Know How to Regrow—Axon Regeneration in the Zebrafish Spinal Cord
Abstract
1. Introduction
2. Axon Regeneration and Functional Recovery after SCI in Zebrafish
Differential Regenerative Capacity of Zebrafish Axons
3. A Breadth of Zebrafish SCI Paradigms
3.1. Mechanical Lesion
3.2. Alternative Lesion Paradigms
4. Neuron-Extrinsic and -Intrinsic Factors Define Successful Axon Regeneration in Zebrafish
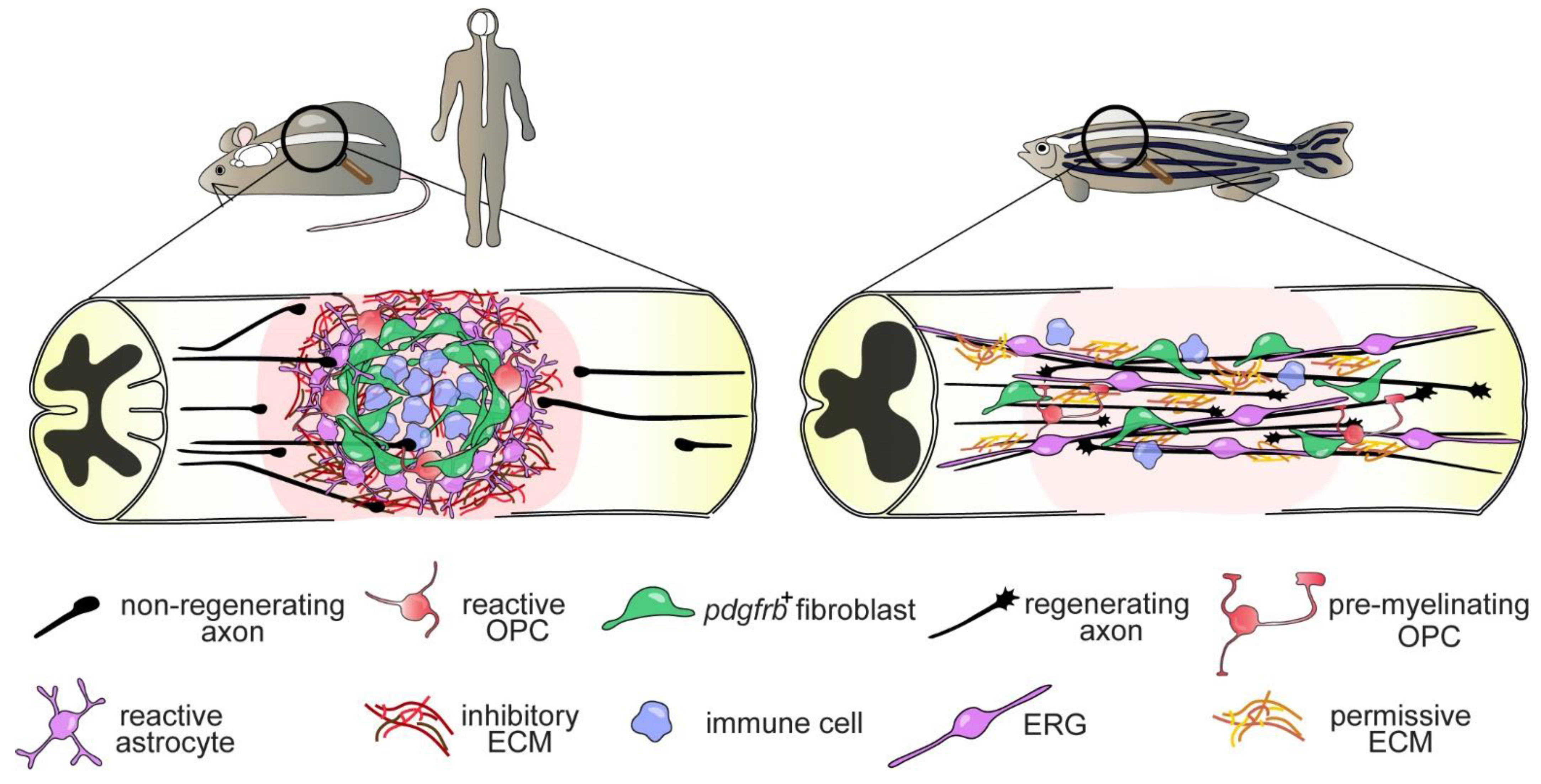
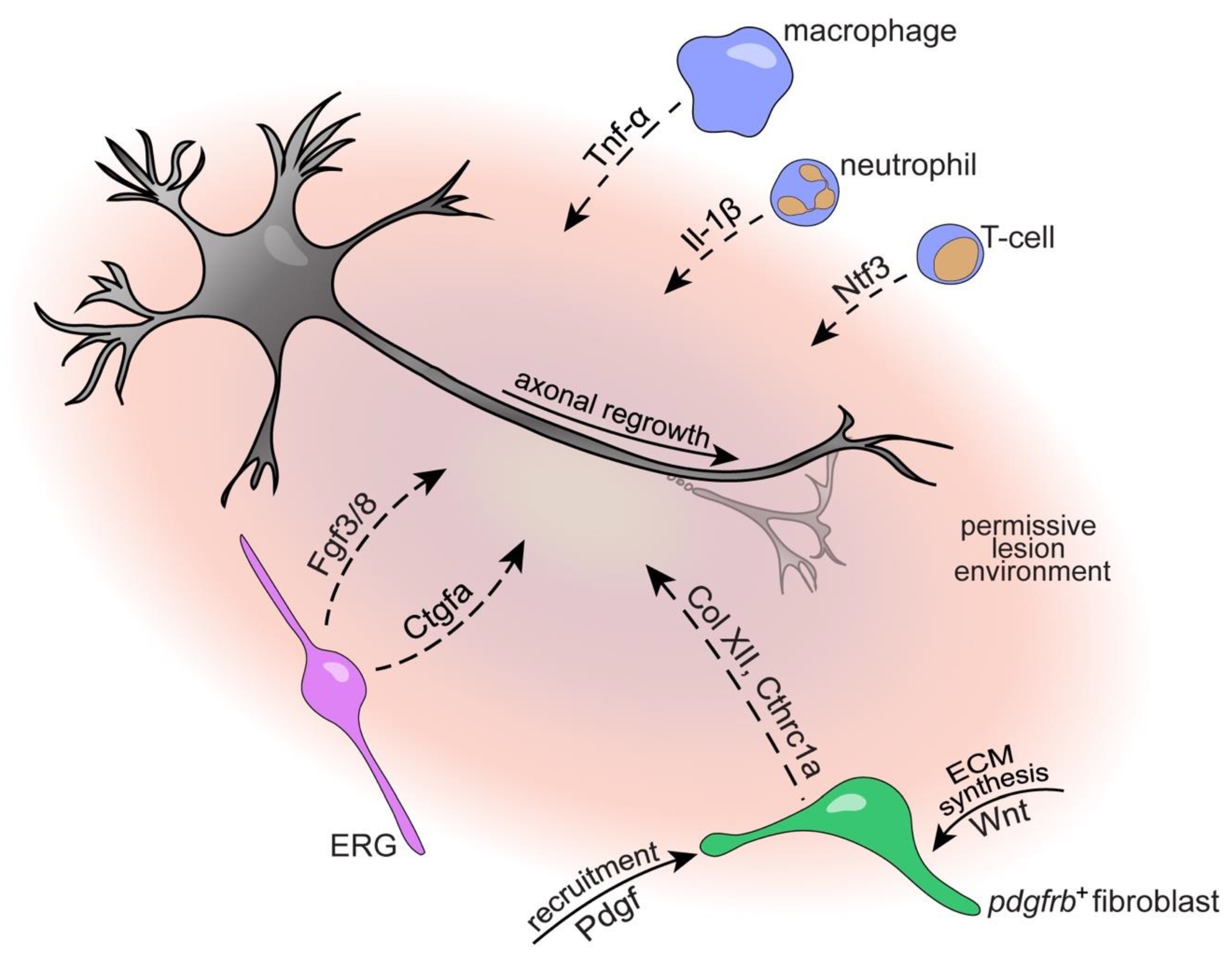
4.1. Immune System
4.2. Oligodendrocytes and Oligodendrocyte Progenitor Cells
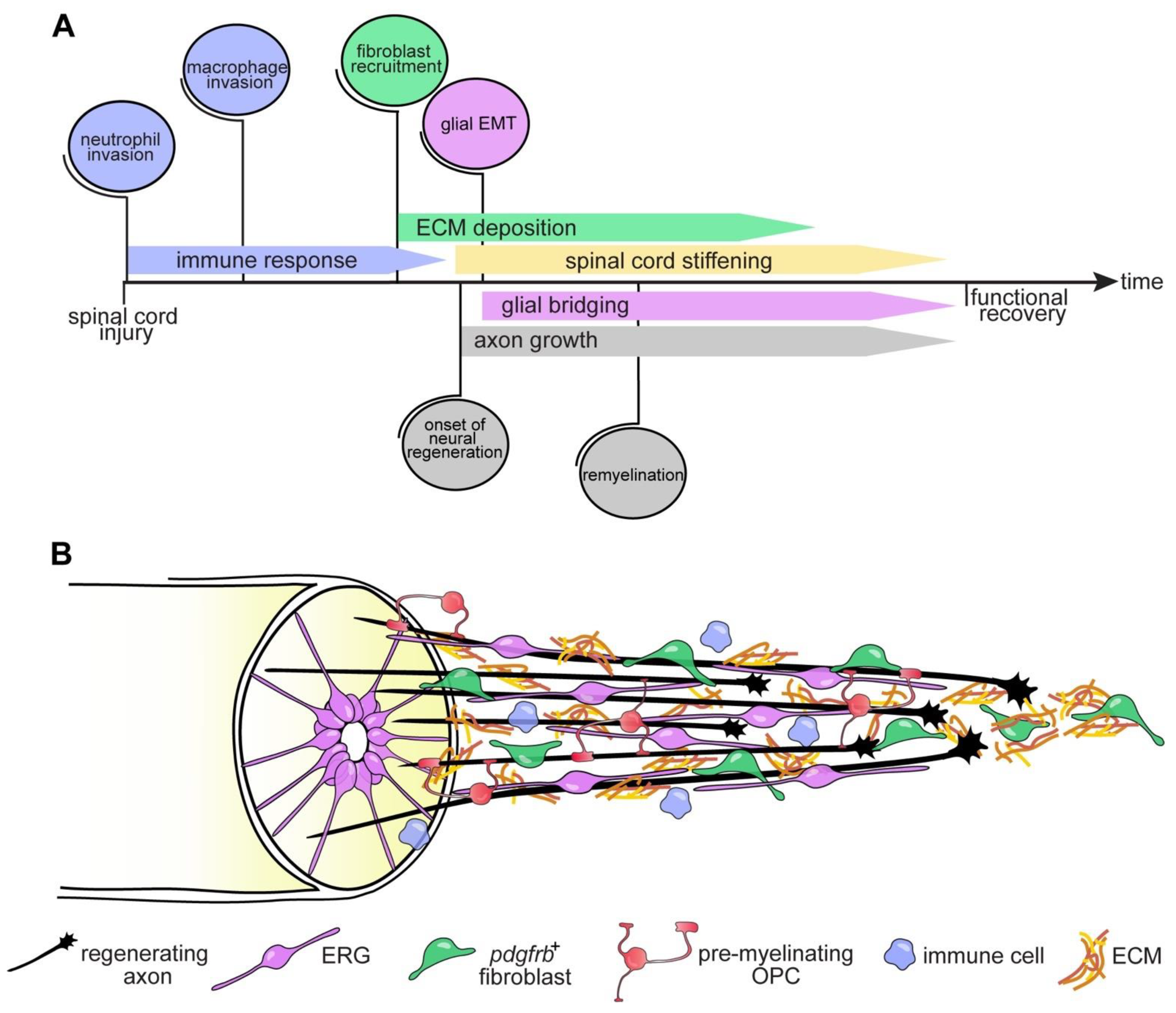
4.3. The Glial Cell and Fibroblast Response to SCI
4.3.1. Ependymo-Radial Glia
4.3.2. Fibroblasts
5. Intrinsic Regenerative Capacity
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar]
- Boulland, J.-L.; Lambert, F.M.; Züchner, M.; Ström, S.; Glover, J.C. A Neonatal Mouse Spinal Cord Injury Model for Assessing Post-Injury Adaptive Plasticity and Human Stem Cell Integration. PLoS ONE 2013, 8, e71701. [Google Scholar] [CrossRef]
- Züchner, M.; Kondratskaya, E.; Sylte, C.B.; Glover, J.C.; Boulland, J. Rapid recovery and altered neurochemical dependence of locomotor central pattern generation following lumbar neonatal spinal cord injury. J. Physiol. 2018, 596, 281–303. [Google Scholar] [CrossRef]
- Fry, E.J.; Stolp, H.B.; Lane, M.A.; Dziegielewska, K.M.; Saunders, N.R. Regeneration of supraspinal axons after complete transection of the thoracic spinal cord in neonatal opossums (Monodelphis domestica). J. Comp. Neurol. 2003, 466, 422–444. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.J.; Nelson, B.H.; Valenzuela, J.I.; Keirstead, H.S.; Shull, S.E.; Ethell, D.W.; Steeves, J.D. Functional repair of transected spinal cord in embryonic chick. Restor. Neurol. Neurosci. 1991, 2, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Faret, G.; González-Pinto, K.; Cebrián-Silla, A.; Peñailillo, J.; García-Verdugo, J.M.; Larraín, J. Cellular response to spinal cord injury in regenerative and non-regenerative stages in Xenopus laevis. Neural Dev. 2021, 16, 2. [Google Scholar] [CrossRef]
- Streeter, K.; Sunshine, M.; Brant, J.; Sandoval, A.G.W.; Maden, M.; Fuller, D.D. Molecular and histologic outcomes following spinal cord injury in spiny mice, Acomys cahirinus. J. Comp. Neurol. 2019, 528, 1535–1547. [Google Scholar] [CrossRef]
- A Zukor, K.; Kent, D.T.; Odelberg, S.J. Meningeal cells and glia establish a permissive environment for axon regeneration after spinal cord injury in newts. Neural Dev. 2011, 6, 1. [Google Scholar] [CrossRef]
- Mchedlishvili, L.; Epperlein, H.H.; Telzerow, A.; Tanaka, E.M. A clonal analysis of neural progenitors during axolotl spinal cord regeneration reveals evidence for both spatially restricted and multipotent progenitors. Development 2007, 134, 2083–2093. [Google Scholar] [CrossRef]
- Yamada, H.; Miyake, T.; Kitamura, T. Regeneration of Axons in Transection of the Carp Spinal Cord. Zool. Sci. 1995, 12, 325–332. [Google Scholar] [CrossRef]
- Doyle, L.; Stafford, P.; Roberts, B. Recovery of locomotion correlated with axonal regeneration after a complete spinal transection in the eel. Neuroscience 2001, 107, 169–179. [Google Scholar] [CrossRef]
- Sobrido-Cameán, D.; Robledo, D.; Romaus-Sanjurjo, D.; Pérez-Cedrón, V.; Sánchez, L.; Rodicio, M.C.; Barreiro-Iglesias, A. Inhibition of Gamma-Secretase Promotes Axon Regeneration After a Complete Spinal Cord Injury. Front. Cell Dev. Biol. 2020, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, K.L.; Allen, S.R.; Harkenrider, T.L.; Fogerson, S.M.; Guadarrama, E.; Morgan, J.R. Regenerative capacity in the lamprey spinal cord is not altered after a repeated transection. PLoS ONE 2019, 14, e0204193. [Google Scholar] [CrossRef]
- Bernstein, J.J. Relation of spinal cord regeneration to age in adult goldfish. Exp. Neurol. 1964, 9, 161–174. [Google Scholar] [CrossRef]
- Becker, T.; Wullimann, M.F.; Becker, C.; Bernhardt, R.R.; Schachner, M. Axonal regrowth after spinal cord transection in adult zebrafish. J. Comp. Neurol. 1997, 377, 577–595. [Google Scholar] [CrossRef]
- Kroehne, V.; Freudenreich, D.; Hans, S.; Kaslin, J.; Brand, M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 2011, 138, 4831–4841. [Google Scholar] [CrossRef]
- Fausett, B.V.; Goldman, D. A Role for α1 Tubulin-Expressing Muller Glia in Regeneration of the Injured Zebrafish Retina. J. Neurosci. 2006, 26, 6303–6313. [Google Scholar] [CrossRef]
- Bernhardt, R.; Tongiorgi, E.; Anzini, P.; Schachner, M. Increased expression of specific recognition molecules by retinal ganglion cells and by optic pathway glia accompanies the successful regeneration of retinal axons in adult zebrafish. J. Comp. Neurol. 1996, 376, 253–264. [Google Scholar] [CrossRef]
- Thummel, R.; Kassen, S.C.; Montgomery, J.E.; Enright, J.M.; Hyde, D.R. Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev. Neurobiol. 2007, 68, 392–408. [Google Scholar] [CrossRef]
- Cigliola, V.; Becker, C.J.; Poss, K.D. Building bridges, not walls: Spinal cord regeneration in zebrafish. Dis. Model. Mech. 2020, 13, 044131. [Google Scholar] [CrossRef]
- Becker, C.G.; Becker, T. Neuronal Regeneration from Ependymo-Radial Glial Cells: Cook, Little Pot, Cook! Dev. Cell 2015, 32, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Lieberoth, B.C.; Morellini, F.; Feldner, J.; Becker, T.; Schachner, M. L1.1 Is Involved in Spinal Cord Regeneration in Adult Zebrafish. J. Neurosci. 2004, 24, 7837–7842. [Google Scholar] [CrossRef] [PubMed]
- Mokalled, M.H.; Patra, C.; Dickson, A.L.; Endo, T.; Stainier, D.; Poss, K.D. Injury-induced ctgfa directs glial bridging and spinal cord regeneration in zebrafish. Science 2016, 354, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Ohnmacht, J.; Yang, Y.-J.; Maurer, G.W.; Barreiro-Iglesias, A.; Tsarouchas, T.M.; Wehner, D.; Sieger, D.; Becker, C.G.; Becker, T. Spinal motor neurons are regenerated after mechanical lesion and genetic ablation in larval zebrafish. Dev. 2016, 143, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- Briona, L.K.; Dorsky, R.I. Radial glial progenitors repair the zebrafish spinal cord following transection. Exp. Neurol. 2014, 256, 81–92. [Google Scholar] [CrossRef]
- Wehner, D.; Tsarouchas, T.M.; Michael, A.; Haase, C.; Weidinger, G.; Reimer, M.M.; Becker, T.; Becker, C.G. Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Kuscha, V.; Barreiro-Iglesias, A.; Becker, C.; Becker, T. Plasticity of tyrosine hydroxylase and serotonergic systems in the regenerating spinal cord of adult zebrafish. J. Comp. Neurol. 2012, 520, 933–951. [Google Scholar] [CrossRef]
- Becker, T.; Bernhardt, R.R.; Reinhard, E.; Wullimann, M.F.; Tongiorgi, E.; Schachner, M. Readiness of Zebrafish Brain Neurons to Regenerate a Spinal Axon Correlates with Differential Expression of Specific Cell Recognition Molecules. J. Neurosci. 1998, 18, 5789–5803. [Google Scholar] [CrossRef]
- Becker, T.; Becker, C.G. Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J. Comp. Neurol. 2001, 433, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Lieberoth, B.C.; Becker, C.; Schachner, M. Differences in the regenerative response of neuronal cell populations and indications for plasticity in intraspinal neurons after spinal cord transection in adult zebrafish. Mol. Cell. Neurosci. 2005, 30, 265–278. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Sztal, T.E.; Jusuf, P.R.; Hall, T.E.; Nguyen-Chi, M.; Currie, P.D. Fgf-Dependent Glial Cell Bridges Facilitate Spinal Cord Regeneration in Zebrafish. J. Neurosci. 2012, 32, 7477–7492. [Google Scholar] [CrossRef] [PubMed]
- Thiele, T.; Donovan, J.C.; Baier, H. Descending Control of Swim Posture by a Midbrain Nucleus in Zebrafish. Neuron 2014, 83, 679–691. [Google Scholar] [CrossRef]
- Vasudevan, D.; Liu, Y.-C.; Barrios, J.P.; Wheeler, M.K.; Douglass, A.D.; Dorsky, R.I. Regenerated interneurons integrate into locomotor circuitry following spinal cord injury. Exp. Neurol. 2021, 342, 113737. [Google Scholar] [CrossRef] [PubMed]
- Hecker, A.; Anger, P.; Braaker, P.N.; Schulze, W.; Schuster, S. High-resolution mapping of injury-site dependent functional recovery in a single axon in zebrafish. Commun. Biol. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Hu, B.-B.; Chen, M.; Huang, R.-C.; Huang, Y.-B.; Xu, Y.; Yin, W.; Li, L. In vivo imaging of Mauthner axon regeneration, remyelination and synapses re-establishment after laser axotomy in zebrafish larvae. Exp. Neurol. 2018, 300, 67–73. [Google Scholar] [CrossRef]
- Bhatt, D.H.; Otto, S.J.; DePoister, B.; Fetcho, J.R.; Leist, M.; Ghezzi, P.; Grasso, G.; Bianchi, R.; Villa, P.; Fratelli, M.; et al. Cyclic AMP-Induced Repair of Zebrafish Spinal Circuits. Science 2004, 305, 254–258. [Google Scholar] [CrossRef]
- Hecker, A.; Schulze, W.; Oster, J.; Richter, D.O.; Schuster, S. Removing a single neuron in a vertebrate brain forever abolishes an essential behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 3254–3260. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.M.; Sörensen, I.; Kuscha, V.; Frank, R.E.; Liu, C.; Becker, C.G.; Becker, T. Motor Neuron Regeneration in Adult Zebrafish. J. Neurosci. 2008, 28, 8510–8516. [Google Scholar] [CrossRef]
- Wehner, D.; Becker, T.; Becker, C.G. Restoration of anatomical continuity after spinal cord transection depends on Wnt/β-catenin signaling in larval zebrafish. Data Brief 2018, 16, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Tsata, V.; Kroehne, V.; Wehner, D.; Rost, F.; Lange, C.; Hoppe, C.; Kurth, T.; Reinhardt, S.; Petzold, A.; Dahl, A.; et al. Reactive oligodendrocyte progenitor cells (re-)myelinate the regenerating zebrafish spinal cord. Development 2020, 147. [Google Scholar] [CrossRef]
- Briona, L.K.; Dorsky, R.I. Spinal Cord Transection in the Larval Zebrafish. J. Vis. Exp. 2014, 2014, e51479. [Google Scholar] [CrossRef] [PubMed]
- Gollmann-Tepeköylü, C.; Nägele, F.; Graber, M.; Pölzl, L.; Lobenwein, D.; Hirsch, J.; An, A.; Irschick, R.; Röhrs, B.; Kremser, C.; et al. Shock waves promote spinal cord repair via TLR3. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.-W.; Kamei, Y.; Shigenobu, S.; Sheu, J.-C.; Tsai, H.-J. Injury-induced Cavl-expressing cells at lesion rostral side play major roles in spinal cord regeneration. Open Biol. 2021, 11, 200304. [Google Scholar] [CrossRef]
- Hui, S.P.; Dutta, A.; Ghosh, S. Cellular response after crush injury in adult zebrafish spinal cord. Dev. Dyn. 2010, 239, 2962–2979. [Google Scholar] [CrossRef]
- Curado, S.; Anderson, R.M.; Jungblut, B.; Mumm, J.; Schroeter, E.; Stainier, D. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev. Dyn. 2007, 236, 1025–1035. [Google Scholar] [CrossRef]
- Mruk, K.; Ciepla, P.; Piza, P.A.; Alnaqib, M.A.; Chen, J.K. Targeted cell ablation in zebrafish using optogenetic transcriptional control. Development 2020, 147, 183640. [Google Scholar] [CrossRef] [PubMed]
- Anguita-Salinas, C.; Sánchez, M.; Morales, R.A.; Ceci, M.L.; Rojas-Benítez, D.; Allende, M.L. Cellular Dynamics during Spinal Cord Regeneration in Larval Zebrafish. Dev. Neurosci. 2019, 41, 112–122. [Google Scholar] [CrossRef]
- Sagasti, A.; O’Brien, G.S.; Rieger, S.; Martin, S.M.; Cavanaugh, A.M.; Portera-Cailliau, C. Two-photon axotomy and time-lapse confocal imaging in live zebrafish embryos. J. Vis. Exp. 2009, 2009, e1129. [Google Scholar] [CrossRef]
- Sahu, S.; Zhang, Z.; Li, R.; Hu, J.; Shen, H.; Loers, G.; Shen, Y.; Schachner, M. A Small Organic Compound Mimicking the L1 Cell Adhesion Molecule Promotes Functional Recovery after Spinal Cord Injury in Zebrafish. Mol. Neurobiol. 2017, 55, 859–878. [Google Scholar] [CrossRef]
- Grossman, S.; Rosenberg, L.; Wrathall, J. Temporal–Spatial Pattern of Acute Neuronal and Glial Loss after Spinal Cord Contusion. Exp. Neurol. 2001, 168, 273–282. [Google Scholar] [CrossRef]
- Fitch, M.T.; Silver, J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008, 209, 294–301. [Google Scholar] [CrossRef]
- Becker, T.; Becker, C.G. Axonal regeneration in zebrafish. Curr. Opin. Neurobiol. 2014, 27, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jones, N.R.; Blumbergs, P.C.; Heuvel, C.V.D.; Moore, E.J.; Manavis, J.; Sarvestani, G.T.; Ghabriel, M.N. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J. Clin. Neurosci. 2005, 12, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.D.; Salazar, D.L.; Woodruff, T.; Anderson, A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 2010, 133, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, C.T.; Claasen, J.-H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Nelson, C.M.; Lennon, V.A.; Lee, H.; Krug, R.G., 2nd; Kamalova, A.; Madigan, N.N.; Clark, K.J.; Windebank, A.J.; Henley, J.R. Glucocorticoids Target Ependymal Glia and Inhibit Repair of the Injured Spinal Cord. Front.Cell Dev. Biol. 2019, 7, 56. [Google Scholar] [CrossRef]
- Hilla, A.M.; Diekmann, H.; Fischer, D. Microglia Are Irrelevant for Neuronal Degeneration and Axon Regeneration after Acute Injury. J. Neurosci. 2017, 37, 6113–6124. [Google Scholar] [CrossRef]
- Keatinge, M.; Tsarouchas, T.M.; Munir, T.; Porter, N.J.; Larraz, J.; Gianni, D.; Tsai, H.-H.; Becker, C.G.; Lyons, D.A.; Becker, T. CRISPR gRNA phenotypic screening in zebrafish reveals pro-regenerative genes in spinal cord injury. PLoS Genet. 2021, 17, e1009515. [Google Scholar] [CrossRef]
- Hui, S.P.; Sheng, D.Z.; Sugimoto, K.; Gonzalez-Rajal, A.; Nakagawa, S.; Hesselson, D.; Kikuchi, K. Zebrafish Regulatory T Cells Mediate Organ-Specific Regenerative Programs. Dev. Cell 2017, 43, 659–672.e5. [Google Scholar] [CrossRef]
- Huie, J.R.; Ferguson, A.R.; Kyritsis, N.; Pan, J.Z.; Irvine, K.-A.; Nielson, J.L.; Schupp, P.G.; Oldham, M.C.; Gensel, J.C.; Lin, A.; et al. Machine intelligence identifies soluble TNFa as a therapeutic target for spinal cord injury. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, I.; Klugmann, M.; Anderson, T.; Yool, D.; Thomson, C.; Schwab, M.H.; Schneider, A.; Zimmermann, F.; McCulloch, M.; Nadon, N.; et al. Axonal Swellings and Degeneration in Mice Lacking the Major Proteolipid of Myelin. Science 1998, 280, 1610–1613. [Google Scholar] [CrossRef]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nat. Cell Biol. 2012, 487, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Larson, V.A.; Mironova, Y.; Vanderpool, K.G.; Waisman, A.; Rash, J.E.; Agarwal, A.; Bergles, D.E. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. eLife 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Bergles, D.E.; Roberts, J.D.B.; Somogyi, P.; Jahr, C.E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nat. Cell Biol. 2000, 405, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef]
- Mensch, S.; Baraban, M.; Almeida, R.; Czopka, T.; Ausborn, J.; El Manira, A.; Lyons, D. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 2015, 18, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Assinck, P.; Duncan, G.J.; Plemel, J.R.; Lee, M.; Stratton, J.A.; Manesh, S.B.; Liu, J.; Ramer, L.M.; Kang, S.H.; Bergles, D.E.; et al. Myelinogenic Plasticity of Oligodendrocyte Precursor Cells following Spinal Cord Contusion Injury. J. Neurosci. 2017, 37, 8635–8654. [Google Scholar] [CrossRef] [PubMed]
- Duncan, G.J.; Manesh, S.B.; Hilton, B.; Assinck, P.; Liu, J.; Moulson, A.; Plemel, J.R.; Tetzlaff, W. Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Filous, A.R.; Tran, A.; Howell, C.J.; Busch, S.A.; Evans, T.A.; Stallcup, W.B.; Kang, S.H.; Bergles, D.E.; Lee, S.-I.; Levine, J.M.; et al. Entrapment via Synaptic-Like Connections between NG2 Proteoglycan+ Cells and Dystrophic Axons in the Lesion Plays a Role in Regeneration Failure after Spinal Cord Injury. J. Neurosci. 2014, 34, 16369–16384. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Levine, J. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J. Neurosci. 1994, 14, 7616–7628. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.E.; Strittmatter, S.M. Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 2014, 27, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bodrikov, V.; Welte, C.; Wiechers, M.; Weschenfelder, M.; Kaur, G.; Shypitsyna, A.; Pinzon-Olejua, A.; Bastmeyer, M.; Stuermer, C.A.O. Substrate properties of zebrafish Rtn4b/Nogo and axon regeneration in the zebrafish optic nerve. J. Comp. Neurol. 2017, 525, 2991–3009. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Becker, C.; Schachner, M. Expression of protein zero is increased in lesioned axon pathways in the central nervous system of adult zebrafish. Glia 2003, 41, 301–317. [Google Scholar] [CrossRef]
- Hong, L.T.A.; Kim, Y.-M.; Park, H.H.; Hwang, D.H.; Cui, Y.; Lee, E.M.; Yahn, S.; Lee, J.K.; Song, S.-C.; Kim, B.G. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Kwiecien, J.M.; Dabrowski, W.; Dąbrowska-Bouta, B.; Sulkowski, G.; Oakden, W.; Kwiecien-Delaney, C.J.; Yaron, J.R.; Zhang, L.; Schutz, L.; Marzec-Kotarska, B.; et al. Prolonged inflammation leads to ongoing damage after spinal cord injury. PLoS ONE 2020, 15, e0226584. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef]
- Anderson, M.A.; Ao, Y.; Sofroniew, M.V. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014, 565, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Barnabé-Heider, F.; Göritz, C.; Sabelström, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisén, J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010, 7, 470–482. [Google Scholar] [CrossRef]
- Meletis, K.; Barnabé-Heider, F.; Carlén, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal Cord Injury Reveals Multilineage Differentiation of Ependymal Cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef]
- Jones, L.L.; Margolis, R.U.; Tuszynski, M.H. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp. Neurol. 2003, 182, 399–411. [Google Scholar] [CrossRef]
- Shaw, D.K.; Saraswathy, V.M.; Zhou, L.; McAdow, A.R.; Burris, B.; Butka, E.; Morris, S.A.; Dietmann, S.; Mokalled, M.H. Localized EMT reprograms glial progenitors to promote spinal cord repair. Dev. Cell 2021, 56, 613–626.e7. [Google Scholar] [CrossRef] [PubMed]
- Dervan, A.G.; Roberts, B.L. Reaction of spinal cord central canal cells to cord transection and their contribution to cord regeneration. J. Comp. Neurol. 2003, 458, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Nona, S.N.; Stafford, C.A. Gliali repair at the lesion site in regenerating goldfish spinal cord: An immunohistochemical study using species-specific antibodies. J. Neurosci. Res. 1995, 42, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Matteoli, M.; Parpura, V.; Mothet, J.; Zorec, R. Astrocytes as secretory cells of the central nervous system: Idiosyncrasies of vesicular secretion. EMBO J. 2016, 35, 239–257. [Google Scholar] [CrossRef]
- Conrad, S.; Schluesener, H.J.; Adibzahdeh, M.; Schwab, J.M. Spinal cord injury induction of lesional expression of profibrotic and angiogenic connective tissue growth factor confined to reactive astrocytes, invading fibroblasts and endothelial cells. J. Neurosurg. Spine 2005, 2, 319–326. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, Q.-J.; Sun, J.-C.; Yang, Y.; Wang, H.-B.; Zhang, Q.; Shi, J.-G. Lentivirus-mediated silencing of the CTGF gene suppresses the formation of glial scar tissue in a rat model of spinal cord injury. Spine J. 2018, 18, 164–172. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Tang, J.K.K.Y.; Siegel, A.L.; Nguyen, P.; Kaslin, J.; Currie, P.D.; Jusuf, P.R. Different Fgfs have distinct roles in regulating neurogenesis after spinal cord injury in zebrafish. Neural Dev. 2018, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Wehner, D.; Cizelsky, W.; Vasudevaro, M.D.; Ozhan, G.; Haase, C.; Kagermeier-Schenk, B.; Röder, A.; Dorsky, R.I.; Moro, E.; Argenton, F.; et al. Wnt/β-catenin signaling defines organizing centers that orchestrate growth and differentiation of the regenerating zebrafish caudal fin. Cell Rep. 2014, 6, 467–481. [Google Scholar]
- Cavone, L.; McCann, T.; Drake, L.K.; Aguzzi, E.A.; Oprişoreanu, A.-M.; Pedersen, E.; Sandi, S.; Selvarajah, J.; Tsarouchas, T.M.; Wehner, D.; et al. A unique macrophage subpopulation signals directly to progenitor cells to promote regenerative neurogenesis in the zebrafish spinal cord. Dev. Cell 2021. [Google Scholar] [CrossRef]
- Dias, D.O.; Göritz, C. Fibrotic scarring following lesions to the central nervous system. Matrix Biol. 2018, 68–69, 561–570. [Google Scholar] [CrossRef]
- Göritz, C.; Dias, D.O.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisén, J. A Pericyte Origin of Spinal Cord Scar Tissue. Science 2011, 333, 238–242. [Google Scholar]
- Soderblom, C.; Luo, X.; Blumenthal, E.; Bray, E.; Lyapichev, K.; Ramos, J.; Krishnan, V.; Lai-Hsu, C.; Park, K.K.; Tsoulfas, P.; et al. Perivascular Fibroblasts Form the Fibrotic Scar after Contusive Spinal Cord Injury. J. Neurosci. 2013, 33, 13882–13887. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.O.; Kim, H.; Holl, D.; Solnestam, B.W.; Lundeberg, J.; Carlén, M.; Göritz, C.; Frisén, J. Reducing Pericyte-Derived Scarring Promotes Recovery after Spinal Cord Injury. Cell 2018, 173, 153–165.e22. [Google Scholar] [CrossRef]
- Hellal, F.; Hurtado, A.; Ruschel, J.; Flynn, K.C.; Laskowski, C.J.; Umlauf, M.; Kapitein, L.C.; Strikis, D.; Lemmon, V.; Bixby, J.; et al. Microtubule Stabilization Reduces Scarring and Causes Axon Regeneration after Spinal Cord Injury. Science 2011, 331, 928–931. [Google Scholar] [CrossRef]
- Ruschel, J.; Hellal, F.; Flynn, K.C.; Dupraz, S.; Elliott, D.A.; Tedeschi, A.; Bates, M.; Sliwinski, C.; Brook, G.; Dobrindt, K.; et al. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 2015, 348, 347–352. [Google Scholar] [CrossRef]
- Stichel, C.C.; Hermanns, S.; Luhmann, H.J.; Lausberg, F.; Niermann, H.; D’Urso, D.; Servos, G.; Hartwig, H.-G.; Müller, H.W. Inhibition of collagen IV deposition promotes regeneration of injured CNS axons. Eur. J. Neurosci. 1999, 11, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Klapka, N.; Hermanns, S.; Straten, G.; Masanneck, C.; Duis, S.; Hamers, F.P.T.; Müller, D.; Zuschratter, W.; Müller, H.W. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur. J. Neurosci. 2005, 22, 3047–3058. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, E.J.; Moon, L.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nat. Cell Biol. 2002, 416, 636–640. [Google Scholar] [CrossRef]
- Tsata, V.; Möllmert, S.; Schweitzer, C.; Kolb, J.; Möckel, C.; Böhm, B.; Rosso, G.; Lange, C.; Lesche, M.; Hammer, J.; et al. A switch in pdgfrb cell-derived ECM composition prevents inhibitory scarring and promotes axon regeneration in the zebrafish spinal cord. Dev. Cell 2021, 56, 509–524.e9. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; Cahill, M.A.; Stuermer, C.A.O. Fibroblasts at the transection site of the injured goldfish optic nerve and their potential role during retinal axonal regeneration. J. Comp. Neurol. 1995, 360, 599–631. [Google Scholar] [CrossRef][Green Version]
- Muhl, L.; Genové, G.; Leptidis, S.; Liu, J.; He, L.; Mocci, G.; Sun, Y.; Gustafsson, S.; Buyandelger, B.; Chivukula, I.V.; et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat. Commun. 2020, 11, 3953. [Google Scholar] [CrossRef]
- Currie, J.D.; Kawaguchi, A.; Traspas, R.M.; Schuez, M.; Chara, O.; Tanaka, E.M. Live Imaging of Axolotl Digit Regeneration Reveals Spatiotemporal Choreography of Diverse Connective Tissue Progenitor Pools. Dev. Cell 2016, 39, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.S.; Hoi, K.K.; Phan, T.M.; Ray, C.A.; Berndt, J.D.; Moon, R.T. Wnt/β-catenin signaling promotes regeneration after adult zebrafish spinal cord injury. Biochem. Biophys. Res. Commun. 2016, 477, 952–956. [Google Scholar] [CrossRef]
- Briona, L.K.; Poulain, F.E.; Mosimann, C.; Dorsky, R.I. Wnt/ß-catenin signaling is required for radial glial neurogenesis following spinal cord injury. Dev. Biol. 2015, 403, 15–21. [Google Scholar] [CrossRef]
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.; Fawcett, J.; Franze, K. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017, 8, 14787. [Google Scholar] [CrossRef]
- Koser, D.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.; Sheridan, G.K.; Svoboda, H.; Viana, M.; Costa, L.D.F.; Guck, J.; et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016, 19, 1592–1598. [Google Scholar] [CrossRef]
- Möllmert, S.; Kharlamova, M.A.; Hoche, T.; Taubenberger, A.V.; Abuhattum, S.; Kuscha, V.; Kurth, T.; Brand, M.; Guck, J. Zebrafish Spinal Cord Repair Is Accompanied by Transient Tissue Stiffening. Biophys. J. 2020, 118, 448–463. [Google Scholar] [CrossRef]
- Cho, Y.; Sloutsky, R.; Naegle, K.M.; Cavalli, V. Injury-Induced HDAC5 Nuclear Export Is Essential for Axon Regeneration. Cell 2013, 155, 894–908. [Google Scholar] [CrossRef]
- Plunet, W.; Kwon, B.K.; Tetzlaff, W. Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy. J. Neurosci. Res. 2002, 68, 1–6. [Google Scholar] [CrossRef]
- Park, K.K.; Liu, K.; Hu, Y.; Smith, P.D.; Wang, C.; Cai, B.; Xu, B.; Connolly, L.; Kramvis, I.; Sahin, M.; et al. Promoting Axon Regeneration in the Adult CNS by Modulation of the PTEN/mTOR Pathway. Sci. 2008, 322, 963–966. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Bu, X.; Wei, Y.; Lou, X. The major vault protein is dispensable for zebrafish organ regeneration. Heliyon 2020, 6, e05422. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.-C.; Lin, J.-F.; Ma, L.-P.; Shen, Y.-Q.; Schachner, M. Major vault protein promotes locomotor recovery and regeneration after spinal cord injury in adult zebrafish. Eur. J. Neurosci. 2012, 37, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Zhou, Z.-L.; Hao, Q.; Zhao, L.; Cui, C.; Huang, S.-B.; Yang, Y.-L.; Shen, Y.-Q. Activating Transcription Factor 6 Contributes to Functional Recovery After Spinal Cord Injury in Adult Zebrafish. J. Mol. Neurosci. 2021, 71, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.-X.; Yao, L.; Cui, C.; Zhao, H.-D.; Liu, C.-J.; Li, Y.-H.; Wang, L.-F.; Huang, S.-B.; Shen, Y.-Q. Semaphorin4D promotes axon regrowth and swimming ability during recovery following zebrafish spinal cord injury. Neuroscience 2017, 351, 36–46. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, M.; Hu, B.; Huang, R.; Hu, B. In vivo Imaging of Mitochondrial Transport in Single-Axon Regeneration of Zebrafish Mauthner Cells. Front. Cell. Neurosci. 2017, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Xie, Y.; Ordaz, J.D.; Huh, A.J.; Huang, N.; Wu, W.; Liu, N.; Chamberlain, K.A.; Sheng, Z.-H.; Xu, X.-M. Restoring Cellular Energetics Promotes Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Cell Metab. 2020, 31, 623–641.e8. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-Q.; Chen, M.; Ren, D.-L.; Hu, B. Dual Oxidase Mutant Retards Mauthner-Cell Axon Regeneration at an Early Stage via Modulating Mitochondrial Dynamics in Zebrafish. Neurosci. Bull. 2020, 36, 1500–1512. [Google Scholar] [CrossRef]
- Bremer, J.; Marsden, K.C.; Miller, A.; Granato, M. The ubiquitin ligase PHR promotes directional regrowth of spinal zebrafish axons. Commun. Biol. 2019, 2, 195. [Google Scholar] [CrossRef]
- Hans, S.; Zöller, D.; Hammer, J.; Stucke, J.; Spieß, S.; Kesavan, G.; Kroehne, V.; Eguiguren, J.S.; Ezhkova, D.; Petzold, A.; et al. Cre-Controlled CRISPR mutagenesis provides fast and easy conditional gene inactivation in zebrafish. Nat. Commun. 2021, 12, 1125. [Google Scholar] [CrossRef]
- Wehner, D.; Jahn, C.; Weidinger, G. Use of the TetON System to Study Molecular Mechanisms of Zebrafish Regeneration. J. Vis. Exp. 2015, 2015, e52756. [Google Scholar] [CrossRef] [PubMed]
- Knopf, F.; Schnabel, K.; Haase, C.; Pfeifer, K.; Anastassiadis, K.; Weidinger, G. Dually inducible TetON systems for tissue-specific conditional gene expression in zebrafish. Proc. Natl. Acad. Sci. USA 2010, 107, 19933–19938. [Google Scholar] [CrossRef]
- Campbell, L.J.; Willoughby, J.J.; Jensen, A.M. Two Types of Tet-On Transgenic Lines for Doxycycline-Inducible Gene Expression in Zebrafish Rod Photoreceptors and a Gateway-Based Tet-On Toolkit. PLoS ONE 2012, 7, e51270. [Google Scholar] [CrossRef]
- Lange, C.; Rost, F.; Machate, A.; Reinhardt, S.; Lesche, M.; Weber, A.; Kuscha, V.; Dahl, A.; Rulands, S.; Brand, M. Single cell sequencing of radial glia progeny reveals diversity of newborn neurons in the adult zebrafish brain. Development 2020, 147, 1855951. [Google Scholar] [CrossRef] [PubMed]
- Raj, B.; Wagner, D.E.; McKenna, A.; Pandey, S.; Klein, A.M.; Shendure, J.; Gagnon, J.A.; Schier, A.F. Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat. Biotechnol. 2018, 36, 442–450. [Google Scholar] [CrossRef]
- Schlüßler, R.; Möllmert, S.; Abuhattum, S.; Cojoc, G.; Müller, P.; Kim, K.; Möckel, C.; Zimmermann, C.; Czarske, J.; Guck, J. Mechanical Mapping of Spinal Cord Growth and Repair in Living Zebrafish Larvae by Brillouin Imaging. Biophys. J. 2018, 115, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Chapela, D.; Sousa, S.; Martins, I.; Cristóvão, A.M.; Pinto, P.; Corte-Real, S.; Saúde, L. A zebrafish drug screening platform boosts the discovery of novel therapeutics for spinal cord injury in mammals. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Early, J.J.; Marshall-Phelps, K.L.; Williamson, J.M.; Swire, M.; Kamadurai, H.; Muskavitch, M.; Lyons, D.A. An automated high-resolution in vivo screen in zebrafish to identify chemical regulators of myelination. eLife 2018, 7, e35136. [Google Scholar] [CrossRef]
- Tica, J.; Didangelos, A. Comparative Transcriptomics of Rat and Axolotl After Spinal Cord Injury Dissects Differences and Similarities in Inflammatory and Matrix Remodeling Gene Expression Patterns. Front. Neurosci. 2018, 12, 808. [Google Scholar] [CrossRef] [PubMed]
 |  | |||||
|---|---|---|---|---|---|---|
| Cell Type | Mechanisms of Growth-Modulation Inhibitory; Inhibitory; 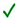 Promoting/Non-Inhibitory; Promoting/Non-Inhibitory; 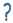 Unknown Unknown | Reference | ||||
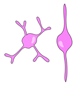 | astrocyte glia/ ERG |  | glial scarring; secretion of inhibitory ECM |  | glial bridging; physical and trophic support (?) | [23,25,31,79,81,88] |
 | fibroblast |  | fibrous scarring; secretion of inhibitory ECM |  | no fibrous scarring; secretion of growth- promoting ECM | [97,98,99,100,103,104,106] |
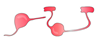 | OPC/ oligodendrocyte |  | growth cone entrapment; low remyelination, secretion of inhibitory factors, formation of dystrophic endbulbs |  | no growth cone entrapment (?); remyelination (?), secretion of growth- promoting factors (?) | [40,71,72,73,76] |
 | microglia |  | prolonged inflammation; secretion of pro-inflammatory cytokines |  | limited inflammation; phagocytosis, debris removal | [53,54,59] |
 | macrophage/ neutrophil/ T-cell |  | prolonged inflammation; secretion of pro-inflammatory cytokines |  | limited inflammation; phagocytosis, secretion of growth- promoting factors (?) | [24,55,56,57,60,61] |
 | mechanical properties |  | mechanical barrier |  | growth-permissive mechanical tissue properties; tissue stiffening | [112,114] |
 | neuron-intrinsic factors |  | limited growth capacity; failure of growth cone formation and neurite extension |  | elevated growth capacity (?); upregulation of growth-promoting molecules | [22,28,34,36,115,116,117,118,119,120,121,122,123,124,125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsata, V.; Wehner, D. Know How to Regrow—Axon Regeneration in the Zebrafish Spinal Cord. Cells 2021, 10, 1404. https://doi.org/10.3390/cells10061404
Tsata V, Wehner D. Know How to Regrow—Axon Regeneration in the Zebrafish Spinal Cord. Cells. 2021; 10(6):1404. https://doi.org/10.3390/cells10061404
Chicago/Turabian StyleTsata, Vasiliki, and Daniel Wehner. 2021. "Know How to Regrow—Axon Regeneration in the Zebrafish Spinal Cord" Cells 10, no. 6: 1404. https://doi.org/10.3390/cells10061404
APA StyleTsata, V., & Wehner, D. (2021). Know How to Regrow—Axon Regeneration in the Zebrafish Spinal Cord. Cells, 10(6), 1404. https://doi.org/10.3390/cells10061404






