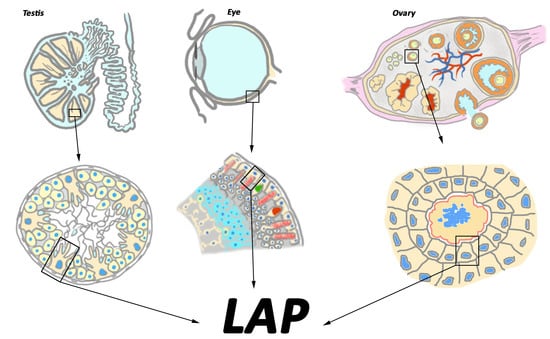
MERTK-Mediated LC3-Associated Phagocytosis (LAP) of Apoptotic Substrates in Blood-Separated Tissues: Retina, Testis, Ovarian Follicles
Abstract
1. Phagocytosis, General Information, and Types of Phagocyte Cells
2. Non-Professional Phagocytes from Blood-Separated Tissues and Their Substrates
3. Autophagy: A Lysosome-Related Degradation Pathway Sharing Similarities with Phagocytosis
4. Autophagy in Non-Professional Phagocytes from Blood-Separated Tissues
5. Non-Canonical Roles of Autophagy
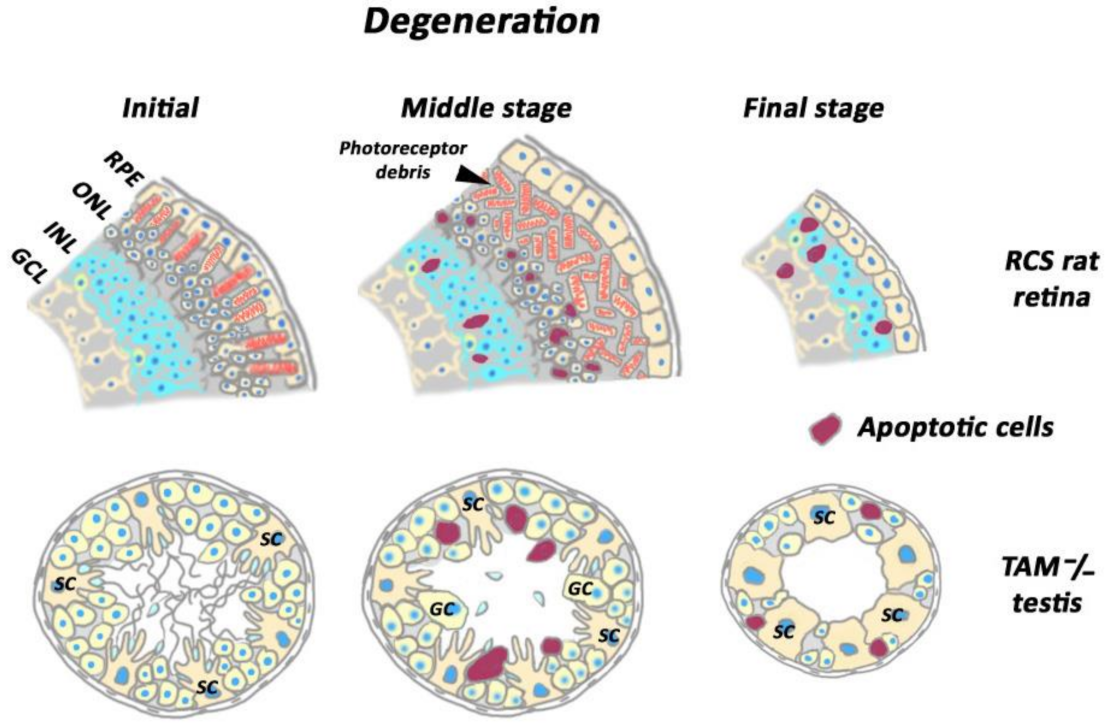
6. Ablation of Phagocytic Activity by Resident Non-Professional Phagocytes Causes Blindness in the Retina and Compromised Fertility in the Testis and the Ovaries
7. MERTK Is a Member of the TAM Family of Receptor Tyrosine Kinases
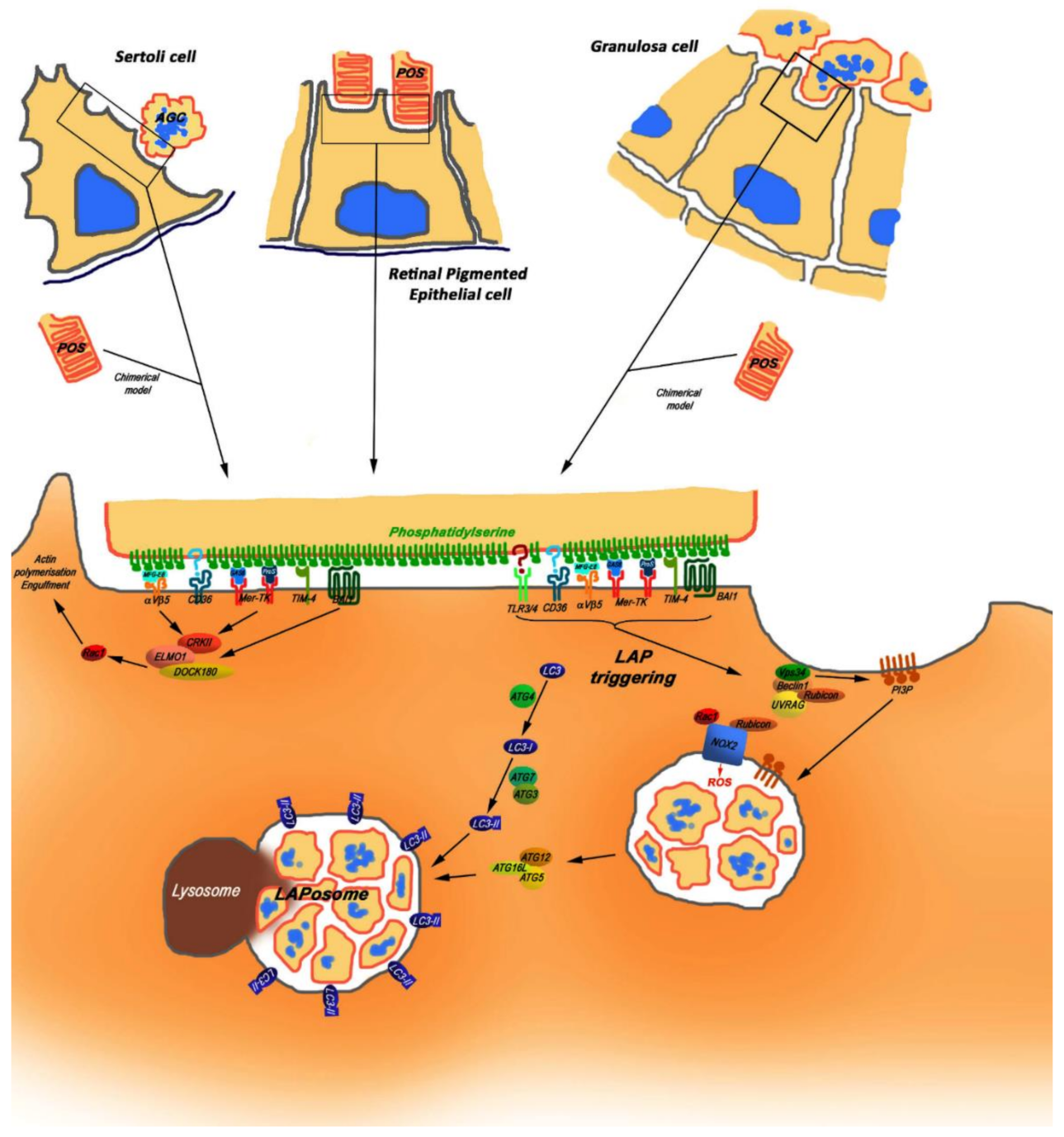
8. MERTK Cooperates with Other Phagocytosis Receptors to Provide the Ingestion of Apoptotic Substrates
9. MERTK-Mediated Phagocytosis in Blood-Separated Tissues Is an Autophagy-Assisted Process Termed LAP
10. LAP Is a Degradation Process Different from Both Phagocytosis and Autophagy
11. Klotho Is a Regulator of MERTK Expression
12. TAM Receptors as Negative Regulators of Inflammation: Two Possible Explanations
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itoh, M.; Yano, A.; Li, X.; Miyamoto, K.; Takeuchi, Y. Limited uptake of foreign materials by resident macrophages in murine ovarian tissues. J. Reprod. Immunol. 1999, 43, 55–66. [Google Scholar] [CrossRef]
- Penberthy, K.K.; Lysiak, J.J.; Ravichandran, K.S. Rethinking Phagocytes: Clues from the Retina and Testes. Trends Cell. Biol. 2018, 28, 317–327. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012, 7, 61–98. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Kevany, B.M.; Palczewsky, K. Phagocytosis of retinal rod and cone photoreceptors. Physiology 2010, 25, 8–15. [Google Scholar] [CrossRef]
- Henson, P.M.; Bratton, D.L.; Fadok, V.A. The phosphatidylserine receptor: A crucial molecular switch? Nat. Rev. Mol. Cell. Biol. 2001, 2, 627–633. [Google Scholar] [CrossRef]
- Helming, l.; Winter, J.; Gordon, S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J. Cell. Sci. 2009, 122, 453–459. [Google Scholar] [CrossRef]
- Yefimova, M.G.; Messaddeq, N.; Meunier, A.C.; Cantereau, A.; Jegou, B.; Bourmeyster, N. Phagocytosis by Sertoli Cells: Analysis of Main Phagocytosis Steps by Confocal and Electron Microscopy. Methods Mol. Biol. 2018, 1748, 85–101. [Google Scholar]
- Rabinovitch, M. Professional and non-professional phagocytes: An introduction. Trends Cell Biol. 1995, 5, 85–87. [Google Scholar] [CrossRef]
- Lemke, G.; Rothlin, C.V. Immunobiology of the TAM receptors. Nat. Rev. Immunol. 2008, 8, 327–336. [Google Scholar] [CrossRef]
- Nguyen-Legros, J.; Hicks, D. Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int. Rev. Cytol. 2000, 196, 245–313. [Google Scholar] [PubMed]
- Bok, D.; Young, R.W. Phagocytic Properties of the Retinal Pigment Epithelium. In The Retinal Pigment Epithelium; Zinn, K.M., Marmor, M.F., Eds.; Harvard University Press: Cambridge, MA, USA, 1979; pp. 148–174. [Google Scholar]
- Young, R.W. Biological renewal, applications to the eye. Trans. Ophthalmol. Soc. UK 1982, 102, 42–75. [Google Scholar]
- Clermont, Y. Quantitative analysis of spermatogenesis of the rat: A revised model for the renewal of spermatogonia. Am. J. Anat. 1962, 111, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Huckins, C. The morphology and kinetics of spermatogonial degeneration in normal adult rats: an analysis using a simplified classification of the germinal epithelium. Anat. Rec. 1978, 190, 905–926. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Rodriguez, J.; Martinez-Garcia, C. Apoptosis is physiologically restricted to a specialized cytoplasmic compartment in rat spermatids. Biol. Reprod. 1999, 61, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Pineau, C.; Le Magueresse, B.; Courtens, J.L.; Jégou, B. Study in vitro of the phagocytic function of Sertoli cells in the rat. Cell Tissue Res. 1991, 264, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Panneerdoss, S.; Viswanadhapalli, S.; Abdelfattah, N.; Onyeagucha, B.C.; Timilsina, S.; Mohammad, T.A.; Chen, Y.; Drake, M.; Vuori, K.; Kumar, T.R.; et al. Cross-talk between miR-471-5p and autophagy component proteins regulates LC3-associated phagocytosis (LAP) of apoptotic germ cells. Nat. Commun. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Jégou, B. The Sertoli-germ cell communication network in mammals. Int. Rev. Cytol. 1993, 147, 25–96. [Google Scholar]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Yefimova, M.G.; Lefevre, C.; Bashamboo, A.; Eozenou, C.; Burel, A.; Lavault, M.T.; Meunier, A.C.; Pimentel, C.; Veau, S.; Neyroud, A.S.; et al. Granulosa cells provide elimination of apoptotic oocytes through unconventional autophagy-assisted phagocytosis. Hum. Reprod. 2020, 35, 1346–1362. [Google Scholar] [CrossRef]
- Mork, L.; Maatouk, D.M.; McMahon, J.A.; Guo, J.J.; Zhang, P.; McMahon, A.P.; Capel, B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol. Reprod. 2012, 86, 37. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adams, C.M.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Boya, P.; Esteban-Martínez, L.; Serrano-Puebla, A.; Gómez-Sintes, R.; Villarejo-Zori, B. Autophagy in the eye: Development, degeneration, and aging. Prog. Retin. Eye Res. 2016, 55, 206–245. [Google Scholar] [CrossRef]
- Ravikumar, B.; Sarkar, S.; Davies, J.E.; Futter, M.; Garcia-Arencibia, M.; Green-Thompson, Z.W.; Jimenez-Sanchez, M.; Korolchuk, V.I.; Lichtenberg, M.; Luo, S.; et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev. 2010, 90, 1383–1435. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.A.; Deretic, V. Toll-like receptors in control of immunological autophagy. Cell Death Diff. 2009, 16, 976–983. [Google Scholar] [CrossRef]
- Wirawan, E.; Vanden Berghe, T.; Lippens, S.; Agostinis, P.; Vandenabeele, P. Autophagy: For better or for worse. Cell Res. 2012, 22, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef]
- Hurley, J.H.; Young, L.N. Mechanisms of Autophagy Initiation. Annu. Rev. Biochem. 2017, 86, 225–244. [Google Scholar] [CrossRef]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1- dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Wei, Y.; Pattingre, S.; Sinha, S.; Bassik, M.; Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 2008, 30, 678–688. [Google Scholar] [CrossRef]
- Shi, C.S.; Kehrl, J.H. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J. Biol. Chem. 2008, 283, 33175–33182. [Google Scholar] [CrossRef]
- Sarkar, S. Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: Autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem. Soc. Trans. 2013, 41, 1103–1130. [Google Scholar] [CrossRef]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and exosomes in the aged retinal pigment epithelium: Possible relevance to drusen formation and age-related macular degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef]
- Mitter, S.K.; Rao, H.V.; Qi, X.; Cai, J.; Sugrue, A.; Dunn, W.A., Jr.; Grant, M.B.; Boulton, M.E. Autophagy in the retina: A potential role in age-related macular degeneration. Adv. Exp. Med. Biol. 2012, 723, 83–90. [Google Scholar]
- Chen, Y.; Sawada, O.; Kohno, H.; Le, Y.Z.; Subauste, C.; Maeda, T.; Maeda, A. Autophagy protects the retina from light-induced degeneration. J. Biol. Chem. 2013, 288, 7506–7518. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013, 9, 973–984. [Google Scholar] [CrossRef]
- Lin, W.; Xu, G. Autophagy: A Role in the Apoptosis, Survival, Inflammation, and Development of the Retina. Ophthalmic Res. 2019, 61, 65–72. [Google Scholar] [CrossRef]
- Flores-Bellver, M.; Bonet-Ponce, L.; Barcia, J.M.; Garcia-Verdugo, J.M.; Martinez-Gil, N.; Saez-Atienzar, S.; Sancho-Pelluz, J.; Jordan, J.; Galindo, M.F.; Romero, F.J. Autophagy and mitochondrial alterations in human retinal pigment epithelial cells induced by ethanol: Implications of 4-hydroxy-nonenal. Cell Death Dis. 2014, 5, e1328. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, K.K.; Tong, Y.; Zhou, Y.L.; Wang, Y.X.; Zhao, P.Q.; Wang, Z.Y. Exogenous NAD(+) decreases oxidative stress and protects H2O2-treated RPE cells against necrotic death through the up-regulation of autophagy. Sci. Rep. 2016, 31, 26322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Copland, D.A.; Theodoropoulou, S.; Chiu, H.A.; Barba, M.D.; Mak, K.W.; Mack, M.; Nicholson, L.B.; Dick, A.D. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Sci. Rep. 2016, 6, 20639. [Google Scholar] [CrossRef]
- Corcelle, E.; Nebout, M.; Bekri, S.; Gauthier, N.; Hofman, P.; Poujeol, P.; Fénichel, P.; Mograbi, B. Disruption of autophagy at the maturation step by the carcinogen lindane is associated with the sustained mitogen-activated protein kinase/extracellular signal-regulated kinase activity. Cancer Res. 2006, 66, 6861–6870. [Google Scholar] [CrossRef]
- Yefimova, M.G.; Messaddeq, N.; Harnois, T.; Meunier, A.C.; Clarhaut, J.; Noblanc, A.; Weickert, J.L.; Cantereau, A.; Philippe, M.; Bourmeyster, N.; et al. A Chimerical phagocytosis model reveals the recruitment by Sertoli cells of autophagy for the degradation of ingested illegitimated substrate. Autophagy 2013, 9, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, H.Z.; Xu, L.M.; Huang, Y.R.; Dai, H.L.; Kang, X.N. Testosterone regulates the autophagic clearance of androgen-binding protein in rat Sertoli cells. Sci. Rep. 2015, 5, 8894. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Shang, Y.; Liu, W.; Song, Z.; Zhao, H.; Wang, L.; Jia, P.; Gao, F.; Xu, Z.; et al. Autophagy is required for ectoplasmic specialization assembly in sertoli cells. Autophagy 2016, 12, 814–832. [Google Scholar] [CrossRef]
- Horibe, A.; Eid, N.; Ito, Y.; Hamaoka, H.; Tanaka, Y.; Kondo, Y. Upregulated Autophagy in Sertoli Cells of Ethanol-Treated Rats Is Associated with Induction of Inducible Nitric Oxide Synthase (iNOS), Androgen Receptor Suppression and Germ Cell Apoptosis. Int. J. Mol. Sci. 2017, 18, 1061. [Google Scholar] [CrossRef]
- Horibe, A.; Eid, N.; Ito, Y.; Otsuki, Y.; Kondo, Y. Ethanol-Induced Autophagy in Sertoli Cells Is Specifically Marked at Androgen-Dependent Stages of the Spermatogenic Cycle: Potential Mechanisms and Implications. Int. J. Mol. Sci. 2019, 20, 184. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, X.; Mei, S. Autophagy in Ovarian Follicular Development and Atresia. Int. J. Biol. Sci. 2019, 15, 726–737. [Google Scholar] [CrossRef]
- Song, Z.H.; Yu, H.Y.; Wang, P.; Mao, G.K.; Liu, W.X.; Li, M.N.; Wang, H.N.; Shang, Y.L.; Liu, C.; Xu, Z.L.; et al. Germ cell-specific Atg7 knockout results in primary ovarian insufficiency in female mice. Cell Death Dis. 2015, 6, e1589. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jo, M.W.; Lee, E.Y.; Yoon, B.K.; Choi, D.S. The role of autophagy in follicular development and atresia in rat granulosa cells. Fertil. Steril. 2010, 93, 2532–2537. [Google Scholar] [CrossRef]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.C. Protective mechanism of FSH against oxidative damage in mouse ovarian granulosa cells by repressing autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef]
- Duerrschmidt, N.; Zabirnyk, O.; Nowicki, M.; Ricken, A.; Hmeidan, F.A.; Blumenauer, V.; Borlak, J.; Spanel-Borowski, K. Lectin-like oxidized low-density lipoprotein receptor-1-mediated autophagy in human granulosa cells as an alternative of programmed cell death. Endocrinology 2006, 147, 3851–3860. [Google Scholar] [CrossRef]
- Choi, J.; Jo, M.; Lee, E.; Choi, D. AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction 2013, 147, 73–80. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Green, D.R. LC3-associated phagocytosis at a glance. J. Cell Sci. 2019, 132, jcs222984. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Teubner, B.J.W.; Tummers, B.; Boada-Romero, E.; Harris, L.; Yang, M.; Guy, C.S.; Zakharenko, S.S.; Green, D.R. LC3-Associated Endocytosis Facilitates β-Amyloid Clearance and Mitigates Neurodegeneration in Murine Alzheimer’s Disease. Cell 2019, 178, 536–551. [Google Scholar] [CrossRef]
- Duran, J.M.; Anjard, C.; Stefan, C.; Loomis, W.F.; Malhotra, V. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 2010, 188, 527–536. [Google Scholar] [CrossRef]
- Manjithaya, R.; Anjard, C.; Loomis, W.F.; Subramani, S. Unconventional secretion of Pichia pastorisAcb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J. Cell Biol. 2010, 188, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Dupont, N.; Jiang, S.; Pilli, M.; Ornatowski, W.; Bhattacharya, D.; Deretic, V. Autophagybased unconventional secretory pathway for extracellular delivery of IL-1beta. EMBO J. 2011, 30, 4701–4711. [Google Scholar] [CrossRef] [PubMed]
- Bruns, C.; McCaffery, J.M.; Curwin, A.J.; Duran, J.M.; Malhotra, V. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J. Cell Biol. 2011, 195, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Garcia, D.; Curwin, A.J.; Popoff, J.F.; Bruns, C.; Duran, J.M.; Malhotra, V. Remodeling of secretory compartments creates CUPS during nutrient starvation. J. Cell Biol. 2014, 207, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.X.; Yang, G.; Hao, F.; Urba, W.J.; Hu, H.M. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008, 68, 6889–6895. [Google Scholar] [CrossRef] [PubMed]
- Uhl, M.; Kepp, O.; Jusforgues-Saklani, H.; Vicencio, J.M.; Kroemer, G.; Albert, M.L. Autophagy within the antigen donor cell facilitates efficient antigen cross-priming of virus-specific CD8+T cells. Cell Death Differ. 2009, 16, 991–1005. [Google Scholar] [CrossRef]
- Yi, Y.; Zhou, Z.; Shu, S.; Fang, Y.; Twitty, C.; Hilton, T.L.; Aung, S.; Urba, W.J.; Fox, B.A.; Hu, H.M.; et al. Autophagy-assisted antigen cross-presentation: Autophagosome as the argo of shared tumor-specific antigens and DAMPs. Oncoimmunology 2012, 1, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.C.; Campbell, D.A.; Tansley, K. Hereditary degeneration of rat retina. Br. J. Ophthalmol. 1938, 22, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.B.; Szamier, R.B. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science 1977, 197, 1001–1003. [Google Scholar] [CrossRef]
- LaVail, M.M.; Battelle, B.A. Influence of eye pigmentation and light deprivation on inherited retinal dystrophy in the rat. Exp. Eye Res. 1975, 21, 167–192. [Google Scholar] [CrossRef]
- Matthes, M.T.; LaVail, M.M. Inherited retinal dystrophy in the RCS rat: Composition of the outer segment debris zone. Prog. Clin. Biol. Res. 1989, 314, 315–330. [Google Scholar]
- Tso, M.O.; Zhang, C.; Abler, A.S.; Chang, C.J.; Wong, F.; Chang, G.Q.; Lam, T.T. Apoptosis leads to photoreceptor degeneration in inherited retinal dystrophy of RCS rats. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2693–2699. [Google Scholar]
- Mullen, R.J.; LaVail, M.M. Inherited retinal dystrophy: Primary defect in pigment epithelium determined with experimental rat chimeras. Science 1976, 192, 799–801. [Google Scholar] [CrossRef]
- Roque, R.S.; Imperial, C.J.; Caldwell, R.B. Microglial cells invade the outer retina as photoreceptors degenerate in Royal College of Surgeons rats. Investig. Ophthalmol. Vis. Sci. 1996, 37, 196–203. [Google Scholar]
- Vollrath, D.; Feng, W.; Duncan, J.L.; Yasumura, D.; D’Cruz, P.M.; Chappelow, A.; Matthes, M.T.; Kay, M.A.; LaVail, M.M. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc. Natl. Acad. Sci. USA 2001, 98, 12584–12589. [Google Scholar] [CrossRef] [PubMed]
- Yefimova, M.G.; Jeanny, J.C.; Keller, N.; Sergeant, C.; Guillonneau, X.; Beaumont, C.; Courtois, Y. Impaired retinal iron homeostasis associated with defective phagocytosis in Royal College of Surgeons rats. Investig. Ophthalmol. Vis. Sci. 2002, 43, 537–545. [Google Scholar]
- Yefimova, M.G.; Jeanny, J.C.; Guillonneau, X.; Keller, N.; Nguyen-Legros, J.; Sergeant, C.; Guillou, F.; Courtois, Y. Iron, ferritin, transferrin, and transferrin receptor in the adult rat retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2343–2351. [Google Scholar]
- Bigot, K.; Gondouin, P.; Bénard, R.; Montagne, P.; Youale, J.; Piazza, M.; Picard, E.; Bordet, T.; Behar-Cohen, F. Transferrin Non-Viral Gene Therapy for Treatment of Retinal Degeneration. Pharmaceutics 2020, 12, 836. [Google Scholar] [CrossRef]
- Lashay, A.; Mahmoudzadeh, R.; Heidari Keshel, S.; Naderi, A.; Omidi, R.; Asadi Amoli, F. Role of Schwann Cells in Preservation of Retinal Tissue Through Reduction of Oxidative Stress. Med. Hypothesis Discov. Innov. Ophthalmol. 2019, 8, 323–332. [Google Scholar] [PubMed]
- Picard, E.; Le Rouzic, Q.; Oudar, A.; Berdugo, M.; El Sanharawi, M.; Andrieu-Soler, C.; Naud, M.C.; Jonet, L.; Latour, C.; Klein, C.; et al. Targeting iron-mediated retinal degeneration by local delivery of transferrin. Free Radic. Biol. Med. 2015, 89, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; LaVail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000, 9, 645–651. [Google Scholar] [CrossRef]
- Nandrot, E.; Dufour, E.M.; Provost, A.C.; Péquignot, M.O.; Bonnel, S.; Gogat, K.; Marchant, D.; Rouillac, C.; Sépulchre de Condé, B.; Bihoreau, M.T.; et al. Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol. Dis. 2000, 7, 586–599. [Google Scholar] [CrossRef]
- Nandrot, E.F.; Dufour, E.M. Mertk in daily retinal phagocytosis: A history in the making. Adv. Exp. Med. Biol. 2010, 664, 133–140. [Google Scholar]
- Duncan, J.L.; LaVail, M.M.; Yasumura, D.; Matthes, M.T.; Yang, H.; Trautmann, N.; Chappelow, A.V.; Feng, W.; Earp, H.S.; Matsushima, G.K.; et al. An RCS-like retinal dystrophy phenotype in mer knockout mice. Investig. Ophthalmol. Vis. Sci. 2003, 44, 826–838. [Google Scholar] [CrossRef]
- Gal, A.; Li, Y.; Thompson, D.A.; Weir, J.; Orth, U.; Jacobson, S.G.; Apfelstedt-Sylla, E.; Vollrath, D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 2000, 26, 270–271. [Google Scholar] [CrossRef]
- Tschernutter, M.; Jenkins, S.A.; Waseem, N.H.; Saihan, Z.; Holder, G.E.; Bird, A.C.; Bhattacharya, S.S.; Ali, R.R.; Webster, A.R. Clinical characterisation of a family with retinal dystrophy caused by mutation in the Mertk gene. Br. J. Ophthalmol. 2006, 90, 718–723. [Google Scholar] [CrossRef]
- Lu, Q.; Gore, M.; Zhang, Q.; Camenish, T.; Boast, S.; Casagranda, F.; Lai, C.; Skinner, M.K.; Klein, R.; Matsushima, G.K.; et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 1999, 398, 723–728. [Google Scholar] [CrossRef]
- Xiong, W.; Chen, Y.; Wang, H.; Wang, H.; Wu, H.; Lu, Q.; Han, D. Gas6 and the Tyro3 receptor tyrosine kinase subfamily regulate the phagocytosis function of Sertoli cells. Reproduction 2008, 135, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Qi, N.; Wu, H.; Xiong, W.; Ma, J.; Lu, Q.; Han, D. Functions of TAM RTKs in regulating spermatogenesis and male fertility in mice. Reproduction 2009, 138, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Jégou, B. The Sertoli cell in vivo and in vitro. Cell Biol. Toxicol. 1992, 8, 49–54. [Google Scholar] [CrossRef]
- Wang, F.; Han, D. Sertoli cell phagocytosis: an essential event for spermatogenesis. In Male reproductive health; Wu, W., Ziglioli, F., Maestroni, U., Eds.; IntechOpen: London, UK, 2019; pp. 69–84. [Google Scholar]
- Burstyn-Cohen, T.; Maimon, A. TAM receptors, Phosphatidylserine, inflammation, and Cancer. Cell Commun. Signal 2019, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wang, H.; Wu, H.; Chen, Y.; Han, D. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction 2009, 137, 469–479. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef]
- Hatzirodos, N.; Hummitzsch, K.; Irving-Rodgers, H.F.; Harland, M.L.; Morris, S.E.; Rodgers, R.J. Transcriptome profiling of granulosa cells from bovine ovarian follicles during atresia. BMC Genom. 2014, 15, 40. [Google Scholar] [CrossRef]
- Dai, W.; Pan, H.; Hassanain, H.; Gupta, S.L.; Murphy, M.J., Jr. Molecular cloning of a novel receptor tyrosine kinase, tif, highly expressed in human ovary and testis. Oncogene 1994, 9, 975–979. [Google Scholar]
- Varnum, B.C.; Young, C.; Elliott, G.; Garcia, A.; Bartley, T.D.; Fridell, Y.W.; Hunt, R.W.; Trail, G.; CloGCton, C.; Toso, R.J.; et al. Axl receptor tyrosine kinase stimulated by the vitamin K-dependent protein encoded by growth arrest-specific gene 6. Nature 1995, 373, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Strick, D.J.; Vollrath, D. Focus on molecules: MERTK. Exp. Eye Res. 2010, 91, 786–787. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.S.; McMahon, E.J.; Pop, S.M.; Reap, E.A.; Caricchio, R.; Cohen, P.L.; Earp, H.S.; Matsushima, G.K. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 2001, 411, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.M.; Camenisch, T.D.; Lemke, G.; Earp, H.S.; Matsushima, G.K. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J. Immunol. 2007, 178, 5635–5642. [Google Scholar] [CrossRef]
- Van der Meer, J.H.; van der Poll, T.; van ‘t Veer, C. TAM receptors, Gas6, and protein S: Roles in inflammation and hemostasis. Blood 2014, 123, 2460–2469. [Google Scholar] [CrossRef]
- Cohen, P.L.; Caricchio, R.; Abraham, V.; Camenish, T.D.; Jennette, J.C.; Roubey, R.A.S.; Earp, H.H.S.; Matsushima, G.; Reap, E. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J. Exp. Med. 2002, 196, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; Lee, C.Y.D.; Wilkinson, B.L.; Jiang, Q.; Koenigcknecht-Talboo, J.L.; Varnum, B.; Landreth, G.E. Regulation of microglial phagocytosis and inflammatory gene expression by GAS6, acting on the Axl/Mer family of tyrosine kinases. J. Neuroimmune Pharmacol. 2008, 3, 130–140. [Google Scholar] [CrossRef]
- Hafizi, S.; Dahlbäck, B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006, 273, 5231–5244. [Google Scholar] [CrossRef]
- Pierce, A.; Bliesner, B.; Xu, M.; Nielsen-Preiss, S.; Lemke, G.; Tobet, S.; Wierman, M.E. Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration. Mol. Endocrinol. 2008, 22, 2481–2495. [Google Scholar] [CrossRef]
- Salian-Mehta, S.; Xu, M.; Wierman, M.E. AXL and MET crosstalk to promote gonadotropin releasing hormone (GnRH) neuronal cell migration and survival. Mol. Cell. Endocrinol. 2013, 374, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Salian-Mehta, S.; Xu, M.; Pierce, A.; Bliesner, B.; Tobet, S.; Wierman, M.E. Loss of Growth arrest specific gene 6 (Gas6) results in altered GnRH neuron migration, delayed vaginal opening and sexual maturation in mice. Mol. Cell. Endocrinol. 2014, 393, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Linger, R.M.A.; Keating, A.K.; Earp, H.S.; Graham, D.K. TAM receptor tyrosine kinases: Biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Canc. Res. 2008, 100, 35–83. [Google Scholar]
- Nagata, K.; Ohashi, K.; Nakano, T.; Arita, H.; Zong, C.; Hanafusa, H.; Mizuno, K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J. Biol. Chem. 1996, 271, 30022–30027. [Google Scholar] [CrossRef]
- Caberoy, N.B. Synergistic interaction of tubby and tubby-like protein 1 (Tulp1). Adv. Exp. Med. Biol. 2014, 801, 503–509. [Google Scholar]
- Caberoy, N.B.; Alvarado, G.; Bigcas, J.L.; Li, W. Galectin-3 is a new MerTKspecific eat-me signal. J. Cell Phys. 2012, 227, 401–407. [Google Scholar] [CrossRef]
- Nagata, S.; Suzuki, J.; Segawa, K.; Fujii, T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016, 23, 952–961. [Google Scholar] [CrossRef]
- North, M.A.; Naggert, J.K.; Yan, Y.; Noben-Trauth, K.; Nishina, P.M. Molecular characterization of TUB, TULP1 and TULP2 members of a novel tubby gene family and their possible relation to ocular diseases. Proc. Natl. Acad. Sci. USA 1997, 94, 3128–3133. [Google Scholar] [CrossRef]
- Wollina, U.; Schreiber, G.; Görnig, M.; Feldrappe, S.; Burchert, M.; Gabius, H.J. Sertoli cell expression of galectin-1 and -3 and accessible binding sites in normal human testis and Sertoli cell only-syndrome. Histol. Histopathol. 1999, 14, 779–784. [Google Scholar]
- Chan, M.C.; Mather, J.P.; McCray, G.; Lee, W.M. Identification and regulation of receptor tyrosine kinases Rse and Mer and their ligand Gas6 in testicular somatic cells. J. Androl. 2000, 21, 291–302. [Google Scholar]
- Uehara, F.; Ohba, N.; Ozawa, M. Isolation and characterization of galectins in the mammalian retina. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2164–2172. [Google Scholar]
- Deschildre, C.; Li, J.W.; Chater, S.; Dacheux, F.; Selva, J.; Albert, M.; Bailly, M.; Hatey, F.; Benahmed, M. Expression of galectin-3 and its regulation in the testis. Int. J. Androl. 2007, 30, 28–40. [Google Scholar] [CrossRef]
- Kim, M.K.; Sung, C.O.; Do, I.G.; Jeon, H.K.; Song, T.J.; Park, H.S.; Lee, Y.Y.; Kim, B.G.; Lee, J.W.; Bae, D.S. Overexpression of Galectin-3 and its clinical significance in ovarian carcinoma. Int. J. Clin. Oncol. 2011, 16, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Jackson, P.K. The tubby family proteins. Genome Biol. 2011, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Nandrot, E.F. Opposite Roles of MerTK Ligands Gas6 and Protein S During Retinal Phagocytosis. Adv. Exp. Med. Biol. 2018, 1074, 577–583. [Google Scholar] [PubMed]
- Kim, K.H.; Kim, E.Y.; Ko, J.J.; Lee, K.A. Gas6 is a reciprocal regulator of mitophagy during mammalian oocyte maturation. Sci. Rep. 2019, 9, 10343. [Google Scholar] [CrossRef]
- Shimada, C.; Xu, R.; Al-Alem, L.; Stasenko, M.; SprigGC, D.R.; Rueda, B.R. Galectins and Ovarian Cancer. Cancers 2020, 12, 1421. [Google Scholar] [CrossRef]
- Prasad, D.; Rothlin, C.V.; Burrola, P.; Burstyn-Cohen, T.; Lu, Q.; Garcia de Frutos, P.; Lemke, G. TAM receptor function in the retinal pigment epithelium. Mol. Cell. Neurosci. 2006, 33, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Burstyn-Cohen, T.; Lew, E.D.; Través, P.G.; Burrola, P.G.; Hash, J.C.; Lemke, G. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron 2012, 76, 1123–1132. [Google Scholar] [CrossRef]
- Poiriers, F.; Robertson, E.J. Normal development of mice carrying a null mutation in gene encoding the L14 S-type lectin. Development 1993, 119, 1229–1236. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Ogilvie, J.M.; Lett, J.M.; Hudges, R.M.; LaRegina, M.C.; Olson, L.M. The murine tub (rd5) mutation is not associated with a primary axonemal defect. Cell Tissue Res 1998, 291, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Strick, D.J.; Feng, W.; Vollrath, D. Mertk drives myosin II redistribution during retinal pigment epithelium phagocytosis. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Shelby, S.J.; Feathers, K.L.; Ganios, A.M.; Jia, L.; Miller, J.M.; Thompson, D.A. MERTK signaling in the retinal pigment epithelium regulates the tyrosine phosphorylation of GDP dissociation inhibitor alpha from the GDI/CHM family of RAB GTPase effectors. Exp. Eye Res. 2015, 140, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, D.; Yasumura, D.; Benchorin, G.; Matthes, M.T.; Feng, W.; Nguyen, N.M.; Sedano, C.D.; Calton, M.A.; LaVail, M.M. Tyro3 Modulates Mertk-Associated Retinal Degeneration. PLoS Genet. 2015, 11, e1005723. [Google Scholar] [CrossRef]
- Finnemann, S.C.; Silverstein, R.L. Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J. Exp. Med. 2001, 194, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Nandrot, E.F.; Kim, Y.; Brodie, S.E.; Huang, X.; Sheppard, D.; Finnemann, S.C. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J. Exp. Med. 2004, 200, 1539–1545. [Google Scholar] [CrossRef]
- Mao, Y.; Finnemann, S.C. Essential diurnal Rac1 activation during retinal phagocytosis requiresavb5 integrin but not tyrosine kinases focal adhesion kinase or Mer tyrosine kinase. Mol. Biol. Cell. 2012, 23, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Bulloj, A.; Duan, W.; Finnemann, S.C. PI 3-kinase independent role for AKT in F-actin regulation during outer segment phagocytosis by RPE cells. Exp. Eye Res. 2013, 113, 9–18. [Google Scholar] [CrossRef]
- Gillot, I.; Jehi-Petri, C.; Gounon, P.; Luquet, S.; Rassoulzadegan, M.; Grimaldi, P.; Vidal, F. Germ cells and fatty acids induce translocation of CD36 scavenger receptor to the plasma membrane of Sertoli cells. J. Cell. Sci. 2005, 118, 3027–3035. [Google Scholar] [CrossRef][Green Version]
- Carr, M.E., Jr.; Steingold, K.A.; Zekert, S.L. Protein S levels during the normal menstrual cycle and during estrogen therapy for premature ovarian failure. Am. J. Med. Sci. 1993, 306, 212–217. [Google Scholar] [CrossRef]
- Ensslin, M.A.; Shur, B.D. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell 2003, 114, 405–417. [Google Scholar] [CrossRef]
- Sun, W.; Fujimoto, J.; Tamaya, T. Coexpression of Gas6/Axl in human ovarian cancers. Oncology 2004, 66, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D.; Huet-Calderwood, C.; Le Bellego, F.; Fabre, S.; Monget, P.; Calderwood, D.A. Integrins in the ovary. Semin. Reprod. Med. 2006, 24, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Naka, M.; Kusakabe, K.; Takeshita, A.; Nakagawa, H.; Ito, Y.; Shibata, M.A.; Otsuki, Y. Phagocytosis mechanism of apoptotic granulosa cells regulated by milk-fat globule-EGF factor 8. Med. Mol. Morphol. 2009, 42, 143–149. [Google Scholar] [CrossRef]
- Petrik, J.J.; Gentry, P.A.; Feige, J.J.; LaMarre, J. Expression and localization of thrombospondin-1 and -2 and their cell-surface receptor, CD36, during rat follicular development and formation of the corpus luteum. Biol. Reprod. 2002, 67, 1522–1531. [Google Scholar] [CrossRef][Green Version]
- Osz, K.; Ross, M.; Petrik, J. The thrombospondin-1 receptor CD36 is an important mediator of ovarian angiogenesis and folliculogenesis. Reprod. Biol. Endocrinol. 2014, 12, 21. [Google Scholar] [CrossRef]
- Siu, M.K.; Cheng, C.Y. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin protein complex in the regulation of ectoplasmic specialization dynamics in the testis. Biol. Reprod. 2004, 70, 945–964. [Google Scholar] [CrossRef]
- Wong, C.H.; Xia, W.; Lee, N.P.Y.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Regulation of ectoplasmic specialization dynamics in the seminiferous epithelium by focal adhesion- associated proteins in testosterone-suppressed rat testis. Endocrinology 2005, 146, 1192–1204. [Google Scholar] [CrossRef]
- Tancioni, I.; Uryu, S.; Sulzmaier, F.J.; Shah, N.R.; Lawson, C.; Miller, N.L.; Jean, C.; Chen, X.L.; Ward, K.K.; Schlaepfer, D.D. FAK Inhibition disrupts aβ5 integrin signaling axis controlling anchorage-independent ovarian carcinoma growth. Mol. Cancer Ther. 2014, 13, 2050–2061. [Google Scholar] [CrossRef]
- Tyasi, T.L.; Sun, X.; Shan, X.; Liswaniso, S.; Chimbaka, I.M.; Qin, N.; Xu, R. Effects of RAC1 on Proliferation of Hen Ovarian Prehierarchical Follicle Granulosa Cells. Animals 2020, 10, 1589. [Google Scholar] [CrossRef]
- Kim, J.Y.; Zhao, H.; Martinez, J.; Doggett, T.A.; Kolesnikov, A.V.; Tang, P.H.; Ablonczy, Z.; Chan, C.C.; Zhou, Z.; Green, D.R.; et al. Noncanonical autophagy promotes the visual cycle. Cell 2013, 154, 365–376. [Google Scholar] [CrossRef]
- Heckmann, B.L.; Boada-Romero, E.; Cunha, L.D.; Magne, J.; Green, D.R. LC3-Associated phagocytosis and inflammation. J. Mol. Biol. 2017, 429, 3561–3576. [Google Scholar] [CrossRef]
- Martinez, J. LAP it up, fuzz ball: A short history of LC3-associated phagocytosis. Curr. Opin. Immunol. 2018, 55, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Malireddi, R.K.; Lu, Q.; Cunha, L.D.; Pelletier, S.; Gingras, S.; Orchard, R.; Guan, J.L.; Tan, H.; Peng, J.; et al. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell. Biol. 2015, 17, 893–906. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Overholtzer, M. LAP: The protector against autoimmunity. Cell Res. 2016, 26, 865–866. [Google Scholar] [CrossRef] [PubMed]
- Masud, S.; Prajsnar, T.K.; Torraca, V.; Lamers, G.E.M.; Benning, M.; Van Der Vaart, M.; Meijer, A.H. Macrophages target Salmonella by Lc3-associated phagocytosis in a systemic infection model. Autophagy 2019, 15, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Feliciano, L.; Doggett, T.A.; Zhou, Z.; Ferguson, T.A. RUBCN/rubicon and EGFR regulate lysosomal degradative processes in the retinal pigment epithelium (RPE) of the eye. Autophagy 2017, 13, 2072–2085. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.O.; Swanson, M.S. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell. Microbiol. 2005, 7, 765–778. [Google Scholar] [CrossRef]
- Martinez, J.; Almendinger, J.; Oberst, A.; Ness, R.; Dillon, C.P.; Fitzgerald, P.; Hengartner, M.O.; Green, D.R. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17396–17401. [Google Scholar] [CrossRef]
- Wirawan, E.; Lippens, S.; Vanden Berghe, T.; Romagnoli, A.; Fimia, G.M.; Piacentini, M.; Vandenabeele, P. Beclin1: A role in membrane dynamics and beyond. Autophagy 2012, 8, 6–17. [Google Scholar] [CrossRef]
- Simon, A.; Clarke, A. Non-canonical autophagy LAPs lupus. Cell Death Differ. 2016, 23, 1267–1268. [Google Scholar] [CrossRef][Green Version]
- Almedawar, S.; Vafia, K.; Schreiter, S.; Neumann, K.; Khattak, S.; Kurth, T.; Ader, M.; Karl, M.O.; Tsang, S.H.; Tanaka, E.M. MERTK-Dependent Ensheathment of Photoreceptor Outer Segments by Human Pluripotent Stem Cell-Derived Retinal Pigment Epithelium. Stem. Cell Rep. 2020, 3, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Nakagawa, A.; Nagaosa, K.; Shiratsuchi, A.; Nakanishi, Y. Phosphatidylserine binding of class B scavenger receptor type I, a phagocytosis receptor of testicular Sertoli cells. J. Biol. Chem. 2002, 277, 27559–27566. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Shiratsuchi, A. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: Mechanisms and consequences. Biol. Pharm. Bull. 2004, 27, 13–16. [Google Scholar] [CrossRef]
- Steinberg, R.H.; Wood, I.; Hogan, M.J. Pigment epithelial ensheathment and phagocytosis of extrafoveal cones in human retina. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1977, 277, 459–471. [Google Scholar]
- Myers, K.V.; Amend, S.R.; Pienta, K.J. Targeting Tyro3, Axl and MerTK (TAM receptors): Implications for macrophages in the tumor microenvironment. Mol. Cancer 2019, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Giroud, P.; Renaudineau, S.; Gudefin, L.; Calcei, A.; Menguy, T.; Rozan, C.; Mizrahi, J.; Caux, C.; Duong, V.; Valladeau-Guilemond, J. Expression of TAM-R in Human Immune Cells and Unique Regulatory Function of MerTK in IL-10 Production by Tolerogenic DC. Front. Immunol. 2020, 11, 564133. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Kokkinaki, M.; Abu-Asab, M.; Gunawardena, N.; Ahern, G.; Javidnia, M.; Young, J.; Golestaneh, N. Klotho regulates retinal pigment epithelial functions and protects against oxidative stress. J. Neurosci. 2013, 33, 16346–16359. [Google Scholar] [CrossRef] [PubMed]
- Imura, A.; Iwano, A.; Tohyama, O.; Tsuji, Y.; Nozaki, K.; Hashimoto, N.; Fujimori, T.; Nabeshima, Y. Secreted Klotho protein in sera and CSF: Implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004, 565, 143–147. [Google Scholar] [CrossRef]
- Wolf, G. Lipofuscin and macular degeneration. Nutr. Rev. 2003, 61, 342–346. [Google Scholar] [CrossRef]
- Saftari, L.N.; Kwon, O.S. Ageing vision and falls: A review. J. Physiol. Anthropol. 2018, 37, 11. [Google Scholar] [CrossRef]
- Lim, K.; Groen, A.; Molostvov, G.; Lu, T.; Lilley, K.S.; Snead, D.; James, S.; Wilkinson, I.B.; Ting, S.; Hsiao, L.L.; et al. α-Klotho Expression in Human Tissues. J. Clin. Endocrinol. Metab. 2015, 100, E1308–E1318. [Google Scholar] [CrossRef] [PubMed]
- Li, S.A.; Watanabe, M.; Yamada, H.; Nagai, A.; Kinuta, M.; Takei, K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell. Struct. Funct. 2004, 29, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Toyama, R.; Fujimori, T.; Nabeshima, Y.; Itoh, Y.; Tsuji, Y.; Osamura, R.Y.; Nabeshima, Y. Impaired regulation of gonadotropins leads to the atrophy of the female reproductive system in klotho-deficient mice. Endocrinology 2006, 147, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Fan, L.; Yu, Q.; Luo, S.; Wu, X.; Tang, J.; Kang, G.; Tang, L. Abnormality of Klotho Signaling Is Involved in Polycystic Ovary Syndrome. Reprod. Sci. 2018, 25, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hao, Y.; Zhong, Q.; Hang, J.; Zhao, Y.; Qiao, J. Low KLOTHO level related to aging is associated with diminished ovarian reserve. Fertil. Steril. 2020, 114, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, Y.; Huang, Y.; Chen, J.; Yu, Z.; Chen, C.; Lai, L. miR-15b induces premature ovarian failure in mice via inhibition of α-Klotho expression in ovarian granulosa cells. Free Radic. Biol. Med. 2019, 141, 383–392. [Google Scholar] [CrossRef]
- Nelson, L.M. Clinical practice. Primary ovarian insufficiency. N. Engl. J. Med. 2009, 360, 606–614. [Google Scholar] [CrossRef]
- Gleicher, N.; Kushnir, V.A.; Barad, D.H. Prospectively assessing risk for premature ovarian senescence in young females: A new paradigm. Reprod. Biol. Endocrinol. 2015, 13, 34. [Google Scholar] [CrossRef]
- Lu, Q.; Lemke, G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 2001, 293, 306–311. [Google Scholar] [CrossRef]
- Camenisch, T.D.; Koller, B.H.; Earp, H.S.; Matsushima, G.K. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 1999, 162, 3498–3503. [Google Scholar] [PubMed]
- Rothlin, C.V.; Ghosh, S.; Zuniga, E.I.; Oldstone, M.B.; Lemke, G. TAM receptors are pleiotropic inhibitors of the innate immunity response. Cell 2007, 131, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Cunha, L.D.; Park, S.; Yang, M.; Lu, Q.; Orchard, R.; Li, Q.Z.; Yan, M.; Janke, L.; Guy, C.; et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 2016, 533, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Asare, P.F.; Roscioli, E.; Hurtado, P.R.; Tran, H.B.; Mah, C.Y.; Hodge, S. LC3-Associated Phagocytosis (LAP): A Potentially Influential Mediator of Efferocytosis-Related Tumor Progression and Aggressiveness. Front. Oncol. 2020, 10, 1298. [Google Scholar] [CrossRef]
- Kyger, M.; Worley, A.; Adamus, G. Autoimmune responses against photoreceptor antigens during retinal degeneration and their role in macrophage recruitment into retinas of RCS rats. J. Neuroimmunol. 2013, 254, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Qi, N.; Shang, T.; Wu, H.; Deng, T.; Han, D. Sertoli cell-initiated testicular innate immune response through Toll-like receptor-3 activation is negatively regulated by Tyro3, Axl and Mer receptors. Endocrinology 2010, 151, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, N.; Chen, Q.; Yan, K.; Liu, Z.; Zhang, X.; Liu, P.; Chen, Y.; Han, D. Breakdown of immune homeostasis in the testis of mice lacking Tyro3, Axl and Mer receptor tyrosine kinases. Immunol. Cell. Biol. 2013, 91, 416–426. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Deng, T.; Xiong, W.; Lui, P.; Li, N.; Chen, Y.; Han, D. Damaged spermatogenic cells induce inflammatory gene expression in mouse Sertoli cells through the activation of Toll-like receptors 2 and 4. Mol. Cell. Endocrinol. 2013, 365, 162–173. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signaling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef]
- Mandrup-Poulsen, T.; Nerup, J.; Reimers, J.I.; Pociot, F.; Andersen, H.U.; Karlsen, A.; Bjerre, U.; Bergholdt, R. Cytokines and the endocrine system. I. The immunoendocrine network. Eur. J. Endocrinol. 1995, 133, 660–671. [Google Scholar] [CrossRef]
- Vinatier, D.; Dufour, P.; Tordjeman-Rizzi, N.; Prolongeau, J.F.; Depret-Moser, S.; Monnier, J.C. Immunological aspects of ovarian function: Role of the cytokines. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 63, 155–168. [Google Scholar] [CrossRef]
- Dejucq, N.; Dugast, I.; Ruffault, A.; van der Meide, P.H.; Jégou, B. Interferon-alpha and -gamma expression in the rat testis. Endocrinology 1995, 136, 4925–4931. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Beutler, B. Intracellular Toll-like receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Y.; Deng, W.W.; Sun, B. Negative regulation of Toll-like receptor signaling pathway. Microbes Infect. 2009, 11, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kawai, T.; Akira, S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012, 33, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Jégou, B.; Cudicini, C.; Gomez, E.; Stéphan, J.P. Interleukin-1, interleukin-6 and the germ cell-Sertoli cell cross-talk. Reprod. Fertil. Dev. 1995, 7, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Syed, V.; Stéphan, J.P.; Gérard, N.; Legrand, A.; Parvinen, M.; Bardin, C.W.; Jégou, B. Residual bodies activate Sertoli cell interleukin-1 alpha (IL-1 alpha) release, which triggers IL-6 production by an autocrine mechanism, through the lipoxygenase pathway. Endocrinology 1995, 136, 3070–3078. [Google Scholar] [CrossRef]
- Cudicini, C.; Lejeune, H.; Gomez, E.; Bosmans, E.; Ballet, F.; Saez, J.; Jégou, B. Human Leydig cells and Sertoli cells are producers of interleukins-1 and -6. J. Clin. Endocrinol. Metab. 1997, 82, 1426–1433. [Google Scholar] [CrossRef]
- Dejucq, N.; Chousterman, S.; Jégou, B. The testicular antiviral defense system: Localization, expression, and regulation of 2’5’ oligoadenylate synthetase, double-stranded RNA-activated protein kinase, and Mx proteins in the rat seminiferous tubule. J. Cell. Biol. 1997, 139, 865–873. [Google Scholar] [CrossRef]
- Stéphan, J.P.; Syed, V.; Jégou, B. Regulation of Sertoli cell IL-1 and IL-6 production in vitro. Mol. Cell. Endocrinol. 1997, 134, 109–118. [Google Scholar] [CrossRef]
- Dejucq, N.; Lienard, M.O.; Guillaume, E.; Dorval, I.; Jégou, B. Expression of interferons-alpha and -gamma in testicular interstitial tissue and spermatogonia of the rat. Endocrinology 1998, 139, 3081–3087. [Google Scholar] [CrossRef] [PubMed]
- Jégou, B.; Stéphan, J.P.; Cudicini, C.; Gomez, E.; Bauché, F.; Piquet-Pellorce, C.; Touzalin, A.M. The Sertoli cell-germ cell interactions and the seminiferous tubule interleukin-1 and interleukin-6 system. Results Probl. Cell. Differ. 2000, 28, 53–68. [Google Scholar] [PubMed]
- Dejucq, N.; Jégou, B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol. Mol. Biol. Rev. 2001, 65, 208–231. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.M.; Mruk, D.D.; Lee, W.M.; Cheng, C.Y. Cytokines and junction restructuring events during spermatogenesis in the testis: An emerging concept of regulation. Cytokines Growth Factor Rev. 2009, 20, 329–338. [Google Scholar] [CrossRef]
- Bagavant, H.; Adams, S.; Terranova, P.; Chang, A.; Kraemer, F.W.; Lou, Y.; Kasai, K.; Luo, A.M.; Tung, K.S. Autoimmune ovarian inflammation triggered by proinflammatory (Th1) T cells is compatible with normal ovarian function in mice. Biol. Reprod. 1999, 61, 635–642. [Google Scholar] [CrossRef]
- Sarapik, A.; Velthut, A.; Haller-Kikkatalo, K.; Faure, G.C.; Béné, M.C.; de Carvalho Bittencourt, M.; Massin, F.; Uibo, R.; Salumets, A. Follicular proinflammatory cytokines and chemokines as markers of IVF success. Clin. Dev. Immunol. 2012, 2012, 606459. [Google Scholar] [CrossRef]
- Büscher, U.; Chen, F.C.; Kentenich, H.; Schmiady, H. Cytokines in the follicular fluid of stimulated and non-stimulated human ovaries; is ovulation a suppressed inflammatory reaction? Hum. Reprod. 1999, 14, 162–166. [Google Scholar] [CrossRef]
- Warma, A.; Descarreaux, M.; Chorfi, Y.; Dupras, R.; Rémillard, R.; Ndiaye, K. Interleukins’ expression profile changes in granulosa cells of preovulatory follicles during the postpartum period in dairy cows. Cytokine X 2020, 2, 100022. [Google Scholar] [CrossRef]
- Suescun, M.O.; Rival, C.; Theas, M.S.; Calandra, R.S.; Lustig, L. Involvement of tumor necrosis factor-α in the pathogenesis of autoimmune orchitis in rats. Biol. Reprod. 2003, 68, 2114–2121. [Google Scholar] [CrossRef]
- Huleihel, M.; Lunenfeld, E. Regulation of spermatogenesis by paracrine/autocrine testicular factors. Asian J. Androl. 2004, 6, 259–268. [Google Scholar]
- Guazzone, V.A.; Jacobo, P.; Theas, M.S.; Lustig, L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc. Res. Tech. 2009, 72, 620–628. [Google Scholar] [CrossRef]
- Rival, C.; Theas, M.S.; Guazzone, V.A.; Lustig, L. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J. Reprod. Immunol. 2006, 70, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, X.; Hu, J.; Cui, L.; Zhao, S.; Jiao, X.; Qin, Y. Dysregulated cytokine profile associated with biochemical premature ovarian insufficiency. Am. J. Reprod. Immunol. 2020, 84, e13292. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Zhang, Y.; Chen, Q.; Yan, K.; Han, D. Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology 2012, 135, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Alciato, F.; Sainaghi, P.P.; Sola, D.; Castello, L.; Avanzi, G.C. TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J. Leukoc. Biol. 2010, 5, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef]
- Pagani, S.; Bellan, M.; Mauro, D.; Castello, L.M.; Avanzi, G.C.; Lewis, M.J.; Sainaghi, P.P.; Pitzalis, C.; Nerviani, A. New Insights into the Role of Tyro3, Axl, and Mer Receptors in Rheumatoid Arthritis. Dis. Markers. 2020, 2020, 1614627. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Utheim, T.P.; Guo, C.; Xiao, M.; Liu, Y.; Yin, Z.; Ma, J. Correlation of cytokine levels and microglial cell infiltration during retinal degeneration in RCS rats. PLoS ONE 2013, 8, e82061. [Google Scholar] [CrossRef]
- Yoshida, N.; Ikeda, Y.; Notomi, S.; Ishikawa, K.; Murakami, Y.; Hisatomi, T.; Enaida, H.; Ishibashi, T. Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology 2013, 120, e5–e12. [Google Scholar] [CrossRef]
- Ozaki, E.; Campbell, M.; Kiang, A.S.; Humphries, M.; Doyle, S.L.; Humphries, P. Inflammation in age-related macular degeneration. Adv. Exp. Med. Biol. 2014, 801, 229–235. [Google Scholar]
- Inana, G.; Murat, C.; An, W.; Yao, X.; Harris, I.R.; Cao, J. RPE phagocytic function declines in age-related macular degeneration and is rescued by human umbilical tissue derived cells. J. Transl. Med. 2018, 16, 63. [Google Scholar] [CrossRef]
- Riccioli, A.; Starace, D.; Galli, R.; Fuso, A.; Scarpa, S.; Palombi, F.; De Cesaris, P.; Ziparo, E.; Filippini, A. Sertoli cells initiate testicular innate immune responses through TLR activation. J. Immunol. 2006, 177, 7122–7130. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Xiong, W.; Chen, S.; Tang, H.; Han, D. Expression patterns and functions of Toll-like receptors in mouse Sertoli cells. Endocrinology 2008, 149, 4402–4412. [Google Scholar] [CrossRef]
- Ernst, E.H.; Amoushahi, M.; Sørensen, A.S.; KraGCtrup, T.W.; Ernst, E.; Lykke-Hartmann, K. Distinct expression patterns of TLR transcripts in human oocytes and granulosa cells from primordial and primary follicles. J. Reprod. Immunol. 2020, 140, 103125. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.V.; Nagineni, C.N.; Chin, M.S.; Hooks, J.J.; Detrick, B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 2004, 153, 7–15. [Google Scholar] [CrossRef]
- Shiose, S.; Chen, Y.; Okano, K.; Roy, S.; Kohno, H.; Tang, J.; Pearlman, E.; Maeda, T.; Palczewski, K.; Maeda, A. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J. Biol. Chem. 2011, 286, 15543–15555. [Google Scholar] [CrossRef]
- Nakamura, H.; Horai, Y.; Suzuki, T.; Okada, A.; Ichinose, K.; Yamasaki, S.; Koji, T.; Kawakami, A. TLR3-mediated apoptosis and activation of phosphorylated Akt in the salivary gland epithelial cells of primary Sjögren’s syndrome patients. Rheumatol. Int. 2013, 33, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Kindzelskii, A.L.; Elner, V.M.; Elner, S.G.; Yang, D.; Hughes, B.A.; Petty, H.R. Toll-like receptor 4 (TLR4) of retinal pigment epithelial cells participates in transmembrane signaling in response to photoreceptor outer segments. J. Gen. Phys. 2004, 124, 139–149. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yefimova, M.G.; Ravel, C.; Rolland, A.D.; Bourmeyster, N.; Jégou, B. MERTK-Mediated LC3-Associated Phagocytosis (LAP) of Apoptotic Substrates in Blood-Separated Tissues: Retina, Testis, Ovarian Follicles. Cells 2021, 10, 1443. https://doi.org/10.3390/cells10061443
Yefimova MG, Ravel C, Rolland AD, Bourmeyster N, Jégou B. MERTK-Mediated LC3-Associated Phagocytosis (LAP) of Apoptotic Substrates in Blood-Separated Tissues: Retina, Testis, Ovarian Follicles. Cells. 2021; 10(6):1443. https://doi.org/10.3390/cells10061443
Chicago/Turabian StyleYefimova, Marina G., Celia Ravel, Antoine D. Rolland, Nicolas Bourmeyster, and Bernard Jégou. 2021. "MERTK-Mediated LC3-Associated Phagocytosis (LAP) of Apoptotic Substrates in Blood-Separated Tissues: Retina, Testis, Ovarian Follicles" Cells 10, no. 6: 1443. https://doi.org/10.3390/cells10061443
APA StyleYefimova, M. G., Ravel, C., Rolland, A. D., Bourmeyster, N., & Jégou, B. (2021). MERTK-Mediated LC3-Associated Phagocytosis (LAP) of Apoptotic Substrates in Blood-Separated Tissues: Retina, Testis, Ovarian Follicles. Cells, 10(6), 1443. https://doi.org/10.3390/cells10061443