen, H.L.; Horvitz, H.R. Both the apoptotic suicide pathway and phagocytosis are required for a programmed cell death in Caenorhabditis elegans. BMC Biol. 2016, 14, 39. [Google Scholar] [CrossRef]
- Timmons, A.K.; Mondragon, A.A.; Schenkel, C.E.; Yalonetskaya, A.; Taylor, J.D.; Moynihan, K.E.; Etchegaray, J.I.; Meehan, T.L.; McCall, K. Phagocytosis genes nonautonomously promote developmental cell death in the Drosophila ovary. Proc. Natl. Acad. Sci. USA 2016, 113, E1246–E1255. [Google Scholar] [CrossRef]
- Etchegaray, J.I.; Timmons, A.K.; Klein, A.P.; Pritchett, T.L.; Welch, E.; Meehan, T.L.; Li, C.; McCall, K. Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells. Development 2012, 139, 4029–4039. [Google Scholar] [CrossRef]
- Mondragon, A.A.; Yalonetskaya, A.; Ortega, A.J.; Zhang, Y.; Naranjo, O.; Elguero, J.; Chung, W.S.; McCall, K. Lysosomal Machinery Drives Extracellular Acidification to Direct Non-apoptotic Cell Death. Cell Rep. 2019, 27, 11–19. [Google Scholar] [CrossRef]
- Pritchett, T.L.; Tanner, E.A.; McCall, K. Cracking open cell death in the Drosophila ovary. Apoptosis 2009, 14, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, V.K.; Timmons, A.K.; McCall, K. Diversity of cell death pathways: Insight from the fly ovary. Trends Cell Biol. 2013, 23, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.S.; Timmons, A.K.; Mondragon, A.A.; McCall, K. The end of the beginning: Cell death in the Germline. Curr. Top. Dev. Biol. 2015, 114, 93–119. [Google Scholar] [CrossRef]
- Giorgi, F.; Deri, P. Cell death in ovarian chambers of Drosophila melanogaster. J. Embryol. Exp. Morphol. 1976, 35, 521–533. [Google Scholar]
- Nezis, I.P.; Stravopodis, D.J.; Papassideri, I.; Robert-Nicoud, M.; Margaritis, L.H. Stage-specific apoptotic patterns during Drosophila oogenesis. Eur. J. Cell Biol. 2000, 79, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Drummond-Barbosa, D.; Spradling, A.C. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 2001, 231, 265–278. [Google Scholar] [CrossRef]
- Buszczak, M.; Cooley, L. Eggs to die for: Cell death during Drosophila oogenesis. Cell Death Differ. 2000, 7, 1071–1074. [Google Scholar] [CrossRef]
- McCall, K. Eggs over easy: Cell death in the Drosophila ovary. Dev. Biol. 2004, 274, 3–14. [Google Scholar] [CrossRef]
- King, R.C. Ovarian Development in Drosophila Melanogaster; Academic Press: New York, NY, USA, 1970; ISBN 9780124081505. [Google Scholar]
- Spradling, A.C. Developmental genetics of oogenesis. In The Development of Drosophila Melanogaster; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1993; ISBN 9780879694234. [Google Scholar]
- Tolwinski, N.S. Introduction: Drosophila-A model system for developmental biology. J. Dev. Biol. 2017, 5, 9. [Google Scholar] [CrossRef]
- Gratz, S.J.; Rubinstein, C.D.; Harrison, M.M.; Wildonger, J.; O’Connor-Giles, K.M. CRISPR-Cas9 genome editing in Drosophila. Curr. Protoc. Mol. Biol. 2015, 111, 31.2.1–31.2.20. [Google Scholar] [CrossRef]
- St Johnston, D. The art and design of genetic screens: Drosophila melanogaster. Nat. Rev. Genet. 2002, 3, 176–788. [Google Scholar] [CrossRef] [PubMed]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.M.; Yandell, M.D.; Wortman, J.R.; Gabor Miklos, G.L.; Nelson, C.R.; Hariharan, I.K.; Fortini, M.E.; Li, P.W.; Apweiler, R.; Fleischmann, W.; et al. Comparative genomics of the eukaryotes. Science 2000, 287, 2204–2215. [Google Scholar] [CrossRef]
- Meehan, T.L.; Yalonetskaya, A.; Joudi, T.F.; McCall, K. Detection of cell death and phagocytosis in the Drosophila ovary. Methods Mol. Biol. 2015, 1328, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Yalonetskaya, A.; Mondragon, A.A.; Elguero, J.; McCall, K. I spy in the developing fly a multitude ofways to die. J. Dev. Biol. 2018, 6, 26. [Google Scholar] [CrossRef]
- Bastock, R.; St Johnston, D. Drosophila oogenesis. Curr. Biol. 2008, 18, R1082–R1087. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Xu, Q.; Xie, Q.; Mio, W.; Deng, W.M. Automatic stage identification of Drosophila egg chamber based on DAPI images. Sci. Rep. 2016, 6, 18850. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, I.; Page-McCaw, A. Wnt signaling in stem cell maintenance and differentiation in the drosophila germarium. Genes (Basel) 2018, 9, 127. [Google Scholar] [CrossRef]
- Ogienko, A.A.; Fedorova, S.A.; Baricheva, E.M. Basic aspects of ovarian development in Drosophila melanogaster. Genetika 2007, 43, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Kirilly, D.; Xie, T. The Drosophila ovary: An active stem cell community. Cell Res. 2007, 17, 15–25. [Google Scholar] [CrossRef]
- Song, X.; Zhu, C.H.; Doan, C.; Xie, T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 2002, 296, 1855–1857. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Spradling, A.C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 2000, 290, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Dej, K.J.; Spradling, A.C. The endocycle controls nurse cell polytene chromosome structure during Drosophila oogenesis. Development 1999, 126, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Margolis, J.; Spradling, A. Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 1995, 121, 3797–3807. [Google Scholar] [CrossRef] [PubMed]
- Horne-Badovinac, S.; Bilder, D. Mass transit: Epithelial morphogenesis in the Drosophila egg chamber. Dev. Dyn. 2005, 232, 559–574. [Google Scholar] [CrossRef]
- Wu, X.; Singh Tanwar, P.; Raftery, L.A. Drosophila follicle cells: Morphogenesis in an eggshell. Semin. Cell Dev. Biol. 2008, 19, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Kolahi, K.S.; White, P.F.; Shreter, D.M.; Classen, A.K.; Bilder, D.; Mofrad, M.R.K. Quantitative analysis of epithelial morphogenesis in Drosophila oogenesis: New insights based on morphometric analysis and mechanical modeling. Dev. Biol. 2009, 331, 129–139. [Google Scholar] [CrossRef]
- McGregor, J.R.; Xi, R.; Harrison, D.A. JAK signaling is somatically required for follicle cell differentiation in Drosophila. Development 2002, 129, 705–717. [Google Scholar] [CrossRef]
- Torres, I.L.; López-Schier, H.; St. Johnston, D. A notch/delta-dependent relay mechanism establishes anterior-posterior polarity in Drosophila. Dev. Cell 2003, 5, 547–558. [Google Scholar] [CrossRef]
- Borensztejn, A.; Mascaro, A.; Wharton, K.A. JAK/STAT signaling prevents excessive apoptosis to ensure maintenance of the interfollicular stalk critical for Drosophila oogenesis. Dev. Biol. 2018, 438, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Besse, F.; Pret, A.M. Apoptosis-mediated cell death within the ovarian polar cell lineage of Drosophila melanogaster. Development 2003, 130, 1017–1027. [Google Scholar] [CrossRef]
- Hou, Y.C.C.; Chittaranjan, S.; Barbosa, S.G.; McCall, K.; Gorski, S.M. Effector caspase Dcp-1 and IAP protein Bruce regulate starvation-induced autophagy during Drosophila melanogaster oogenesis. J. Cell Biol. 2008, 182, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Nezis, L.P.; Lamark, T.; Velentzas, A.D.; Rusten, T.E.; Bjoørkoøy, G.; Johansen, T.; Papassideri, I.S.; Stravopodis, D.J.; Margaritis, L.H.; Stenmark, H.; et al. Cell death during Drosophila melanogaster early oogenesis is mediated through autophagy. Autophagy 2009, 5, 298–302. [Google Scholar] [CrossRef]
- Bolobolova, E.U.; Dorogova, N.V.; Fedorova, S.A. Major Scenarios of Genetically Regulated Cell Death during Oogenesis in Drosophila melanogaster. Russ. J. Genet. 2020, 56, 655–665. [Google Scholar] [CrossRef]
- Khammari, A.; Agnès, F.; Gandille, P.; Pret, A.M. Physiological apoptosis of polar cells during Drosophila oogenesis is mediated by Hid-dependent regulation of Diap1. Cell Death Differ. 2011, 18, 793–805. [Google Scholar] [CrossRef]
- Chao, S.H.; Nagoshi, R.N. Induction of apoptosis in the germline and follicle layer of Drosophila egg chambers. Mech. Dev. 1999, 88, 159–172. [Google Scholar] [CrossRef]
- De Lorenzo, C.; Strand, D.; Mechler, B.M. Requirement of Drosophila l(2)gl function for survival of the germline cells and organization of the follicle cells in a columnar epithelium during oogenesis. Int. J. Dev. Biol. 1999, 43, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J.; Chavdoula, E.D.; Nezis, I.P.; Margaritis, L.H. Cell death induced by GSM 900-MHz and DCS 1800-MHz mobile telephony radiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 626, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kacsoh, B.Z.; Bozler, J.; Ramaswami, M.; Bosco, G. Social communication of predator-induced changes in Drosophila behavior and germline physiology. Elife 2015, 4, e07423. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, T.L.; McCall, K. Role of the insulin/Tor signaling network in starvation-induced programmed cell death in Drosophila oogenesis. Cell Death Differ. 2012, 19, 1069–1079. [Google Scholar] [CrossRef]
- Laundrie, B.; Peterson, J.S.; Baum, J.S.; Chang, J.C.; Fileppo, D.; Thompson, S.R.; McCall, K. Germline cell death is inhibited by P-element insertions disrupting the dcp-1/pita nested gene pair in Drosophila. Genetics 2003, 165, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Yalonetskaya, A.; Mondragon, A.A.; Hintze, Z.J.; Holmes, S.; McCall, K. Nuclear degradation dynamics in a nonapoptotic programmed cell death. Cell Death Differ. 2020, 27, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Hedgecock, E.M.; Sulston, J.E.; Thomson, J.N. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science 1983, 220, 1277–1279. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.E.; Jacobson, D.M.; Horvitz, H.R. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics 1991, 129, 79–94. [Google Scholar] [CrossRef]
- Mangahas, P.M.; Zhou, Z. Clearance of apoptotic cells in Caenorhabditis elegans. Semin. Cell Dev. Biol. 2005, 16, 295–306. [Google Scholar] [CrossRef]
- Kinchen, J.M.; Cabello, J.; Kilngele, D.; Wong, K.; Felchtinger, R.; Schnabel, H.; Schnabel, R.; Hengartner, M.O. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature 2005, 434, 93–99. [Google Scholar] [CrossRef]
- Franc, N.C. Phagocytosis of apoptotic cells in mammals, caenorhabditis elegans and Drosophila melanogaster: Molecular mechanisms and physiological consequences. Front. Biosci. 2002, 7, d1298–d1313. [Google Scholar]
- Freeman, M.R.; Delrow, J.; Kim, J.; Johnson, E.; Doe, C.Q. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron 2003, 38, 567–580. [Google Scholar] [CrossRef]
- Santoso, C.S.; Meehan, T.L.; Peterson, J.S.; Cedano, T.M.; Turlo, C.V.; McCall, K. The ABC transporter Eato Promotes cell clearance in the Drosophila melanogaster Ovary. G3 Genes Genomes Genet. 2018, 8, 833–843. [Google Scholar] [CrossRef]
- Meehan, T.L. Analysis of Engulfment and Cell Corpse Processing by Epithelial Cells in the Drosophila Ovary; ProQuest Dissertations Publishing: Morrisville, NC, USA, 2016. [Google Scholar]
- Meehan, T.L.; Joudi, T.F.; Timmons, A.K.; Taylor, J.D.; Habib, C.S.; Peterson, J.S.; Emmanuel, S.; Franc, N.C.; McCall, K. Components of the engulfment machinery have distinct roles in corpse processing. PLoS ONE 2016, 11, e0158217. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, H.; Silva, E.A.; Thompson, J.; Guillou, A.; Yates, J.R.; Buchon, N.; Franc, N.C. The pallbearer E3 ligase promotes actin remodeling via RAC in efferocytosis by degrading the ribosomal protein S6. Dev. Cell 2015, 32, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Lebo, D.P.V.; Chirn, A.; Taylor, J.D.; Levan, A.; Torres, V.D.; Agreda, E.; Serizier, S.B.; Lord, A.K.; Jenkins, V.K.; McCall, K. An RNAi screen of the kinome in epithelial follicle cells of the Drosophila melanogaster ovary reveals genes required for proper germline death and clearance. G3 Genes Genomes Genet. 2021, 11, jkaa066. [Google Scholar] [CrossRef]
- Peterson, J.S.; Barkett, M.; McCall, K. Stage-specific regulation of caspase activity in Drosophila oogenesis. Dev. Biol. 2003, 260, 113–123. [Google Scholar] [CrossRef]
- Osterfield, M.; Berg, C.A.; Shvartsman, S.Y. Epithelial Patterning, Morphogenesis, and Evolution: Drosophila Eggshell as a Model. Dev. Cell 2017, 41, 337–348. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.; Steller, H. Requirement for DCP-1 caspase during Drosophila oogenesis. Science 1998, 279, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.M.I.; Hafen, E.; Köhler, K. The lack of autophagy triggers precocious activation of notch signaling during Drosophila oogenesis. BMC Dev. Biol. 2012, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.S.; Arama, E.; Steller, H.; McCall, K. The Drosophila caspases Strica and Dronc function redundantly in programmed cell death during oogenesis. Cell Death Differ. 2007, 14, 1508–1517. [Google Scholar] [CrossRef]
- Nezis, I.P.; Shravage, B.V.; Sagona, A.P.; Lamark, T.; Bjørkøy, G.; Johansen, T.; Rusten, T.E.; Brech, A.; Baehrecke, E.H.; Stenmark, H. Autophagic degradation of dBruce controls DNA fragmentation in nurse cells during late Drosophila melanogaster oogenesis. J. Cell Biol. 2010, 190, 523–531. [Google Scholar] [CrossRef]
- Mazzalupo, S.; Cooley, L. Illuminating the role of caspases during Drosophila oogenesis. Cell Death Differ. 2006, 13, 1950–1959. [Google Scholar] [CrossRef]
- Peterson, J.S.; McCall, K. Combined Inhibition of Autophagy and Caspases Fails to Prevent Developmental Nurse Cell Death in the Drosophila melanogaster Ovary. PLoS ONE 2013, 8, e76046. [Google Scholar] [CrossRef]
- Candelas, P. Follicle Cell Actin Dynamics and Calcium Bursts During Nurse Cell Death in Drosophila melanogaster. Master’s Thesis, Boston University, Boston, MA, USA, 2019. [Google Scholar]
- Weavers, H.; Evans, I.R.; Martin, P.; Wood, W. Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell 2016, 165, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Wood, W. Wound healing: Calcium flashes illuminate early events. Curr. Biol. 2012, 22, R14–R16. [Google Scholar] [CrossRef] [PubMed]
- Razzell, W.; Evans, I.R.; Martin, P.; Wood, W. Calcium flashes orchestrate the wound inflammatory response through duox activation and hydrogen peroxide release. Curr. Biol. 2013, 23, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.P.; Tanner, E.A.; Mateos San Martín, D.; Blute, T.; Kinser, R.D.; Dolph, P.J.; McCall, K. Cell-autonomous requirement for DNaseII in nonapoptotic cell death. Cell Death Differ. 2009, 16, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Mondragon, A. Investigation of Non-Autonomous Control of Cell Death and Corpse Clearance in the Ovary of Drosophila melanogaster. Ph.D. Thesis, Boston University, Boston, MA, USA, 2018. [Google Scholar]
- Zhang, T.; Zhou, Q.; Ogmundsdottir, M.H.; Möller, K.; Siddaway, R.; Larue, L.; Hsing, M.; Kong, S.W.; Goding, C.R.; Palsson, A.; et al. Mitf is a master regulator of the v-ATPase, forming a control module for cellular homeostasis with v-ATPase and TORC1. J. Cell Sci. 2015, 128, 2938–2950. [Google Scholar] [CrossRef] [PubMed]
- Etchegaray, J.; Elguero, E.J.; Tran, J.A.; Sinatra, V.; Feany, M.B.; McCall, K. Defective phagocytic corpse processing results in neurodegeneration and can be rescued by TORC1 activation. J. Neurosci. 2016, 36, 3170–3183. [Google Scholar] [CrossRef] [PubMed]
- Ali-Murthy, Z.; Fetter, R.D.; Wang, W.; Yang, B.; Royer, L.A.; Kornberg, T.B. Elimination of nurse cell nuclei that shuttle into oocytes during oogenesis. J. Cell Biol. 2021, 220, e202012101. [Google Scholar] [CrossRef]
- Nezis, I.P.; Stravopodis, D.J.; Margaritis, L.H.; Papassideri, I.S. Autophagy is required for the degeneration of the ovarian follicular epithelium in higher diptera. Autophagy 2006, 2, 297–298. [Google Scholar] [CrossRef]
- Nezis, I.P.; Stravopodis, D.J.; Margaritis, L.H.; Papassideri, I.S. Programmed cell death of follicular epithelium during the late developmental stages of oogenesis in the fruit flies Bactrocera oleae and Ceratitis capitata (Diptera, Tephritidae) is mediated by autophagy. Dev. Growth Differ. 2006, 48, 189–198. [Google Scholar] [CrossRef]
- Yang, H.; Yamashita, Y.M. The regulated elimination of transit-amplifying cells preserves tissue homeostasis during protein starvation in Drosophila testis. Development 2015, 142, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Yacobi-Sharon, K.; Namdar, Y.; Arama, E. Alternative germ cell death pathway in drosophila involves HtrA2/Omi, lysosomes, and a caspase-9 counterpart. Dev. Cell 2013, 25. [Google Scholar] [CrossRef] [PubMed]
- Melcarne, C.; Lemaitre, B.; Kurant, E. Phagocytosis in Drosophila: From molecules and cellular machinery to physiology. Insect Biochem. Mol. Biol. 2019, 109. [Google Scholar] [CrossRef] [PubMed]
- Denton, D.; Aung-Htut, M.T.; Kumar, S. Developmentally programmed cell death in Drosophila. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Breton, S.; Brown, D. Regulation of luminal acidification by the V-ATPase. Physiology 2013, 28, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Young, S.G.; Fielding, C.J. The ABCs of cholesterol efflux. Nat. Genet. 1999, 22, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Wilson, A.; Marcil, M.; Clee, S.M.; Zhang, L.H.; Roomp, K.; Van Dam, M.; Yu, L.; Brewer, C.; Collins, J.A.; Molhuizen, H.O.F.; et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 1999, 22, 336–345. [Google Scholar] [CrossRef]
- Li, A.C.; Glass, C.K. The macrophage foam cell as a target for therapeutic intervention. Nat. Med. 2002, 8, 1235–1242. [Google Scholar] [CrossRef]
- Monks, J.; Rosner, D.; Geske, F.J.; Lehman, L.; Hanson, L.; Neville, M.C.; Fadok, V.A. Epithelial cells as phagocytes: Apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ. 2005, 12, 107–114. [Google Scholar] [CrossRef]
- Seeberg, J.C.; Loibl, M.; Moser, F.; Schwegler, M.; Büttner-Herold, M.; Daniel, C.; Engel, F.B.; Hartmann, A.; Schlötzer-Schrehardt, U.; Goppelt-Struebe, M.; et al. Non-professional phagocytosis: A general feature of normal tissue cells. Sci. Rep. 2019, 9, 11875. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebo, D.P.V.; McCall, K. Murder on the Ovarian Express: A Tale of Non-Autonomous Cell Death in the Drosophila Ovary. Cells 2021, 10, 1454. https://doi.org/10.3390/cells10061454
Lebo DPV, McCall K. Murder on the Ovarian Express: A Tale of Non-Autonomous Cell Death in the Drosophila Ovary. Cells. 2021; 10(6):1454. https://doi.org/10.3390/cells10061454
Chicago/Turabian StyleLebo, Diane Patricia Vig, and Kimberly McCall. 2021. "Murder on the Ovarian Express: A Tale of Non-Autonomous Cell Death in the Drosophila Ovary" Cells 10, no. 6: 1454. https://doi.org/10.3390/cells10061454
APA StyleLebo, D. P. V., & McCall, K. (2021). Murder on the Ovarian Express: A Tale of Non-Autonomous Cell Death in the Drosophila Ovary. Cells, 10(6), 1454. https://doi.org/10.3390/cells10061454






