Future Perspectives of Therapeutic, Diagnostic and Prognostic Aptamers in Eye Pathological Angiogenesis
Abstract
1. Introduction
2. Mediators of Ocular Angiogenesis
3. Blood–Retinal Barrier (BRB) Dysfunction
4. Physiological Angiogenesis and Pathological Angiogenesis
5. Ocular Immunotherapy in Pathological Angiogenesis
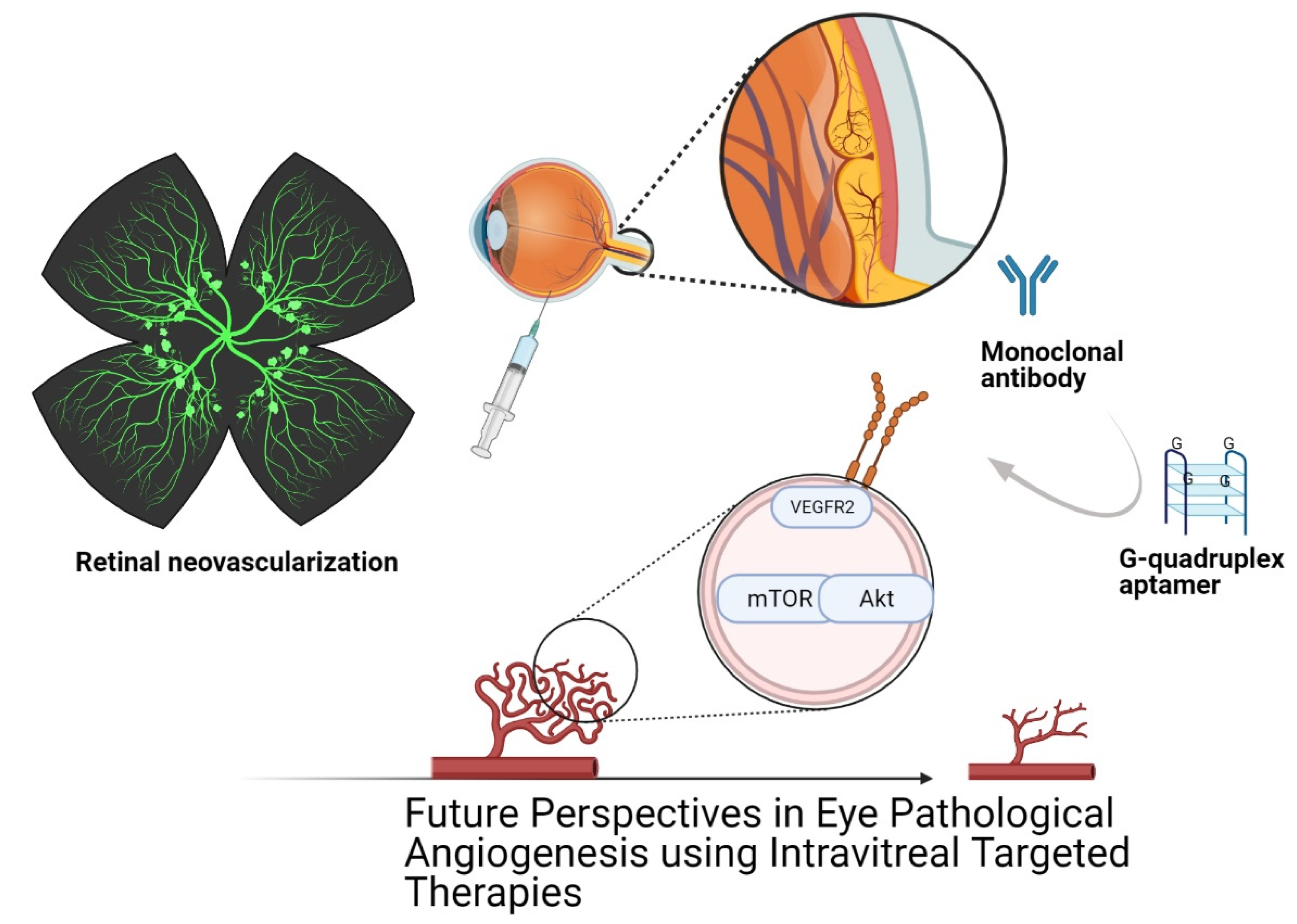
6. Aptamers: Novel Oligonucleotide Therapy
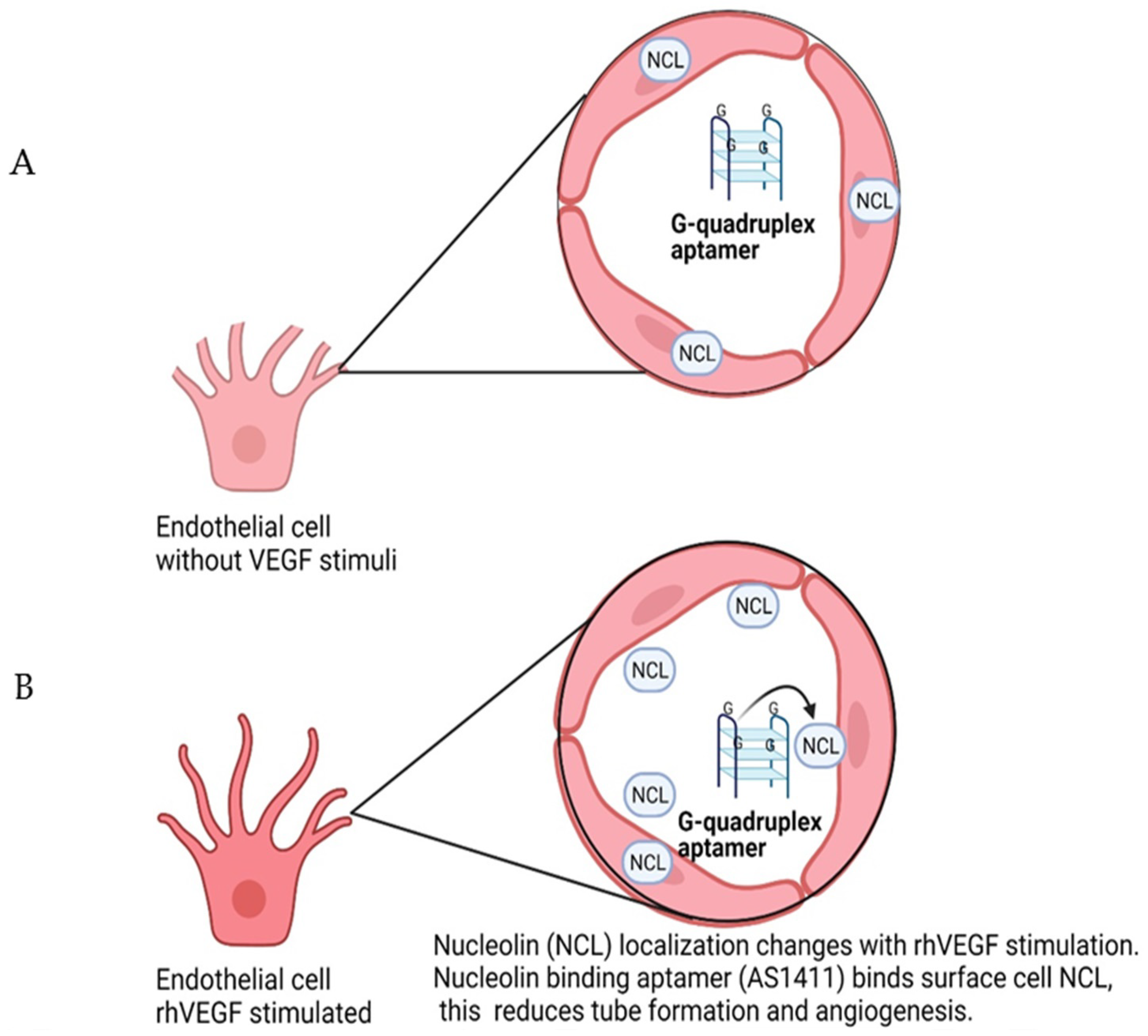
7. Nucleolin-Binding Aptamer, AS1411
8. Aptamers as Diagnostic, Therapeutic and Prognostic Agents
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular targeted therapy of glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Wang, H.; Xu, T.; Jiang, Y.; Xu, H.; Yan, Y.; Fu, D.; Chen, J. The Challenges and the Promise of Molecular Targeted Therapy in Malignant Gliomas. Neoplasia 2015, 17, 239–255. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Le, B.T.; Raguraman, P.; Kosbar, T.R.; Fletcher, S.; Wilton, S.D.; Veedu, R.N. Antisense Oligonucleotides Targeting Angiogenic Factors as Potential Cancer Therapeutics. Mol. Ther. Nucl. Acids 2019, 14, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Shen, L. Molecular targeted therapy of cancer: The progress and future prospect. Front. Lab. Med. 2017, 1, 69–75. [Google Scholar] [CrossRef]
- Cirenajwis, H.; Ekedahl, H.; Lauss, M.; Harbst, K.; Carneiro, A.; Enoksson, J.; Rosengren, F.; Werner-Hartman, L.; Törngren, T.; Kvist, A. Molecular stratification of metastatic melanoma using gene expression profiling: Prediction of survival outcome and benefit from molecular targeted therapy. Oncotarget 2015, 6, 12297. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Panina, Y.A.; Yakimov, A.S.; Komleva, Y.K.; Morgun, A.V.; Lopatina, O.L.; Malinovskaya, N.A.; Shuvaev, A.N.; Salmin, V.V.; Taranushenko, T.E.; Salmina, A.B. Plasticity of adipose tissue-derived stem cells and regulation of angiogenesis. Front. Physiol. 2018, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Wang, C.; Xia, W.-R.; Zheng, J.-Y.; Yang, J.; Liu, B.; Liu, J.-Q.; Liu, L.-F. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1α/VEGF axis. Free Radic. Biol. Med. 2018, 126, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Leblond, A.; Allanore, Y.; Avouac, J. Targeting synovial neoangiogenesis in rheumatoid arthritis. Autoimmun. Rev. 2017, 16, 594–601. [Google Scholar] [CrossRef]
- Guérard, S.; Pouliot, R. The role of angiogenesis in the pathogenesis of psoriasis: Mechanisms and clinical implications. J. Clin. Exp. Dermatol Res. S 2012. [Google Scholar] [CrossRef]
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2019, 110, 775–785. [Google Scholar] [CrossRef]
- Parma, L.; Baganha, F.; Quax, P.H.; de Vries, M.R. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 107–115. [Google Scholar] [CrossRef]
- Suraya, R.; Nagano, T.; Katsurada, M.; Sekiya, R.; Kobayashi, K.; Nishimura, Y. Molecular mechanism of asthma and its novel molecular target therapeutic agent. Respir. Investig. 2021, 59, 291–301. [Google Scholar] [CrossRef]
- Lenke, L.; Martínez de la Escalera, G.; Clapp, C.; Bertsch, T.; Triebel, J. A Dysregulation of the Prolactin/Vasoinhibin Axis Appears to Contribute to Preeclampsia. Front. Endocrinol. 2020, 10, 893. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Acquah, C.; Jeevanandam, J.; Tan, K.X.; Danquah, M.K. Engineered Aptamers for Enhanced COVID-19 Theranostics. Cell. Mol. Bioeng. 2021, 14, 209–221. [Google Scholar] [CrossRef]
- Sene, A.; Chin-Yee, D.; Apte, R.S. Seeing through VEGF: Innate and adaptive immunity in pathological angiogenesis in the eye. Trends Mol. Med. 2015, 21, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Monickaraj, F.; Balasubramanyam, M.; Rema, M.; Mohan, V. Imbalanced levels of angiogenic and angiostatic factors in vitreous, plasma and postmortem retinal tissue of patients with proliferative diabetic retinopathy. J. Diabetes Complicat. 2012, 26, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Kim, S.J.; Ho, A.C.; Schoenberger, S.D.; Bakri, S.J.; Ehlers, J.P.; Thorne, J.E. Therapies for Macular Edema Associated with Central Retinal Vein Occlusion: A Report by the American Academy of Ophthalmology. Ophthalmology. 2015, 122, 769–778. [Google Scholar] [CrossRef]
- Cayabyab, R.; Ramanathan, R. Retinopathy of prematurity: Therapeutic strategies based on pathophysiology. Neonatology 2016, 109, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Martinez, L.; Yilmaz-Ozcan, S.; Yemisci, M.; Schallek, J.; Kılıç, K.; Villafranca-Baughman, D.; Can, A.; Di Polo, A.; Dalkara, T. Retinal ischemia induces α-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion. Acta Neuropathol. Commun. 2019, 7, 134. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Domingueti, C.P.; Dusse, L.M.S.A.; Carvalho, M.d.G.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Tazhibi, M.; Sarrafzade, S.; Amini, M. Retinopathy risk factors in type II diabetic patients using factor analysis and discriminant analysis. J. Educ. Health Promot. 2014, 3, 85. [Google Scholar] [CrossRef]
- Fenwick, E.K.; Xie, J.; Man, R.E.K.; Sabanayagam, C.; Lim, L.; Rees, G.; Wong, T.Y.; Lamoureux, E.L. Combined poor diabetes control indicators are associated with higher risks of diabetic retinopathy and macular edema than poor glycemic control alone. PLoS ONE 2017, 12, e0180252. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Novotny, W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005, 333, 328–335. [Google Scholar] [CrossRef]
- Yang, X.; Scott, H.A.; Monickaraj, F.; Xu, J.; Ardekani, S.; Nitta, C.F.; Cabrera, A.; McGuire, P.G.; Mohideen, U.; Das, A.; et al. Basement membrane stiffening promotes retinal endothelial activation associated with diabetes. FASEB J. 2015, 30, 601–611. [Google Scholar] [CrossRef]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin–TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635. [Google Scholar] [CrossRef] [PubMed]
- Samson, F.P.; He, W.; Sripathi, S.R.; Patrick, A.T.; Madu, J.; Chung, H.; Frost, M.C.; Jee, D.; Gutsaeva, D.R.; Jahng, W.J. Dual switch mechanism of erythropoietin as an antiapoptotic and pro-angiogenic determinant in the retina. ACS Omega 2020, 5, 21113–21126. [Google Scholar] [CrossRef]
- Ahir, B.K.; Engelhard, H.H.; Lakka, S.S. Tumor development and angiogenesis in adult brain tumor: Glioblastoma. Mol. Neurobiol. 2020, 57, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Haefliger, J.-A.; Allagnat, F.; Hamard, L.; Le Gal, L.; Meda, P.; Nardelli-Haefliger, D.; Génot, E.; Alonso, F. Targeting Cx40 (connexin40) expression or function reduces angiogenesis in the developing mouse retina. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2136–2146. [Google Scholar] [CrossRef]
- Kovacs, K.; Marra, K.V.; Yu, G.; Wagley, S.; Ma, J.; Teague, G.C.; Nandakumar, N.; Lashkari, K.; Arroyo, J.G. Angiogenic and Inflammatory Vitreous Biomarkers Associated With Increasing Levels of Retinal Ischemia. Investig. Ophth. Vis. Sci. 2015, 56, 6523–6530. [Google Scholar] [CrossRef] [PubMed]
- Zeilbeck, L.F.; Mueller, B.B.; Leopold, S.A.; Senturk, B.; Langmann, T.; Tamm, E.R.; Ohlmann, A. Norrin mediates angiogenic properties via the induction of insulin-like growth factor-1. Exp. Eye Res. 2016, 145, 317–326. [Google Scholar] [CrossRef]
- Cabral, T.; Mello, L.G.M.; Lima, L.H.; Polido, J.; Regatieri, C.V.; Belfort, R.; Mahajan, V.B. Retinal and choroidal angiogenesis: A review of new targets. Int. J. Retin. Vitr. 2017, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Arden, G.B.; Sivaprasad, S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011, 7, 291–304. [Google Scholar] [CrossRef]
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323. [Google Scholar] [CrossRef]
- Yamamoto, H.; Rundqvist, H.; Branco, C.; Johnson, R.S. Autocrine VEGF Isoforms Differentially Regulate Endothelial Cell Behavior. Front. Cell Dev. Biol. 2016, 4, 99. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Drolet, D.W.; Green, L.S.; Gold, L.; Janjic, N. Fit for the eye: Aptamers in ocular disorders. Nucleic Acid Ther. 2016, 26, 127–146. [Google Scholar] [CrossRef]
- Harhaj, N.S.; Felinski, E.A.; Wolpert, E.B.; Sundstrom, J.M.; Gardner, T.W.; Antonetti, D.A. VEGF Activation of Protein Kinase C Stimulates Occludin Phosphorylation and Contributes to Endothelial Permeability. Investig. Ophth. Vis. Sci. 2006, 47, 5106–5115. [Google Scholar] [CrossRef]
- Murakami, T.; Frey, T.; Lin, C.; Antonetti, D.A. Protein kinase cβ phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes 2012, 61, 1573–1583. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Lin, C.-M.; Keil, J.M.; Sundstrom, J.M.; Smith, C.D.; Antonetti, D.A. Novel atypical PKC inhibitors prevent vascular endothelial growth factor-induced blood-retinal barrier dysfunction. Biochem. J. 2012, 446, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Wang, J.J.; Gao, G.; Shao, C.; Mott, R.; Ma, J.-X. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006, 20, 323–325. [Google Scholar] [CrossRef]
- Reichhart, N.; Strauss, O. Ion channels and transporters of the retinal pigment epithelium. Exp. Eye Res. 2014, 126, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Villarroel, M.; Corraliza, L.; Hernández, C.; Garcia-Ramírez, M. The Retinal Pigment Epithelium: Something More than a Constituent of the Blood-Retinal Barrier—Implications for the Pathogenesis of Diabetic. J. Biomed. Biotechnol. 2010, 2010, 190724. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.; Freeman, S.A. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front. Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef] [PubMed]
- Ponnalagu, M.; Subramani, M.; Jayadev, C.; Shetty, R.; Das, D. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine 2017, 95, 126–135. [Google Scholar] [CrossRef]
- Campbell, M.; Humphries, P. The blood-retina barrier: Tight junctions and barrier modulation. Adv. Exp. Med. Biol. 2012, 763, 70–84. [Google Scholar]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vis. Res. 2017, 139, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, I.; Van Noorden, C.J.F.; Schlingemann, R.O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog. Retin. Eye Res. 2013, 34, 19–48. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.J.M.E. Mechanisms of retinal fluid accumulation and blood-retinal barrier breakdown. Dev. Ophthalmol. 2017, 58, 11–20. [Google Scholar]
- Wang, J.; Chen, S.; Jiang, F.; You, C.; Mao, C.; Yu, J.; Han, J.; Zhang, Z.; Yan, H. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS ONE 2014, 9, e110531. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Zhao, H.; Ren, X.-B. Relationship of VEGF/VEGFR with immune and cancer cells: Staggering or forward? Cancer Biol. Med. 2016, 13, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.; Hartnett, M. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Pollina, E.A.; Legesse-Miller, A.; Haley, E.M.; Goodpaster, T.; Randolph-Habecker, J.; Coller, H.A. Regulating the angiogenic balance in tissues. Cell Cycle 2008, 7, 2056–2070. [Google Scholar] [CrossRef][Green Version]
- Mukwaya, A.; Peebo, B.; Xeroudaki, M.; Ali, Z.; Lennikov, A.; Jensen, L.; Lagali, N. Factors regulating capillary remodeling in a reversible model of inflammatory corneal angiogenesis. Sci. Rep. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Comitato, A.; Subramanian, P.; Turchiano, G.; Montanari, M.; Becerra, S.P.; Marigo, V. Pigment epithelium-derived factor hinders photoreceptor cell death by reducing intracellular calcium in the degenerating retina. Cell Death Dis. 2018, 9, 560. [Google Scholar] [CrossRef]
- Walia, A.; Yang, J.F.; Huang, Y.-H.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim. Biophys. Acta 2015, 1850, 2422–2438. [Google Scholar] [CrossRef] [PubMed]
- Muppala, S.; Frolova, E.; Xiao, R.; Krukovets, I.; Yoon, S.; Hoppe, G.; Vasanji, A.; Plow, E.; Stenina-Adognravi, O. Proangiogenic properties of thrombospondin-4. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, M.; Cammalleri, M.; Martini, D.; Casini, G.; Bagnoli, P. Antiangiogenic role of somatostatin receptor 2 in a model of hypoxia-induced neovascularization in the retina: Results from transgenic mice. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3480–3489. [Google Scholar] [CrossRef][Green Version]
- Clapp, C.; Thebault, S.; Arnold, E.; García, C.; Rivera, J.C.; de la Escalera, G.M. Vasoinhibins: Novel inhibitors of ocular angiogenesis. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E772–E778. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Amaro, C.D.; Moreno-Vega, A.I.; Adan-Castro, E.; Zamora, M.; Garcia-Franco, R.; Ramirez-Neria, P.; Garcia-Roa, M.; Villalpando, Y.; Robles, J.P.; Ramirez-Hernandez, G.; et al. Levosulpiride Increases the Levels of Prolactin and Antiangiogenic Vasoinhibin in the Vitreous of Patients with Proliferative Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2020, 9, 27. [Google Scholar] [CrossRef]
- García, C.; Aranda, J.; Arnold, E.; Thébault, S.; Macotela, Y.; López-Casillas, F.; Mendoza, V.; Quiroz-Mercado, H.; Hernández-Montiel, H.L.; Lin, S.H.; et al. Vasoinhibins prevent retinal vasopermeability associated with diabetic retinopathy in rats via protein phosphatase 2A–dependent eNOS inactivation. J. Clin. Investig. 2008, 118, 2291–2300. [Google Scholar]
- Triebel, J.; Bertsch, T.; Bollheimer, C.; Rios-Barrera, D.; Pearce, C.F.; Hüfner, M.; Escalera, G.M.d.l.; Clapp, C. Principles of the prolactin/vasoinhibin axis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1193–R1203. [Google Scholar] [CrossRef]
- Guerin, C.L.; Loyer, X.; Vilar, J.; Cras, A.; Mirault, T.; Gaussem, P.; Silvestre, J.-S.; Smadja, D.M. Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: Evidence of vasculogenic potential. Thromb Haemost. 2015, 113, 1084–1094. [Google Scholar] [CrossRef]
- Roozbahani, M.; Jamshidian, H.; Mahmoudi, E.; Arshi, A. Angiogenesis: A review of molecular mechanism. Sci. J. Iran. Blood Transfus Organ 2018, 15, 59–70. [Google Scholar]
- Mongiat, M.; Andreuzzi, E.; Tarticchio, G.; Paulitti, A. Extracellular Matrix, a Hard Player in Angiogenesis. Int. J. Mol. Sci. 2016, 17, 1822. [Google Scholar] [CrossRef]
- Jakobsson, L.; Franco, C.A.; Bentley, K.; Collins, R.T.; Ponsioen, B.; Aspalter, I.M.; Rosewell, I.; Busse, M.; Thurston, G.; Medvinsky, A.; et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010, 12, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Haupt, F.; Krishnasamy, K.; Napp, L.C.; Augustynik, M.; Limbourg, A.; Gamrekelashvili, J.; Bauersachs, J.; Haller, H.; Limbourg, F.P. Retinal myeloid cells regulate tip cell selection and vascular branching morphogenesis via Notch ligand Delta-like 1. Sci. Rep. 2019, 9, 9798. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.; Rogers, P. Human endometrial angiogenesis. Reproduction 2001, 121, 181–186. [Google Scholar] [CrossRef]
- Felmeden, D.C.; Blann, A.D.; Lip, G.Y.H. Angiogenesis: Basic pathophysiology and implications for disease. Eur. Heart J. 2003, 24, 586–603. [Google Scholar] [CrossRef]
- Siemerink, M.J.; Klaassen, I.; Van Noorden, C.J.F.; Schlingemann, R.O. Endothelial tip cells in ocular angiogenesis: Potential target for anti-angiogenesis therapy. J. Histochem. Cytochem. 2013, 61, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Yetkin-Arik, B.; Vogels, I.M.C.; Neyazi, N.; van Duinen, V.; Houtkooper, R.H.; van Noorden, C.J.F.; Klaassen, I.; Schlingemann, R.O. Endothelial tip cells in vitro are less glycolytic and have a more flexible response to metabolic stress than non-tip cells. Sci. Rep. 2019, 9, 10414. [Google Scholar] [CrossRef]
- Chen, W.; Xia, P.; Wang, H.; Tu, J.; Liang, X.; Zhang, X.; Li, L. The endothelial tip-stalk cell selection and shuffling during angiogenesis. J Cell Commun. Signal. 2019, 13, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Goveia, J.; Zecchin, A.; Rodriguez, F.M.; Moens, S.; Stapor, P.; Carmeliet, P. Endothelial cell differentiation by SOX17: Promoting the tip cell or stalking its neighbor instead? Circ. Res. 2014, 115, 205–207. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013, 3, a006569. [Google Scholar] [CrossRef]
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106. [Google Scholar] [CrossRef]
- Jones, B.W.; Kondo, M.; Terasaki, H.; Lin, Y.; McCall, M.; Marc, R.E. Retinal remodeling. Jpn. J. Ophthalmol. 2012, 56, 289–306. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Madonna, R.; Giovannelli, G.; Confalone, P.; Renna, F.V.; Geng, Y.-J.; De Caterina, R. High glucose-induced hyperosmolarity contributes to COX-2 expression and angiogenesis: Implications for diabetic retinopathy. Cardiovasc. Diabetol. 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Nejabati, H.R.; Latifi, Z.; Ghasemnejad, T.; Fattahi, A.; Nouri, M. Placental growth factor (PlGF) as an angiogenic/inflammatory switcher: Lesson from early pregnancy losses. Gynecol. Endocrinol. 2017, 33, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Gong, J.; Xu, Z.; Wei, Y.; Duh, E.J. Inhibition of pathological retinal angiogenesis by the integrin αvβ3 antagonist tetraiodothyroacetic acid (tetrac). Exp. Eye Res. 2012, 94, 41–48. [Google Scholar] [CrossRef]
- Littlepage, L.E.; Sternlicht, M.D.; Rougier, N.; Phillips, J.; Gallo, E.; Yu, Y.; Williams, K.; Brenot, A.; Gordon, J.I.; Werb, Z. Matrix Metalloproteinases Contribute Distinct Roles in Neuroendocrine Prostate Carcinogenesis, Metastasis, and Angiogenesis Progression. Cancer Res. 2010, 70, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Placencio, V.R.; Ichimura, A.; Miyata, T.; DeClerck, Y.A. Small Molecule Inhibitors of Plasminogen Activator Inhibitor-1 Elicit Anti-Tumorigenic and Anti-Angiogenic Activity. PLoS ONE 2015, 10, e0133786. [Google Scholar] [CrossRef]
- Tang, Y.; Scheef, E.A.; Gurel, Z.; Sorenson, C.M.; Jefcoate, C.R.; Sheibani, N. CYP1B1 and endothelial nitric oxide synthase combine to sustain proangiogenic functions of endothelial cells under hyperoxic stress. Am. J. Physiol. Cell Physiol. 2010, 298, C665–C678. [Google Scholar] [CrossRef]
- Bae, O.N.; Wang, J.M.; Baek, S.H.; Wang, Q.; Yuan, H.; Chen, A.F. Oxidative stress-mediated thrombospondin-2 upregulation impairs bone marrow-derived angiogenic cell function in diabetes mellitus. Arterioscler. Thromb. Vasc. 2013, 33, 1920–1927. [Google Scholar] [CrossRef]
- MacLauchlan, S.; Yu, J.; Parrish, M.; Asoulin, T.A.; Schleicher, M.; Krady, M.M.; Zeng, J.; Huang, P.L.; Sessa, W.C.; Kyriakides, T.R. Endothelial nitric oxide synthase controls the expression of the angiogenesis inhibitor. Proc. Natl. Acad. Sci. USA 2011, 108, E1137. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; DiMaio, T.A.; Scheef, E.A.; Sorenson, C.M.; Sheibani, N. PECAM-1 regulates proangiogenic properties of endothelial cells through modulation of cell-cell and cell-matrix interactions. Am. J. Physiol. Cell Physiol. 2010, 299, C1468–C1484. [Google Scholar] [CrossRef] [PubMed]
- Stapor, P.C.; Sweat, R.S.; Dashti, D.C.; Betancourt, A.M.; Murfee, W.L. Pericyte dynamics during angiogenesis: New insights from new identities. J. Vasc. Res. 2014, 51, 163–174. [Google Scholar] [CrossRef]
- Santos, G.S.P.; Prazeres, P.H.D.M.; Mintz, A.; Birbrair, A. Role of pericytes in the retina. Eye 2018, 32, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Lee, J.; Kim, J.; Kim, K.; Hong, S.; Han, S.; Kubota, Y.; Augustin, H.G.; Ding, L.; Kim, J.W.; et al. Plastic roles of pericytes in the blood–retinal barrier. Nat. Commun. 2017, 8, 15296. [Google Scholar] [CrossRef]
- Newman, A.C.; Nakatsu, M.N.; Chou, W.; Gershon, P.D.; Hughes, C.C.W. The requirement for fibroblasts in angiogenesis: Fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell. 2011, 22, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liaw, L.; Prudovsky, I.; Brooks, P.C.; Vary, C.; Oxburgh, L.; Friesel, R. Fibroblast Growth Factor Signaling in the Vasculature. Curr. Atheroscler. Rep. 2015, 17, 31. [Google Scholar] [CrossRef]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265. [Google Scholar] [CrossRef]
- Ryman, J.T.; Meibohm, B. Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacometr. Syst. Pharmacol. 2017, 6, 576–588. [Google Scholar] [CrossRef]
- Wells, J.A.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.P.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.W.; Bressler, N.M.; Browning, D.J.; et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; de Haas, S.; Kang, Y.K.; Ohtsu, A.; Tebbutt, N.C.; Ming Xu, J.; Peng Yong, W.; Langer, B.; Delmar, P.; Scherer, S.J.; et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 2012, 30, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J. Analysis of the binding affinity of vascular endothelial growth factor A (VEGF) to ranibizumab, aflibercept and bevacizumab. Investig. Ophth. Vis. Sci. 2013, 54, 1961. [Google Scholar]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef]
- Ferrara, N.; Damico, L.; Shams, N.; Lowman, H.; Kim, R. Development of ranibizumab, an anti–vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006, 26, 859–870. [Google Scholar] [CrossRef]
- Ohr, M.; Kaiser, P.K. Aflibercept in wet age-related macular degeneration: A perspective review. Ther. Adv. Chronic. Dis. 2012, 3, 153–161. [Google Scholar] [CrossRef]
- Kiss, S.; Dugel, P.U.; Khanani, A.M.; Broder, M.S.; Chang, E.; Sun, G.H.; Turpcu, A. Endophthalmitis rates among patients receiving intravitreal anti-VEGF injections: A USA claims analysis. Clin. Ophthalmol. 2018, 12, 1625–1635. [Google Scholar] [CrossRef]
- Moschos, M.M.; Chatziralli, I.P.; Kalogeropoulos, C.; Georgountzos, V.; Agapitos, E. Retinal Toxicity of Intravitreal Trastuzumab in a Rabbit Model: Preliminary Results of an Experimental Study. J. Ocul. Pharmacol. Ther. 2013, 29, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Bourcier, T.; Noel, G.; Speeg-Schatz, C.; Gaucher, D. Bilateral macular ischemia and severe visual loss following trastuzumab therapy. Acta Oncol. 2011, 50, 477–478. [Google Scholar] [CrossRef]
- Dugel, P.U.; Jaffe, G.J.; Sallstig, P.; Warburton, J.; Weichselberger, A.; Wieland, M.; Singerman, L. Brolucizumab Versus Aflibercept in Participants with Neovascular Age-Related Macular Degeneration: A Randomized Trial. Ophthalmology 2017, 124, 1296–1304. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, N.; Parachuri, N.; Sharma, R.; Bandello, F.; Kuppermann, B.D.; Loewenstein, A. Brolucizumab and immunogenicity. Eye 2020, 34, 1726–1728. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, J.; Sun, X. Resistance to anti-VEGF therapy in neovascular age-related macular degeneration: A comprehensive review. Drug Des. Dev. Ther. 2016, 10, 1857–1867. [Google Scholar] [CrossRef]
- Tschulakow, A.; Christner, S.; Julien, S.; Ludinsky, M.; van der Giet, M.; Schraermeyer, U. Effects of a single intravitreal injection of aflibercept and ranibizumab on glomeruli of monkeys. PLoS ONE 2014, 9, e113701. [Google Scholar] [CrossRef] [PubMed]
- Eng, V.A.; Rayess, N.; Nguyen, H.V.; Leng, T. Complete RPE and outer retinal atrophy in patients receiving anti-VEGF treatment for neovascular age-related macular degeneration. PLoS ONE 2020, 15, e0232353. [Google Scholar] [CrossRef] [PubMed]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye (London) 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Yazdian-Robati, R.; Bayat, P.; Oroojalian, F.; Zargari, M.; Ramezani, M.; Taghdisi, S.M.; Abnous, K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2019, 155, 1420–1431. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Cai, S.; Yan, J.; Xiong, H.; Liu, Y.; Peng, D.; Liu, Z. Investigations on the interface of nucleic acid aptamers and binding targets. Analyst 2018, 143, 5317–5338. [Google Scholar] [CrossRef]
- Selvakumar, L.S.; Thakur, M.S. Nano RNA aptamer wire for analysis of vitamin B12. Anal. Biochem. 2012, 427, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Mirau, P.A.; Smith, J.E.; Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N.; Naik, R. Structured DNA Aptamer Interactions with Gold Nanoparticles. Langmuir 2018, 34, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Sugiyama, S.; Sakamoto, T.; Miyakawa, S.; Adachi, H.; Takano, K.; Murakami, S.; Inoue, T.; Mori, Y.; Nakamura, Y.; et al. Conformational plasticity of RNA for target recognition as revealed by the 2.15 A crystal structure of a human IgG-aptamer complex. Nucleic Acids Res. 2010, 38, 7822–7829. [Google Scholar] [CrossRef]
- Shirota, H.; Tross, D.; Klinman, D.M. CpG oligonucleotides as cancer vaccine adjuvants. Vaccines 2015, 3, 390–407. [Google Scholar] [CrossRef]
- Fang, X.; Tan, W. Aptamers Generated from Cell-SELEX for Molecular Medicine: A Chemical Biology Approach. Acc. Chem. Res. 2010, 43, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.Y.; Byun, J. Nucleic Acid aptamers: New methods for selection, stabilization, and application in biomedical science. Biomol. Ther. 2013, 21, 423–434. [Google Scholar] [CrossRef]
- Han, J.; Gao, L.; Wang, J.; Wang, J. Application and development of aptamer in cancer: From clinical diagnosis to cancer therapy. J. Cancer 2020, 11, 6902–6915. [Google Scholar] [CrossRef]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Nat. 2013, 5, 34–43. [Google Scholar] [CrossRef]
- Meyer, C.; Hahn, U.; Rentmeister, A. Cell-Specific Aptamers as Emerging Therapeutics. J. Nucleic. Acids. 2011, 2011, 18. [Google Scholar] [CrossRef]
- Wu, Y.; Sefah, K.; Liu, H.; Wang, R.; Tan, W. DNA aptamer-micelle as an efficient detection/delivery vehicle toward cancer cells. Proc. Natl. Acad. Sci. USA 2010, 107, 5–10. [Google Scholar] [CrossRef]
- Sakai, D.; Grad, S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv. Drug Deliv. Rev. 2014, 84, 159–171. [Google Scholar] [CrossRef]
- Conti, B.; Bucolo, C.; Giannavola, C.; Puglisi, G.; Giunchedi, P.; Conte, U. Biodegradable microspheres for the intravitreal administration of acyclovir: In vitro/in vivo evaluation. Eur. J. Pharm. Sci. 1997, 5, 287–293. [Google Scholar] [CrossRef]
- Alkhamis, O.; Canoura, J.; Yu, H.; Liu, Y.; Xiao, Y. Innovative engineering and sensing strategies for aptamer-based small-molecule detection. TrAC Trends Anal. Chem. 2019, 121, 115699. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Wang, X.; Liu, B.; Liu, J. Conjugation of antibodies and aptamers on nanozymes for developing biosensors. Biosens. Bioelectron. 2020, 168, 112537. [Google Scholar] [CrossRef] [PubMed]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Bawab, A.A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2020, 25, 3. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef]
- Adachi, T.; Nakamura, Y. Aptamers: A Review of Their Chemical Properties and Modifications for Therapeutic Application. Molecules 2019, 24, 4229. [Google Scholar] [CrossRef]
- Edington, M.; Connolly, J.; Chong, N.V. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Vivanco-Rojas, O.; García-Bermúdez, M.Y.; Iturriaga-Goyon, E.; Rebollo, W.; Buentello-Volante, B.; Magaña-Guerrero, F.S.; Bates, P.; Pérez-Torres, A.; Garfias, Y. Corneal neovascularization is inhibited with nucleolin-binding aptamer, As1411. Exp. Eye Res. 2020, 193, 107977. [Google Scholar] [CrossRef]
- Prodeus, A.; Abdul-Wahid, A.; Fischer, N.W.; Huang, E.H.B.; Cydzik, M.; Gariépy, J. Targeting the PD-1/PD-L1 Immune Evasion Axis With DNA Aptamers as a Novel Therapeutic Strategy for the Treatment of Disseminated Cancers. Mol. Ther. Nucl. Acids 2015, 4, e237. [Google Scholar] [CrossRef]
- Li, T.; Yao, F.; An, Y.; Li, X.; Duan, J.; Yang, X.-D. Novel Complex of PD-L1 Aptamer and Holliday Junction Enhances Antitumor Efficacy in Vivo. Molecules 2021, 26, 1067. [Google Scholar] [CrossRef]
- Wu, X.; Li, F.; Li, Y.; Yu, Y.; Liang, C.; Zhang, B.; Zhao, C.; Lu, A.; Zhang, G. A PD-L1 Aptamer Selected by Loss-Gain Cell-SELEX Conjugated with Paclitaxel for Treating Triple-Negative Breast Cancer. Med. Sci. Monit. 2020, 26, e925583. [Google Scholar] [CrossRef]
- Rodríguez, P.C.; Zea, A.H.; Ochoa, A.C. Mechanisms of tumor evasion from the immune response. Cancer Chemother. Biol. Response Modif. 2003, 21, 351–364. [Google Scholar] [CrossRef] [PubMed]
- He, X.-Y.; Ren, X.-H.; Peng, Y.; Zhang, J.-P.; Ai, S.-L.; Liu, B.-Y.; Xu, C.; Cheng, S.-X. Aptamer/Peptide-Functionalized Genome-Editing System for Effective Immune Restoration through Reversal of PD-L1-Mediated Cancer Immunosuppression. Adv. Mater. 2020, 32, 2000208. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Mercado, J.; Ramírez-Velázquez, N.; Partido, G.; Zenteno, E.; Chávez, R.; Agundis-Mata, C.; Jiménez-Martínez, M.C.; Garfias, Y. Tissue and cellular characterisation of nucleolin in a murine model of corneal angiogenesis. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1753–1763. [Google Scholar] [CrossRef]
- Turck, N.; Lefebvre, O.; Gross, I.; Gendry, P.; Kedinger, M.; Simon-Assmann, P.; Launay, J.F. Effect of laminin-1 on intestinal cell differentiation involves inhibition of nuclear nucleolin. J. Cell Physiol. 2006, 206, 545–555. [Google Scholar] [CrossRef]
- Reyes-Reyes, E.M.; Akiyama, S.K. Cell-surface nucleolin is a signal transducing P-selectin binding protein for human colon carcinoma cells. Exp. Cell Res. 2008, 314, 2212–2223. [Google Scholar] [CrossRef]
- Kinoshita, D.; Shishido, T.; Takahashi, T.; Yokoyama, M.; Sugai, T.; Watanabe, K.; Tamura, H.; Nishiyama, S.; Takahashi, H.; Arimoto, T. Growth Factor Midkine Aggravates Pulmonary Arterial Hypertension via Surface Nucleolin. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-P.; Wang, X.; Xie, X.-L.; Zhang, G.-P.; Lv, F.-J.; Weng, W.-T.; Qiu, F.; Li, Z.-F.; Lin, J.-S.; Diao, Y. Cell surface expression of nucleolin mediates the antiangiogenic and antitumor activities of kallistatin. Oncotarget 2017, 9, 2220–2235. [Google Scholar] [CrossRef]
- Shi, H.; Huang, Y.; Zhou, H.; Song, X.; Yuan, S.; Fu, Y.; Luo, Y. Nucleolin is a receptor that mediates antiangiogenic and antitumor activity of endostatin. Blood 2007, 110, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Poimenidi, E.; Theodoropoulou, C.; Koutsioumpa, M.; Skondra, L.; Droggiti, E.; van den Broek, M.; Koolwijk, P.; Papadimitriou, E. Vascular endothelial growth factor A (VEGF-A) decreases expression and secretion of pleiotrophin in a VEGF receptor-independent manner. Vasc. Pharmacol. 2016, 80, 11–19. [Google Scholar] [CrossRef]
- Hovanessian, A.G. Midkine, a cytokine that inhibits HIV infection by binding to the cell surface expressed nucleolin. Cell Res. 2006, 16, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, J.F.; O’Brien, A.D. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157:H7. J. Biol. Chem. 2002, 277, 2876–2885. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Watanabe, T.; Suganuma, M. Cell-surface nucleolin acts as a central mediator for carcinogenic, anti-carcinogenic, and disease-related ligands. J. Cancer Res. Clin. Oncol. 2014, 140, 689–699. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, H.; Zhou, H.; Song, X.; Yuan, S.; Luo, Y. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood 2006, 107, 3564–3571. [Google Scholar] [CrossRef]
- Darche, M.; Cossutta, M.; Caruana, L.; Houppe, C.; Gilles, M.-E.; Habert, D.; Guilloneau, X.; Vignaud, L.; Paques, M.; Courty, J.; et al. Antagonist of nucleolin, N6L, inhibits neovascularization in mouse models of retinopathies. FASEB J. 2020, 34, 5851–5862. [Google Scholar] [CrossRef]
- Huang, F.; Wu, Y.; Tan, H.; Guo, T.; Zhang, K.; Li, D.; Tong, Z. Phosphorylation of nucleolin is indispensable to its involvement in the proliferation and migration of non-small cell lung cancer cells. Oncol. Rep. 2019, 41, 590–598. [Google Scholar] [CrossRef]
- Christian, S.; Pilch, J.; Akerman, M.E.; Porkka, K.; Laakkonen, P.; Ruoslahti, E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell Biol. 2003, 163, 871–878. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Bambury, R.M.; Van Allen, E.M.; Drabkin, H.A.; Lara, P.N., Jr.; Harzstark, A.L.; Wagle, N.; Figlin, R.A.; Smith, G.W.; Garraway, L.A.; et al. A phase II trial of AS1411 (a novel nucleolin-targeted DNA aptamer) in metastatic renal cell carcinoma. Investig. New Drugs 2014, 32, 178–187. [Google Scholar] [CrossRef]
- Sletten, T.; Kostas, M.; Bober, J.; Sorensen, V.; Yadollahi, M.; Olsnes, S.; Tomala, J.; Otlewski, J.; Zakrzewska, M.; Wiedlocha, A. Nucleolin Regulates Phosphorylation and Nuclear Export of Fibroblast Growth Factor 1 (FGF1). PLoS ONE 2014, 9, e90687. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Reyes, E.M.; Šalipur, F.R.; Shams, M.; Forsthoefel, M.K.; Bates, P.J. Mechanistic studies of anticancer aptamer AS1411 reveal a novel role for nucleolin in regulating Rac1 activation. Mol. Oncol. 2015, 9, 1392–1405. [Google Scholar] [CrossRef]
- Tajrishi, M.M.; Tuteja, R.; Tuteja, N. Nucleolin: The most abundant multifunctional phosphoprotein of nucleolus. Commun. Integr. Biol. 2011, 4, 267–275. [Google Scholar] [CrossRef]
- Fähling, M.; Steege, A.; Perlewitz, A.; Nafz, B.; Mrowka, R.; Persson, P.B.; Thiele, B.J. Role of nucleolin in posttranscriptional control of MMP-9 expression. Biochim. Biophys. Acta 2005, 1731, 32–40. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, X. Roles of nucleolin. Focus on cancer and anti-cancer therapy. Saudi Med. J. 2016, 37, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Chen, H.L.; Chen, Q.C. In vitro selection of aptamer S1 against MCF-7 human breast cancer cells. Bioorg. Med. Chem. Lett. 2019, 29, 2393–2397. [Google Scholar] [CrossRef]
- Shieh, Y.-A.; Yang, S.-J.; Wei, M.-F.; Shieh, M.-J. Aptamer-Based Tumor-Targeted Drug Delivery for Photodynamic Therapy. ACS Nano 2010, 4, 1433–1442. [Google Scholar] [CrossRef]
- Carvalho, J.; Lopes-Nunes, J.; Lopes, A.C.; Cabral Campello, M.P.; Paulo, A.; Queiroz, J.A.; Cruz, C. Aptamer-guided acridine derivatives for cervical cancer. Org. Biomol. Chem. 2019, 17, 2992–3002. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hou, J.; Liu, X.; Guo, Y.; Wu, Y.; Zhang, L.; Yang, Z. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials 2014, 35, 3840–3850. [Google Scholar] [CrossRef] [PubMed]
- Talreja, D.; Cashman, S.M.; Dasari, B.; Kumar, B.; Kumar-Singh, R. G-quartet oligonucleotide mediated delivery of functional X-linked inhibitor of apoptosis protein into retinal cells following intravitreal injection. Exp. Eye Res. 2018, 175, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Johnson, D.; Abouammoh, M.; Hollands, S.; Brissette, A. Rate of serious adverse effects in a series of bevacizumab and ranibizumab injections. Can. J. Ophthalmol. 2012, 47, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Puche, N.; Glacet, A.; Mimoun, G.; Zourdani, A.; Coscas, G.; Soubrane, G. Intravitreal ranibizumab for macular oedema secondary to retinal vein occlusion: A retrospective study of 34 eyes. Acta Ophthalmol. 2012, 90, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Koutsioumpa, M.; Papadimitriou, E. Cell surface nucleolin as a target for anti-cancer therapies. Recent Pat. Anticancer Drug Discov. 2014, 9, 137–152. [Google Scholar] [CrossRef]
- Lai, W.-Y.; Wang, W.-Y.; Chang, Y.-C.; Chang, C.-J.; Yang, P.-C.; Peck, K. Synergistic inhibition of lung cancer cell invasion, tumor growth and angiogenesis using aptamer-siRNA chimeras. Biomaterials 2014, 35, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Faryammanesh, R.; Lange, T.; Magbanua, E.; Haas, S.; Meyer, C.; Wicklein, D.; Schumacher, U.; Hahn, U. SDA, a DNA aptamer inhibiting E- and P-selectin mediated adhesion of cancer and leukemia cells, the first and pivotal step in transendothelial migration during metastasis formation. PLoS ONE 2014, 9, e93173. [Google Scholar] [CrossRef]
- Kaur, H.; Bruno, J.G.; Kumar, A.; Sharma, T.K. Aptamers in the therapeutics and diagnostics pipelines. Theranostics 2018, 8, 4016. [Google Scholar] [CrossRef]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V.A. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Lennartsson, J.; Westermark, B. Involvement of platelet-derived growth factor ligands and receptors in tumorigenesis. J. Intern. Med. 2018, 283, 16–44. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Paulis, Y.W.; Nowak-Sliwinska, P.; Deumelandt, K.L.; Hosaka, K.; Soetekouw, P.M.; Cimpean, A.M.; Raica, M.; Pauwels, P.; van den Oord, J.J.; et al. Targeting PDGF-mediated recruitment of pericytes blocks vascular mimicry and tumor growth. J. Pathol. 2018, 246, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.F.; Chung, K.-J.; Orlova, V.V.; Choi, E.Y.; Kaul, S.; Kruhlak, M.J.; Alatsatianos, M.; DeAngelis, R.A.; Roche, P.A.; Magotti, P. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood 2010, 116, 4395–4403. [Google Scholar] [CrossRef]
- Leung, E.; Landa, G. Update on current and future novel therapies for dry age-related macular degeneration. Expert Rev. Clin. Pharmacol. 2013, 6, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Jagadish, N.; Surolia, A.; Suri, A. Heat shock protein 70-2 (HSP70-2) a novel cancer testis antigen that promotes growth of ovarian cancer. Am. J. Cancer Res. 2017, 7, 1252–1269. [Google Scholar]
- Annunziata, C.M.; Kleinberg, L.; Davidson, B.; Berner, A.; Gius, D.; Tchabo, N.; Steinberg, S.M.; Kohn, E.C. BAG-4/SODD and associated antiapoptotic proteins are linked to aggressiveness of epithelial ovarian cancer. Clin. Cancer Res. 2007, 13, 6585–6592. [Google Scholar] [CrossRef]
- Venkatesh, V.; Nataraj, R.; Thangaraj, G.S.; Karthikeyan, M.; Gnanasekaran, A.; Kaginelli, S.B.; Kuppanna, G.; Kallappa, C.G.; Basalingappa, K.M. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 2018, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.N.; Tsai, Y.C.; Hsu, C.C.; Liang, Y.L.; Wu, Y.Y.; Kang, C.Y.; Lin, C.H.; Hsu, P.H.; Lee, G.B.; Hsu, K.F. An aptamer interacting with heat shock protein 70 shows therapeutic effects and prognostic ability in serous ovarian cancer. Mol. Ther. Nucleic Acids 2021, 23, 757–768. [Google Scholar] [CrossRef]
- Xue, K.; MacLaren, R.E. Antisense oligonucleotide therapeutics in clinical trials for the treatment of inherited retinal diseases. Expert Opin. Investig. Drugs 2020, 29, 1163–1170. [Google Scholar] [CrossRef]
- Evers, M.M.; Toonen, L.J.A.; van Roon-Mom, W.M.C. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv. Drug Deliv. Rev. 2015, 87, 90–103. [Google Scholar] [CrossRef]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef]
- Weiss, R.H.; Marshall, D.; Howard, L.; Corbacho, A.M.; Cheung, A.T.; Sawai, E.T. Suppression of breast cancer growth and angiogenesis by an antisense oligodeoxynucleotide to p21Waf1/Cip1. Cancer Lett. 2003, 189, 39–48. [Google Scholar] [CrossRef]
- Schmidt, K.S.; Borkowski, S.; Kurreck, J.; Stephens, A.W.; Bald, R.; Hecht, M.; Friebe, M.; Dinkelborg, L.; Erdmann, V.A. Application of locked nucleic acids to improve aptamer in vivo stability and targeting function. Nucleic Acids Res. 2004, 32, 5757–5765. [Google Scholar] [CrossRef] [PubMed]
- Hnik, P.; Boyer, D.S.; Grillone, L.R.; Clement, J.G.; Henry, S.P.; Green, E.A. Antisense Oligonucleotide Therapy in Diabetic Retinopathy. J. Diabetes Sci. Technol. 2009, 3, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Bullard, L.E.; Qi, X.; Penn, J.S. Role for Extracellular Signal-Responsive Kinase-1 and -2 in Retinal Angiogenesis. Investig. Ophth. Vis. Sci. 2003, 44, 1722–1731. [Google Scholar] [CrossRef]
- He, F.; Wen, N.; Xiao, D.; Yan, J.; Xiong, H.; Cai, S.; Liu, Z.; Liu, Y. Aptamer-based targeted drug delivery systems: Current potential and challenges. Curr. Med. Chem. 2020, 27, 2189–2219. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, D.M.; Kim, K.S.; Jung, W.; Kim, D.E. Applications of Cancer Cell-Specific Aptamers in Targeted Delivery of Anticancer Therapeutic Agents. Molecules 2018, 23, 830. [Google Scholar] [CrossRef]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef]
- Ko, H.Y.; Lee, J.H.; Kang, H.; Ryu, S.H.; Song, I.C.; Lee, D.S.; Kim, S. A nucleolin-targeted multimodal nanoparticle imaging probe for tracking cancer cells using an aptamer. J. Nucl. Med. 2010, 51, 98–105. [Google Scholar]
- Amato, R.; Biagioni, M.; Cammalleri, M.; Dal Monte, M.; Casini, G. VEGF as a Survival Factor in Ex Vivo Models of Early Diabetic Retinopathy. Investig. Ophth. Vis. Sci. 2016, 57, 3066–3076. [Google Scholar] [CrossRef]
- Smith, S.J.; Smith, B.D.; Mohney, B.G. Ocular side effects following intravitreal injection therapy for retinoblastoma: A systematic review. Br. J. Ophthalmol. 2014, 98, 292–297. [Google Scholar] [CrossRef]
- El-Sanhouri, A.; Puklin, J.; Patel, C.; Iezzi, R.; Murphy, P.; Abrams, G.; Mahmoud, T. Systemic Side Effects and Risks Associated With Bilateral Anti-VEGF Injections. Investig. Ophth. Vis. Sci. 2008, 49, 2133. [Google Scholar]
- Bunka, D.H.J.; Platonova, O.; Stockley, P.G. Development of aptamer therapeutics. Curr. Opin. Pharmacol. 2010, 10, 557–562. [Google Scholar] [CrossRef]
- Zhang, Z.; Ali, M.M.; Eckert, M.A.; Kang, D.-K.; Chen, Y.Y.; Sender, L.S.; Fruman, D.A.; Zhao, W. A polyvalent aptamer system for targeted drug delivery. Biomaterials 2013, 34, 9728–9735. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, B.; Yu, B.; Zhong, W.; Lu, Y.; Zhang, J.; Liao, J.; Liu, J.; Pu, Y.; Qiu, L. Advances in the development of aptamer drug conjugates for targeted drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1438. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iturriaga-Goyon, E.; Buentello-Volante, B.; Magaña-Guerrero, F.S.; Garfias, Y. Future Perspectives of Therapeutic, Diagnostic and Prognostic Aptamers in Eye Pathological Angiogenesis. Cells 2021, 10, 1455. https://doi.org/10.3390/cells10061455
Iturriaga-Goyon E, Buentello-Volante B, Magaña-Guerrero FS, Garfias Y. Future Perspectives of Therapeutic, Diagnostic and Prognostic Aptamers in Eye Pathological Angiogenesis. Cells. 2021; 10(6):1455. https://doi.org/10.3390/cells10061455
Chicago/Turabian StyleIturriaga-Goyon, Emilio, Beatriz Buentello-Volante, Fátima Sofía Magaña-Guerrero, and Yonathan Garfias. 2021. "Future Perspectives of Therapeutic, Diagnostic and Prognostic Aptamers in Eye Pathological Angiogenesis" Cells 10, no. 6: 1455. https://doi.org/10.3390/cells10061455
APA StyleIturriaga-Goyon, E., Buentello-Volante, B., Magaña-Guerrero, F. S., & Garfias, Y. (2021). Future Perspectives of Therapeutic, Diagnostic and Prognostic Aptamers in Eye Pathological Angiogenesis. Cells, 10(6), 1455. https://doi.org/10.3390/cells10061455





