Structural and Functional Modulation of Perineuronal Nets: In Search of Important Players with Highlight on Tenascins
Abstract
1. Introduction
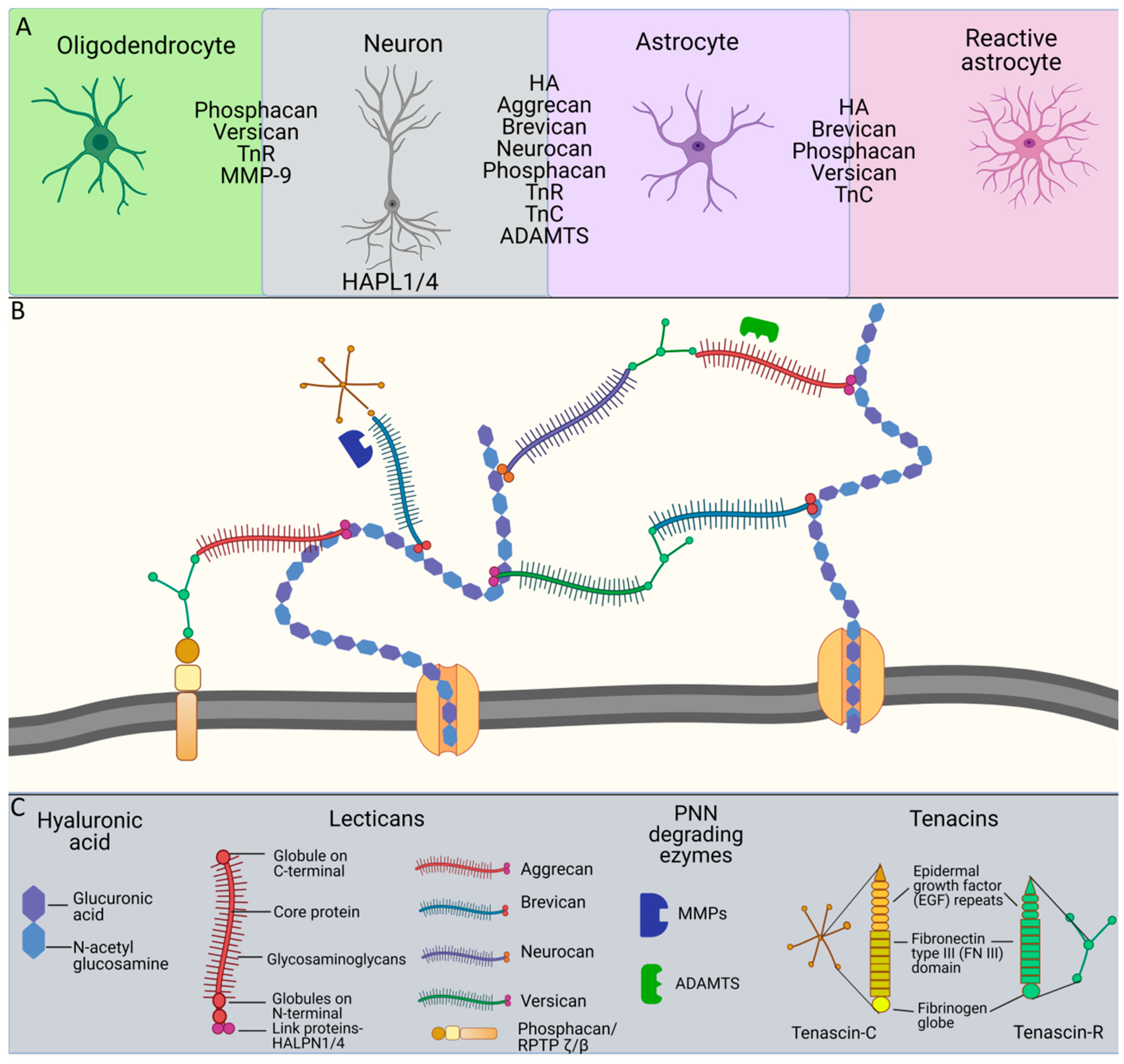
2. Molecular Organization of PNN Structure and Function
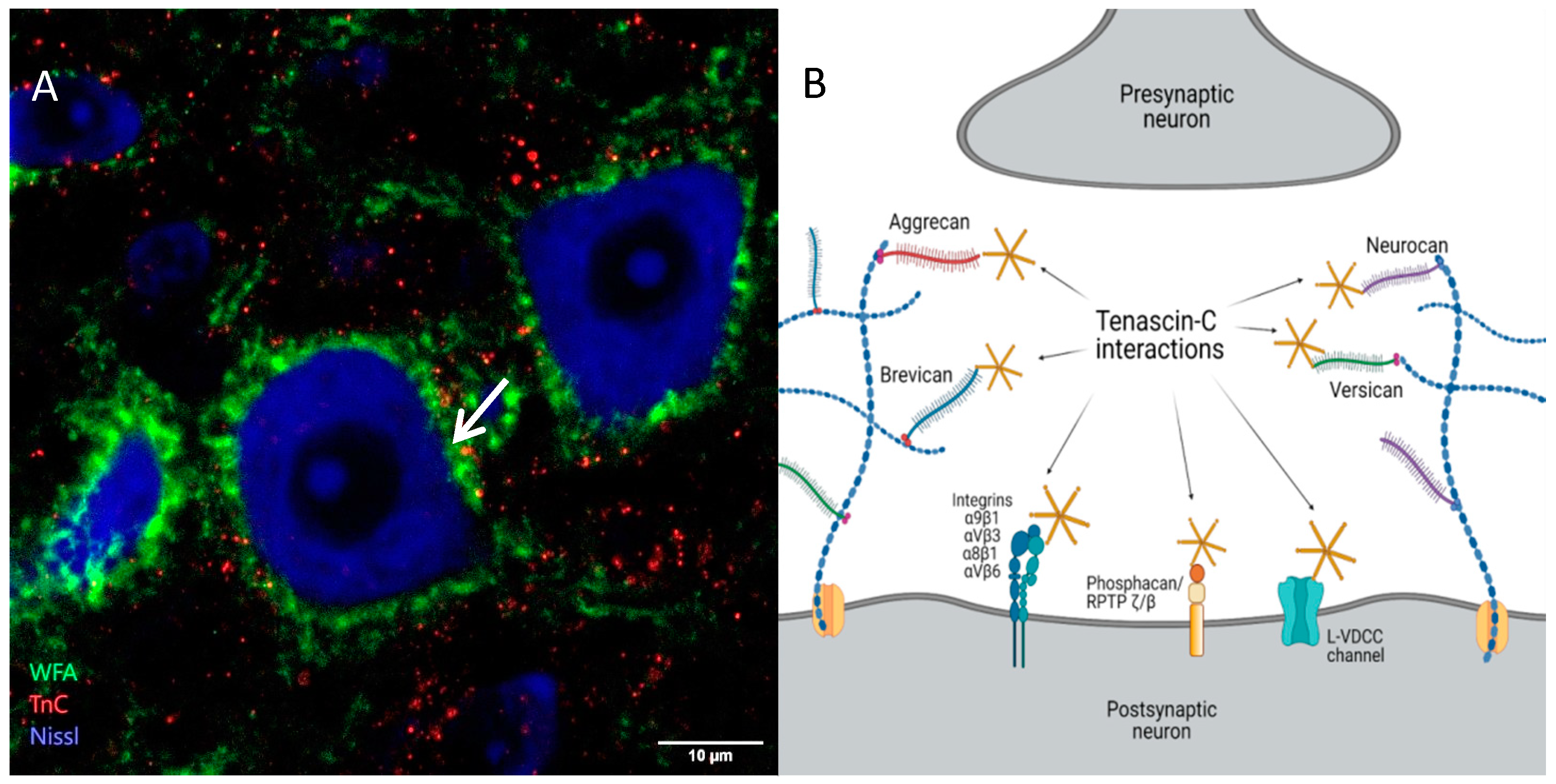
3. Tenascins: Main Properties and Interrelation with Perineuronal Nets
4. Functions of Perineuronal Nets in Neuronal Plasticity
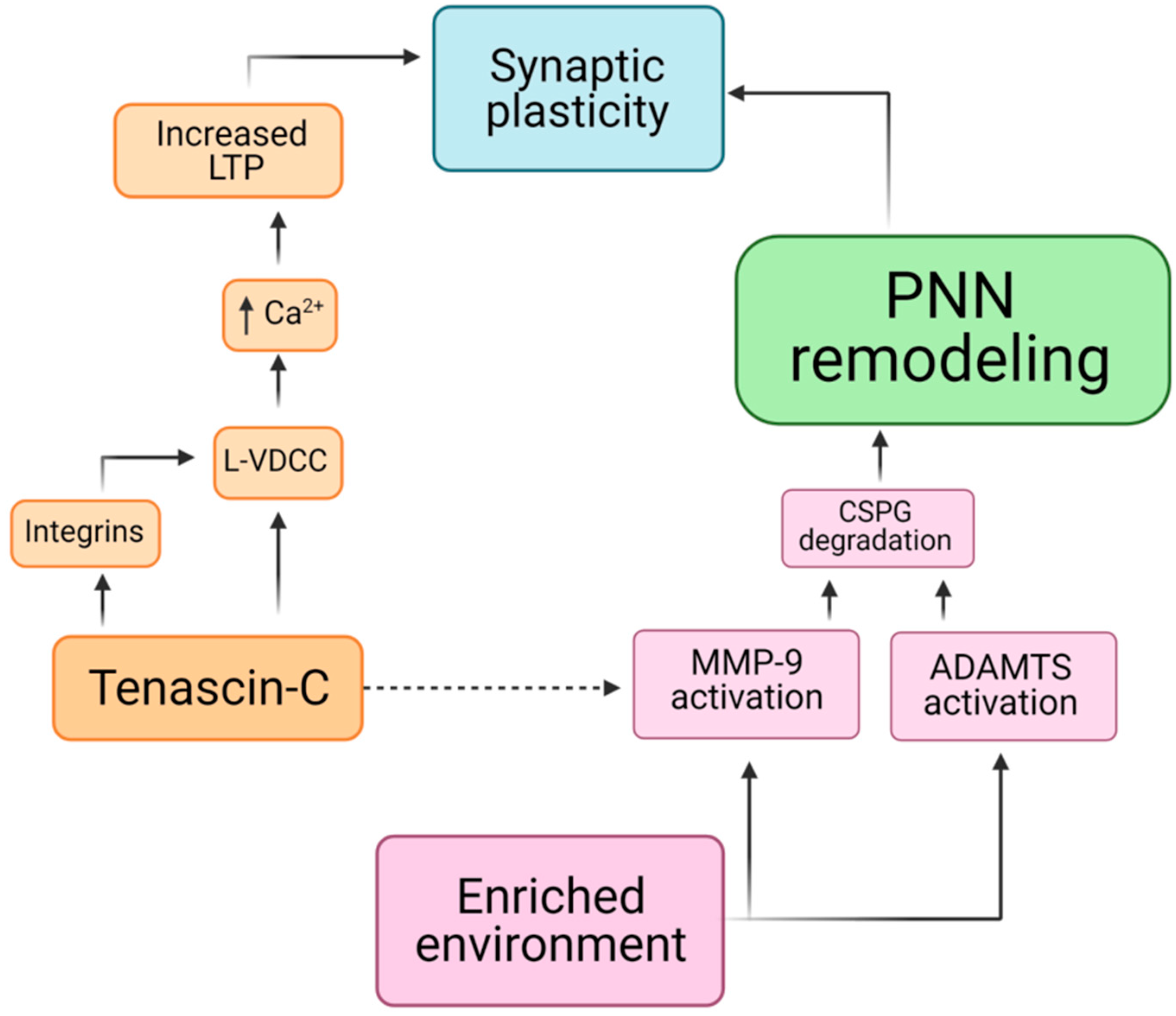
5. Remodeling of Perineuronal Nets
6. Perineuronal Nets and Tenascin-C in Mechanotransduction
7. Perineuronal Nets and Tenascin-C in Regulation of Neurogenesis
8. Perineuronal Nets Visualization Techniques and Ultrastructure Analysis
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Golgi, C. Intorno Alla Struttura Della Cellula Nervosa. Boll. Della Soc. Med. -Chir. Di Pavia 1898, 13, 316. [Google Scholar]
- Celio, M.R.; Spreafico, R.; De Biasi, S.; Vitellaro-Zuccarello, L. Perineuronal Nets: Past and Present. Trends Neurosci. 1998, 21, 510–515. [Google Scholar] [CrossRef]
- Ruoslahti, E. Brain Extracellular Matrix. Glycobiology 1996, 6, 489–492. [Google Scholar] [CrossRef]
- Yamada, J.; Ohgomori, T.; Jinno, S. Perineuronal Nets Affect Parvalbumin Expression in GABAergic Neurons of the Mouse Hippocampus. Eur. J. Neurosci. 2015, 41, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Carceller, H.; Guirado, R.; Ripolles-Campos, E.; Teruel-Marti, V.; Nacher, J. Perineuronal Nets Regulate the Inhibitory Perisomatic Input onto Parvalbumin Interneurons and γ Activity in the Prefrontal Cortex. J. Neurosci. 2020, 40, 5008–5018. [Google Scholar] [CrossRef]
- Wang, D.; Fawcett, J. The Perineuronal Net and the Control of Cns Plasticity. Cell Tissue Res. 2012, 349, 147–160. [Google Scholar] [CrossRef]
- Brückner, G.; Brauer, K.; Härtig, W.; Wolff, J.R.; Rickmann, M.J.; Derouiche, A.; Delpech, B.; Girard, N.; Oertel, W.H.; Reichenbach, A. Perineuronal Nets Provide a Polyanionic, Glia-associated Form of Microenvironment around Certain Neurons in Many Parts of the Rat Brain. Glia 1993, 8, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Balmer, T.S. Perineuronal Nets Enhance the Excitability of Fast-Spiking Neurons. eNeuro 2016, 3, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Suttkus, A.; Holzer, M.; Morawski, M.; Arendt, T. The Neuronal Extracellular Matrix Restricts Distribution and Internalization of Aggregated Tau-Protein. Neuroscience 2016, 313, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Morawski, M.; Arendt, T.; Butz, T. Quantitative Microanalysis of Perineuronal Nets in Brain Tissue. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 210, 395–400. [Google Scholar] [CrossRef]
- Martín-De-Saavedra, M.D.; Del Barrio, L.; Cañas, N.; Egea, J.; Lorrio, S.; Montell, E.; Vergés, J.; García, A.G.; López, M.G. Chondroitin Sulfate Reduces Cell Death of Rat Hippocampal Slices Subjected to Oxygen and Glucose Deprivation by Inhibiting P38, NFκB and INOS. Neurochem. Int. 2011, 58, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Cabungcal, J.H.; Steullet, P.; Morishita, H.; Kraftsik, R.; Cuenod, M.; Hensch, T.K.; Do, K.Q. Perineuronal Nets Protect Fast-Spiking Interneurons against Oxidative Stress. Proc. Natl. Acad. Sci. USA 2013, 110, 9130–9135. [Google Scholar] [CrossRef] [PubMed]
- Soleman, S.; Filippov, M.A.; Dityatev, A.; Fawcett, J.W. Targeting the Neural Extracellular Matrix in Neurological Disorders. Neuroscience 2013, 253, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Mauney, S.A.; Athanas, K.M.; Pantazopoulos, H.; Shaskan, N.; Passeri, E.; Berretta, S.; Woo, T.U.W. Developmental Pattern of Perineuronal Nets in the Human Prefrontal Cortex and Their Deficit in Schizophrenia. Biol. Psychiatry 2013, 74, 427–435. [Google Scholar] [CrossRef]
- Slaker, M.; Barnes, J.; Sorg, B.A.; Grimm, J.W. Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex Following Early and Late Abstinence from Sucrose Self-Administration in Rats. PLoS ONE 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.H.; Lensjø, K.K.; Wigestrand, M.B.; Malthe-Sørenssen, A.; Hafting, T.; Fyhn, M. Removal of Perineuronal Nets Disrupts Recall of a Remote Fear Memory. Proc. Natl. Acad. Sci. USA 2018, 115, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, A.C.; Hare, D.J.; Bussey, T.J.; Saksida, L.M. Perineuronal Nets: Plasticity, Protection, and Therapeutic Potential. Trends Neurosci. 2019, 42, 458–470. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Calabrese, E.J.; Mattson, M.P. Cellular Stress Responses, the Hormesis Paradigm, and Vitagenes: Novel Targets for Therapeutic Intervention in Neurodegenerative Disorders. Antioxid. Redox Signal. 2010, 13, 1763–1811. [Google Scholar] [CrossRef]
- Härtig, W.; Brauer, K.; Brückner, G. Wisteria Floribunda Agglutinin-Labelled Nets Surround Parvalbumin-Containing Neurons. Neuroreport 1992, 3, 869–872. [Google Scholar] [CrossRef]
- Carstens, K.E.; Phillips, M.L.; Pozzo-Miller, L.; Weinberg, R.J.; Dudek, S.M. Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons. J. Neurosci. 2016, 36, 6312–6320. [Google Scholar] [CrossRef] [PubMed]
- Bignami, A.; Hosley, M.; Dahl, D. Hyaluronic Acid and Hyaluronic Acid-Binding Proteins in Brain Extracellular Matrix. Anat. Embryol. (Berl). 1993, 188, 419–433. [Google Scholar] [CrossRef]
- Morawski, M.; Dityatev, A.; Hartlage-Rübsamen, M.; Blosa, M.; Holzer, M.; Flach, K.; Pavlica, S.; Dityateva, G.; Dityateva, G.; Brückner, G.; et al. Tenascin-R Promotes Assembly of the Extracellular Matrix of Perineuronal Nets via Clustering of Aggrecan. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20140046. [Google Scholar] [CrossRef]
- Oohashi, T.; Edamatsu, M.; Bekku, Y.; Carulli, D. The Hyaluronan and Proteoglycan Link Proteins: Organizers of the Brain Extracellular Matrix and Key Molecules for Neuronal Function and Plasticity. Exp. Neurol. 2015, 274, 134–144. [Google Scholar] [CrossRef]
- Yamaguchi, Y. Lecticans: Organizers of the Brain Extracellular Matrix. Cell. Mol. Life Sci. 2000, 57, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Bandtlow, C.E.; Zimmermann, D.R. Proteoglycans in the Developing Brain: New Conceptual Insights for Old Proteins. Physiol. Rev. 2000, 80, 1267–1290. [Google Scholar] [CrossRef] [PubMed]
- Giamanco, K.A.; Morawski, M.; Matthews, R.T. Perineuronal Net Formation and Structure in Aggrecan Knockout Mice. Neuroscience 2010, 170, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.; Lensjø, K.K.; Dinh, T.; Yang, S.; Andrews, M.R.; Hafting, T.; Fyhn, M.; Fawcett, J.W.; Dick, G. Aggrecan Directs Extracellular Matrix-Mediated Neuronal Plasticity. J. Neurosci. 2018, 38, 10102–10113. [Google Scholar] [CrossRef]
- Giamanco, K.A.; Matthews, R.T. Deconstructing the Perineuronal Net: Cellular Contributions and Molecular Composition of the Neuronal Extracellular Matrix. Neuroscience 2012, 218, 367–384. [Google Scholar] [CrossRef]
- Favuzzi, E.; Marques-Smith, A.; Deogracias, R.; Winterflood, C.M.; Sánchez-Aguilera, A.; Mantoan, L.; Maeso, P.; Fernandes, C.; Ewers, H.; Rico, B. Activity-Dependent Gating of Parvalbumin Interneuron Function by the Perineuronal Net Protein Brevican. Neuron 2017, 95, 639–655. [Google Scholar] [CrossRef]
- Gottschling, C.; Wegrzyn, D.; Denecke, B.; Faissner, A. Elimination of the Four Extracellular Matrix Molecules Tenascin-C, Tenascin-R, Brevican and Neurocan Alters the Ratio of Excitatory and Inhibitory Synapses. Sci. Rep. 2019, 9, 1–17. [Google Scholar] [CrossRef]
- Eill, G.J.; Sinha, A.; Morawski, M.; Viapiano, M.S.; Matthews, R.T. The Protein Tyrosine Phosphatase RPTPξ/Phosphacan Is Critical for Perineuronal Net Structure. J. Biol. Chem. 2020, 295, 955–968. [Google Scholar] [CrossRef]
- Miyata, S.; Nishimura, Y.; Hayashi, N.; Oohira, A. Construction of Perineuronal Net-like Structure by Cortical Neurons in Culture. Neuroscience 2005, 136, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.C.F.; Carulli, D.; Fawcett, J.W. In Vitro Modeling of Perineuronal Nets: Hyaluronan Synthase and Link Protein Are Necessary for Their Formation and Integrity. J. Neurochem. 2010, 114, 1447–1459. [Google Scholar] [CrossRef]
- Celio, M.R.; Chiquet-Ehrismann, R. “Perineuronal Nets” around Cortical Interneurons Expressing Parvalbumin Are Rich in Tenascin. Neurosci. Lett. 1993, 162, 137–140. [Google Scholar] [CrossRef]
- Grumet, M.; Milev, P.; Sakurai, T.; Karthikeyan, L.; Bourdon, M.; Margolis, R.K.; Margolis, R.U. Interactions with Tenascin and Differential Effects on Cell Adhesion of Neurocan and Phosphacan, Two Major Chondroitin Sulfate Proteoglycans of Nervous Tissue. J. Biol. Chem. 1994, 269, 12142–12146. [Google Scholar] [CrossRef]
- Day, J.M.; Olin, A.I.; Murdoch, A.D.; Canfield, A.; Sasaki, T.; Timpl, R.; Hardingham, T.E.; Aspberg, A. Alternative Splicing in the Aggrecan G3 Domain Influences Binding Interactions with Tenascin-C and Other Extracellular Matrix Proteins. J. Biol. Chem. 2004, 279, 12511–12518. [Google Scholar] [CrossRef] [PubMed]
- Milev, P.; Fischer, D.; Häring, M.; Schulthess, T.; Margolis, R.K.; Chiquet-Ehrismann, R.; Margolis, R.U. The Fibrinogen-like Globe of Tenascin-C Mediates Its Interactions with Neurocan and Phosphacan/Protein-Tyrosine Phosphatase-ζ/β. J. Biol. Chem. 1997, 272, 15501–15509. [Google Scholar] [CrossRef] [PubMed]
- Rauch, U.; Clement, A.; Retzler, C.; Fröhlich, L.; Fässler, R.; Göhring, W.; Faissner, A. Mapping of a Defined Neurocan Binding Site to Distinct Domains of Tenascin-C. J. Biol. Chem. 1997, 272, 26905–26912. [Google Scholar] [CrossRef] [PubMed]
- Götz, B.; Scholze, A.; Clement, A.; Joester, A.; Schütte, K.; Wigger, F.; Frank, R.; Spiess, E.; Ekblom, P.; Faissner, A. Tenascin-C Contains Distinct Adhesive, Anti-Adhesive, and Neurite Outgrowth Promoting Sites for Neurons. J. Cell Biol. 1996, 132, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Yokosaki, Y.; Matsuura, N.; Higashiyama, S.; Murakami, I.; Obara, M.; Yamakido, M.; Shigeto, N.; Chen, J.; Sheppard, D. Identification of the Ligand Binding Site for the Integrin A9β1 in the Third Fibronectin Type III Repeat of Tenascin-C. J. Biol. Chem. 1998, 273, 11423–11428. [Google Scholar] [CrossRef]
- Schnapp, L.M.; Hatch, N.; Ramos, D.M.; Klimanskaya, I.V.; Sheppard, D.; Pytela, R. The Human Integrin A8β1 Functions as a Receptor for Tenascin, Fibronectin, and Vitronectin. J. Biol. Chem. 1995, 270, 23196–23202. [Google Scholar] [CrossRef]
- Andrews, M.R.; Czvitkovich, S.; Dassie, E.; Vogelaar, C.F.; Faissner, A.; Blits, B.; Gage, F.H.; Ffrench-Constant, C.; Fawcett, J.W. A9 Integrin Promotes Neurite Outgrowth on Tenascin-C and Enhances Sensory Axon Regeneration. J. Neurosci. 2009, 29, 5546–5557. [Google Scholar] [CrossRef]
- Maurel, P.; Rauch, U.; Flad, M.; Margolis, R.K.; Margolis, R.U. Phosphacan, a Chondroitin Sulfate Proteoglycan of Brain That Interacts with Neurons and Neural Cell-Adhesion Molecules, Is an Extracellular Variant of a Receptor-Type Protein Tyrosine Phosphatase. Proc. Natl. Acad. Sci. 1994, 91, 2512–2516. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, U. The Extracellular Matrix Molecule Tenascin-C: Expression In Vivo and Functional Characterization In Vitro. Prog. Neurobiol. 1996, 49, 145–161. [Google Scholar] [CrossRef]
- Brückner, G.; Grosche, J.; Schmidt, S.; Härtig, W.; Margolis, R.U.; Delpech, B.; Seidenbecher, C.I.; Czaniera, R.; Schachner, M. Postnatal Development of Perineuronal Nets in Wild-Type Mice and in a Mutant Deficient in Tenascin-R. J. Comp. Neurol. 2000, 428, 616–629. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular Matrix Assembly: A Multiscale Deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Brodkey, J.A.; Laywell, E.D.; O’Brien, T.F.; Faissner, A.; Stefansson, K.; Dörries, H.U.; Schachner, M.; Steindler, D.A. Focal Brain Injury and Upregulation of a Developmentally Regulated Extracellular Matrix Protein. J. Neurosurg. 1995, 82, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, L.; Chevassus Au Louis, N.; Jorquera, I.; Niquet, J.; Khrestchatisky, M.; Ben-Ari, Y.; Represa, A. Transient Increase of Tenascin-C in Immature Hippocampus: Astroglial and Neuronal Expression. J. Neurocytol. 1996, 25, 53–66. [Google Scholar] [CrossRef]
- Theodosis, D.T.; Pierre, K.; Cadoret, M.A.; Allard, M.; Faissner, A.; Poulain, D.A. Expression of High Levels of the Extracellular Matrix Glycoprotein, Tenascin-C, in the Normal Adult Hypothalamoneurohypophysial System. J. Comp. Neurol. 1997, 379, 386–398. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Stamenkovic, S.; Jaworski, T.; Gawlak, M.; Jovanovic, M.; Jakovcevski, I.; Wilczynski, G.M.; Kaczmarek, L.; Schachner, M.; Radenovic, L.; et al. The Extracellular Matrix Glycoprotein Tenascin-C and Matrix Metalloproteinases Modify Cerebellar Structural Plasticity by Exposure to an Enriched Environment. Brain Struct. Funct. 2017, 222, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, B.; Faissner, A.; Beck, H.; Behle, K.; Wolf, H.K.; Wiestler, O.D.; Blümcke, I. Hippocampal Loss of Tenascin Boundaries in Ammon’s Horn Sclerosis. Glia 1997, 19, 35–46. [Google Scholar] [CrossRef]
- Haunsoø, A.; Ibrahim, M.; Bartsch, U.; Letiembre, M.; Celio, M.R.; Menoud, P.A. Morphology of Perineuronal Nets in Tenascin-R and Parvalbumin Single and Double Knockout Mice. Brain Res. 2000, 864, 142–145. [Google Scholar] [CrossRef]
- Hagihara, K.; Miura, R.; Kosaki, R.; Berglund, E.; Ranscht, B.; Yamaguchi, Y. Immunohistochemical Evidence for the Brevican-Tenascin-R Interaction: Colocalization in Perineuronal Nets Suggests a Physiological Role for the Interaction in the Adult Rat Brain. J. Comp. Neurol. 1999, 410, 256–264. [Google Scholar] [CrossRef]
- Fukamauchi, F.; Mataga, N.; Wang, Y.J.; Sato, S.; Yoshiki, A.; Kusakabe, M. Abnormal Behavior and Neurotransmissions of Tenascin Gene Knockout Mouse. Biochem. Biophys. Res. Commun. 1996, 221, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.R.; Salmen, B.; Bukalo, O.; Rollenhagen, A.; Bösl, M.R.; Morellini, F.; Bartsch, U.; Dityatev, A.; Schachner, M. Impairment of L-Type Ca2+ Channel-Dependent Forms of Hippocampal Synaptic Plasticity in Mice Deficient in the Extracellular Matrix Glycoprotein Tenascin-C. J. Neurosci. 2002, 22, 7177–7194. [Google Scholar] [CrossRef] [PubMed]
- Morellini, F.; Schachner, M. Enhanced Novelty-Induced Activity, Reduced Anxiety, Delayed Resynchronization to Daylight Reversal and Weaker Muscle Strength in Tenascin-C-Deficient Mice. Eur. J. Neurosci. 2006, 23, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.; Milenkovic, I.; Galjak, N.; Todorovic, V.; Andjus, P. Enriched Environment Alters the Behavioral Profile of Tenascin-C Deficient Mice. Behav. Brain Res. 2017, 331, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Irintchev, A.; Rollenhagen, A.; Troncoso, E.; Kiss, J.Z.; Schachner, M. Structural and Functional Aberrations in the Cerebral Cortex of Tenascin-C Deficient Mice. Cereb. Cortex 2005, 15, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Gurevicius, K.; Kuang, F.; Stoenica, L.; Irintchev, A.; Gureviciene, I.; Dityatev, A.; Schachner, M.; Tanila, H. Genetic Ablation of Tenascin-C Expression Leads to Abnormal Hippocampal CA1 Structure and Electrical Activity In Vivo. Hippocampus 2009, 19, 1232–1246. [Google Scholar] [CrossRef] [PubMed]
- Šekeljić, V.; Andjus, P.R. Tenascin-C and Its Functions in Neuronal Plasticity. Int. J. Biochem. Cell Biol. 2012, 44. [Google Scholar] [CrossRef]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef]
- Lledo, P.M.; Alonso, M.; Grubb, M.S. Adult Neurogenesis and Functional Plasticity in Neuronal Circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Mangina, C.A.; Sokolov, E.N. Neuronal Plasticity in Memory and Learning Abilities: Theoretical Position and Selective Review. Int. J. Psychophysiol. 2006, 60, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Navarro, X.; Vivó, M.; Valero-Cabré, A. Neural Plasticity after Peripheral Nerve Injury and Regeneration. Prog. Neurobiol. 2007, 82, 163–201. [Google Scholar] [CrossRef]
- Hensch, T.K. Controlling the Critical Period. Neurosci. Res. 2003, 47, 17–22. [Google Scholar] [CrossRef]
- Pizzorusso, T.; Medini, P.; Berardi, N.; Chierzi, S.; Fawcett, J.W.; Maffei, L. Reactivation of Ocular Dominance Plasticity in the Adult Visual Cortex. Science 2002, 298, 1248–1251. [Google Scholar] [CrossRef]
- Travaglia, A.; Steinmetz, A.B.; Miranda, J.M.; Alberini, C.M. Mechanisms of Critical Period in the Hippocampus Underlie Object Location Learning and Memory in Infant Rats. Learn. Mem. 2018, 25, 176–182. [Google Scholar] [CrossRef]
- Lensjø, K.K.; Christensen, A.C.; Tennøe, S.; Fyhn, M.; Hafting, T. Differential Expression and Cell-Type Specificity of Perineuronal Nets in Hippocampus, Medial Entorhinal Cortex, and Visual Cortex Examined in the Rat and Mouse. eNeuro 2017, 4, 1–18. [Google Scholar] [CrossRef]
- Beurdeley, M.; Spatazza, J.; Lee, H.H.C.; Sugiyama, S.; Bernard, C.; Di Nardo, A.A.; Hensch, T.K.; Prochiantz, A. Otx2 Binding to Perineuronal Nets Persistently Regulates Plasticity in the Mature Visual Cortex. J. Neurosci. 2012, 32, 9429–9437. [Google Scholar] [CrossRef]
- Bernard, C.; Prochiantz, A. Otx2-PNN Interaction to Regulate Cortical Plasticity. Neural Plast. 2016, 2016. [Google Scholar] [CrossRef]
- Mix, A.; Hoppenrath, K.; Funke, K. Reduction in Cortical Parvalbumin Expression Due to Intermittent Theta-Burst Stimulation Correlates with Maturation of the Perineuronal Nets in Young Rats. Dev. Neurobiol. 2015, 75. [Google Scholar] [CrossRef]
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and Function of Dendritic Spines. Annu. Rev. Physiol. 2002, 64, 313–353. [Google Scholar] [CrossRef]
- Dino, M.R.; Harroch, S.; Hockfield, S.; Matthews, R.T. Monoclonal Antibody Cat-315 Detects a Glycoform of Receptor Protein Tyrosine Phosphatase Beta/Phosphacan Early in CNS Development That Localizes to Extrasynaptic Sites Prior to Synapse Formation. Neuroscience 2006, 142, 1055–1069. [Google Scholar] [CrossRef]
- De Vivo, L.; Landi, S.; Panniello, M.; Baroncelli, L.; Chierzi, S.; Mariotti, L.; Spolidoro, M.; Pizzorusso, T.; Maffei, L.; Ratto, G.M. Extracellular Matrix Inhibits Structural and Functional Plasticity of Dendritic Spines in the Adult Visual Cortex. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Miyata, S.; Komatsu, Y.; Yoshimura, Y.; Taya, C.; Kitagawa, H. Persistent Cortical Plasticity by Upregulation of Chondroitin 6-Sulfation. Nat. Neurosci. 2012, 15, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Chondroitin Sulfate Proteoglycans: Key Modulators of Neuronal Plasticity, Long-Term Memory, Neurodegenerative, and Psychiatric Disorders. Rev. Neurosci. 2020, 31, 555–568. [Google Scholar] [CrossRef]
- Frischknecht, R.; Heine, M.; Perrais, D.; Seidenbecher, C.I.; Choquet, D.; Gundelfinger, E.D. Brain Extracellular Matrix Affects AMPA Receptor Lateral Mobility and Short-Term Synaptic Plasticity. Nat. Neurosci. 2009, 12, 897–904. [Google Scholar] [CrossRef]
- Vedunova, M.; Sakharnova, T.; Mitroshina, E.; Perminova, M.; Pimashkin, A.; Zakharov, Y.; Dityatev, A.; Mukhina, I. Seizure-like Activity in Hyaluronidase-Treated Dissociated Hippocampal Cultures. Front. Cell. Neurosci. 2013, 7, 1–10. [Google Scholar] [CrossRef]
- Maroto, M.; Fernández-Morales, J.C.; Padín, J.F.; González, J.C.; Hernández-Guijo, J.M.; Montell, E.; Vergés, J.; De Diego, A.M.G.; García, A.G. Chondroitin Sulfate, a Major Component of the Perineuronal Net, Elicits Inward Currents, Cell Depolarization, and Calcium Transients by Acting on AMPA and Kainate Receptors of Hippocampal Neurons. J. Neurochem. 2013, 125, 205–213. [Google Scholar] [CrossRef]
- Vo, T.; Carulli, D.; Ehlert, E.M.E.; Kwok, J.C.F.; Dick, G.; Mecollari, V.; Moloney, E.B.; Neufeld, G.; de Winter, F.; Fawcett, J.W.; et al. The Chemorepulsive Axon Guidance Protein Semaphorin3A Is a Constituent of Perineuronal Nets in the Adult Rodent Brain. Mol. Cell. Neurosci. 2013, 56, 186–200. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Bozzelli, P.L.; Caccavano, A.; Allen, M.; Balmuth, J.; Vicini, S.; Wu, J.Y.; Conant, K. Disruption of Perineuronal Nets Increases the Frequency of Sharp Wave Ripple Events. Hippocampus 2018, 28, 42–52. [Google Scholar] [CrossRef]
- Lensjø, K.K.; Lepperød, M.E.; Dick, G.; Hafting, T.; Fyhn, M. Removal of Perineuronal Nets Unlocks Juvenile Plasticity through Network Mechanisms of Decreased Inhibition and Increased Gamma Activity. J. Neurosci. 2017, 37, 1269–1283. [Google Scholar] [CrossRef]
- Dityatev, A.; Rusakov, D.A. Molecular Signals of Plasticity at the Tetrapartite Synapse. Curr. Opin. Neurobiol. 2011, 21, 353–359. [Google Scholar] [CrossRef]
- Levy, C.; Brooks, J.M.; Chen, J.; Su, J.; Fox, M.A. Cell-Specific and Developmental Expression of Lectican-Cleaving Proteases in Mouse Hippocampus and Neocortex. J. Comp. Neurol. 2015, 523, 629–648. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.H.; Afroz, S.; Reinhard, S.M.; Palacios, A.R.; Tapia, K.; Binder, D.K.; Razak, K.A.; Ethell, I.M. Genetic Reduction of Matrix Metalloproteinase-9 Promotes Formation of Perineuronal Nets Around Parvalbumin-Expressing Interneurons and Normalizes Auditory Cortex Responses in Developing Fmr1 Knock-Out Mice. Cereb. Cortex 2018, 28, 3951–3964. [Google Scholar] [CrossRef] [PubMed]
- Donato, F.; Rompani, S.B.; Caroni, P. Parvalbumin-Expressing Basket-Cell Network Plasticity Induced by Experience Regulates Adult Learning. Nature 2013, 504, 272–276. [Google Scholar] [CrossRef]
- Missirlis, Y.F. Mechanoepigenetics. Front. Cell Dev. Biol. 2016, 4, 10–13. [Google Scholar] [CrossRef]
- Jiang, F.X.; Lin, D.C.; Horkay, F.; Langrana, N.A. Probing Mechanical Adaptation of Neurite Outgrowth on a Hydrogel Material Using Atomic Force Microscopy. Ann. Biomed. Eng. 2011, 39, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.C.F.; Dick, G.; Wang, D.; Fawcett, J.W. Extracellular Matrix and Perineuronal Nets in CNS Repair. Dev. Neurobiol. 2011, 71, 1073–1089. [Google Scholar] [CrossRef]
- Harris, N.G.; Carmichael, S.T.; Hovda, D.A.; Sutton, R.L. Traumatic Brain Injury Results in Disparate Regions of Chondroitin Sulfate Proteoglycan Expression That Are Temporally Limited. J. Neurosci. Res. 2009, 87, 2937–2950. [Google Scholar] [CrossRef]
- Yi, J.H.; Katagiri, Y.; Susarla, B.; Figge, D.; Symes, A.J.; Geller, H.M. Alterations in Sulfated Chondroitin Glycosaminoglycans Following Controlled Cortical Impact Injury in Mice. J. Comp. Neurol. 2012, 520, 3295–3313. [Google Scholar] [CrossRef] [PubMed]
- Vita, S.M.; Grayson, B.E.; Grill, R.J. Acute Damage to the Blood–Brain Barrier and Perineuronal Net Integrity in a Clinically-Relevant Rat Model of Traumatic Brain Injury. Neuroreport 2020, 31, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, P.; Khoshneviszadeh, M.; Jandke, S.; Schreiber, S.; Dityatev, A. Interplay between Perivascular and Perineuronal Extracellular Matrix Remodelling in Neurological and Psychiatric Diseases. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef]
- Bornstein, P. Matricellular Proteins: An Overview. J. Cell Commun. Signal. 2009, 3, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Kannus, P.; Jozsa, L.; Järvinen, T.A.H.; Järvinen, T.L.N.; Kvist, M.; Natri, A.; Järvinen, M. Location and Distribution of Non-Collagenous Matrix Proteins in Musculoskeletal Tissues of Rat. Histochem. J. 1998, 30, 799–810. [Google Scholar] [CrossRef]
- Oberhauser, A.F.; Marszalek, P.E.; Erickson, H.P.; Fernandez, J.M. The Molecular Elasticity of the Extracellular Matrix Protein Tenascin. Nature 1998, 393, 181–185. [Google Scholar] [CrossRef]
- Chiquet, M.; Tunç-Civelek, V.; Sarasa-Renedo, A. Gene Regulation by Mechanotransduction in Fibroblasts. Appl. Physiol. Nutr. Metab. 2007, 32, 967–973. [Google Scholar] [CrossRef]
- Plotnikov, S.V.; Pasapera, A.M.; Sabass, B.; Waterman, C.M. Force Fluctuations within Focal Adhesions Mediate ECM-Rigidity Sensing to Guide Directed Cell Migration. Cell 2012, 151, 1513–1527. [Google Scholar] [CrossRef]
- Midwood, K.S.; Schwarzbauer, J.E. Tenascin-C Modulates Matrix Contraction via Focal Adhesion Kinase– and Rho-Mediated Signaling Pathways. Mol. Biol. Cell 2002, 13, 3601–3613. [Google Scholar] [CrossRef] [PubMed]
- Nishio, T.; Kawaguchi, S.; Yamamoto, M.; Iseda, T.; Kawasaki, T.; Hase, T. Tenascin-C Regulates Proliferation and Migration of Cultured Astrocytes in a Scratch Wound Assay. Neuroscience 2005, 132, 87–102. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Michael, S.V. Astrocyte Scar Formation Aids CNS Axon Regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rauch, U. Extracellular Matrix Components Associated with Remodeling Processes in Brain. Cell. Mol. Life Sci. 2004, 61, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Hutóczki, G.; Szemcsák, C.D.; Zahuczky, G.; Tóth, J.; Adamecz, Z.; Kenyeres, A.; Bognár, L.; Hanzély, Z.; Klekner, A. Brevican, Neurocan, Tenascin-C and Versican Are Mainly Responsible for the Invasiveness of Low-Grade Astrocytoma. Pathol. Oncol. Res. 2012, 18, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnigh, T.R.; et al. Tissue Mechanics Promote IDH1-Dependent HIF1α-Tenascin C Feedback to Regulate Glioblastoma Aggression. Nat. Cell Biol. 2016, 18, 1336–1345. [Google Scholar] [CrossRef]
- Tajerian, M.; Hung, V.; Nguyen, H.; Lee, G.; Joubert, L.M.; Malkovskiy, A.V.; Zou, B.; Xie, S.; Huang, T.T.; Clark, J.D. The Hippocampal Extracellular Matrix Regulates Pain and Memory after Injury. Mol. Psychiatry 2018, 23, 2302–2313. [Google Scholar] [CrossRef]
- Altman, J.; Das, G.D. Autoradiographic and Histological Evidence of Postnatal Hippocampal Neurogenesis in Rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Ge, S.; Goh, E.L.K.; Sailor, K.A.; Kitabatake, Y.; Ming, G.L.; Song, H. GABA Regulates Synaptic Integration of Newly Generated Neurons in the Adult Brain. Nature 2006, 439, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, J.; Moss, J.; Wen, Z.; Sun, G.J.; Hsu, D.; Zhong, C.; Davoudi, H.; Christian, K.M.; Toni, N.; et al. Parvalbumin Interneurons Mediate Neuronal Circuitry-Neurogenesis Coupling in the Adult Hippocampus. Nat. Neurosci. 2013, 16, 1728–1730. [Google Scholar] [CrossRef]
- Vaden, R.J.; Gonzalez, J.C.; Tsai, M.C.; Niver, A.J.; Fusilier, A.R.; Griffith, C.M.; Kramer, R.H.; Wadiche, J.I.; Overstreet-Wadiche, L. Parvalbumin Interneurons Provide Spillover to Newborn and Mature Dentate Granule Cells. Elife 2020, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Groisman, A.I.; Yang, S.M.; Schinder, A.F. Differential Coupling of Adult-Born Granule Cells to Parvalbumin and Somatostatin Interneurons. bioRxiv 2019, 598615. [Google Scholar] [CrossRef]
- Ikrar, T.; Guo, N.; He, K.; Besnard, A.; Levinson, S.; Hill, A.; Lee, H.K.; Hen, R.; Xu, X.; Sahay, A. Adult Neurogenesis Modifies Excitability of the Dentate Gyrus. Front. Neural Circuits 2013, 7, 1–15. [Google Scholar] [CrossRef]
- Dityatev, A.; Brückner, G.; Dityateva, G.; Grosche, J.; Kleene, R.; Schachner, M. Activity-Dependent Formation and Functions of Chondroitin Sulfate-Rich Extracellular Matrix of Perineuronal Nets. Dev. Neurobiol. 2007, 67, 570–588. [Google Scholar] [CrossRef]
- Ge, S.; Yang, C.; Hsu, K.; Ming, G.; Song, H. A Critical Period for Enhanced Synaptic Plasticity in Newly Generated Neurons of the Adult Brain. Neuron 2007, 54, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Fowke, T.M.; Karunasinghe, R.N.; Bai, J.-Z.; Jordan, S.; Gunn, A.J.; Dean, J.M. Hyaluronan Synthesis by Developing Cortical Neurons In Vitro. Sci. Rep. 2017, 7, 44135. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Matsumoto, S.; Sorg, B.; Sherman, L.S. Distinct Roles for Hyaluronan in Neural Stem Cell Niches and Perineuronal Nets. Matrix Biol. 2019, 78–79, 272–283. [Google Scholar] [CrossRef]
- Su, W.; Foster, S.C.; Xing, R.; Feistel, K.; Olsen, R.H.J.; Acevedo, S.F.; Raber, J.; Sherman, L.S. CD44 Transmembrane Receptor and Hyaluronan Regulate Adult Hippocampal Neural Stem Cell Quiescence and Differentiation. J. Biol. Chem. 2017, 292, 4434–4445. [Google Scholar] [CrossRef]
- Yamada, J.; Nadanaka, S.; Kitagawa, H.; Takeuchi, K.; Jinno, S. Increased Synthesis of Chondroitin Sulfate Proteoglycan Promotes Adult Hippocampal Neurogenesis in Response to Enriched Environment. J. Neurosci. 2018, 38, 8496–8513. [Google Scholar] [CrossRef]
- Mencio, C.P.; Hussein, R.K.; Yu, P.; Geller, H.M. The Role of Chondroitin Sulfate Proteoglycans in Nervous System Development. J. Histochem. Cytochem. 2021, 69, 61–80. [Google Scholar] [CrossRef]
- Sirko, S.; Von Holst, A.; Weber, A.; Wizenmann, A.; Theocharidis, U.; Götz, M.; Faissner, A. Chondroitin Sulfates Are Required for Fibroblast Growth Factor-2-Dependent Proliferation and Maintenance in Neural Stem Cells and for Epidermal Growth Factor-Dependent Migration of Their Progeny. Stem Cells 2010, 28, 775–787. [Google Scholar] [CrossRef]
- Gates, M.A.; Thomas, L.B.; Howard, E.M.; Laywell, E.D.; Sajin, B.; Faissner, A.; Götz, B.; Silver, J.; Steindler, D.A. Cell and Molecular Analysis of the Developing and Adult Mouse Subventricular Zone of the Cerebral Hemispheres. J. Comp. Neurol. 1995, 361, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Faissner, A.; Ffrench-Constant, C. Knockout Mice Reveal a Contribution of the Extracellular Matrix Molecule Tenascin-C to Neural Precursor Proliferation and Migration. Development 2001, 128, 2485–2496. [Google Scholar] [CrossRef]
- Garcion, E.; Halilagic, A.; Faissner, A.; Ffrench-Constant, C. Generation of an Environmental Niche for Neural Stem Cell Development Bythe Extracellular Matrix Molecule Tenascin, C. Development 2004, 131, 3423–3432. [Google Scholar] [CrossRef] [PubMed]
- Kwok, J.C.F.; Foscarin, S.; Fawcett, J.W. Perineuronal Nets: A Special Structure in the Central Nervous System Extracellular Matrix. In Extracellular Matrix; Humana Press: New York, NY, USA, 2015; pp. 23–32. [Google Scholar] [CrossRef]
- Köppe, G.; Brückner, G.; Härtig, W.; Delpech, B.; Bigl, V. Characterization of Proteoglycan-Containing Perineuronal Nets by Enzymatic Treatments of Rat Brain Sections. Histochem. J. 1997, 29, 11–20. [Google Scholar] [CrossRef]
- Seeger, G.; Brauer, K.; Härtig, W.; Brückner, G. Mapping of Perineuronal Nets in the Rat Brain Stained by Colloidal Iron Hydroxide Histochemistry and Lectin Cytochemistry. Neuroscience 1994, 58, 371–388. [Google Scholar] [CrossRef]
- Dzyubenko, E.; Manrique-Castano, D.; Kleinschnitz, C.; Faissner, A.; Hermann, D.M. Topological Remodeling of Cortical Perineuronal Nets in Focal Cerebral Ischemia and Mild Hypoperfusion. Matrix Biol. 2018, 74, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Sigal, Y.M.; Bae, H.; Bogart, L.J.; Hensch, T.K.; Zhuang, X. Structural Maturation of Cortical Perineuronal Nets and Their Perforating Synapses Revealed by Superresolution Imaging. Proc. Natl. Acad. Sci. USA 2019, 116, 7071–7076. [Google Scholar] [CrossRef] [PubMed]
- Arnst, N.; Kuznetsova, S.; Lipachev, N.; Shaikhutdinov, N.; Melnikova, A.; Mavlikeev, M.; Uvarov, P.; Baltina, T.V.; Rauvala, H.; Osin, Y.N.; et al. Spatial Patterns and Cell Surface Clusters in Perineuronal Nets. Brain Res. 2016, 1648, 214–223. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Blazikova, M.; Tucic, M.; Stamenkovic, V.; Andjus, R.P. Analysis of Perineuronal Net Topography in the Hippocampus of Tenascin-C Deficient Mice. In Proceedings of the 12th FENS Forum of Neuroscience, Virtual Forum, Glasgow, UK, 11–15 July 2020. [Google Scholar]
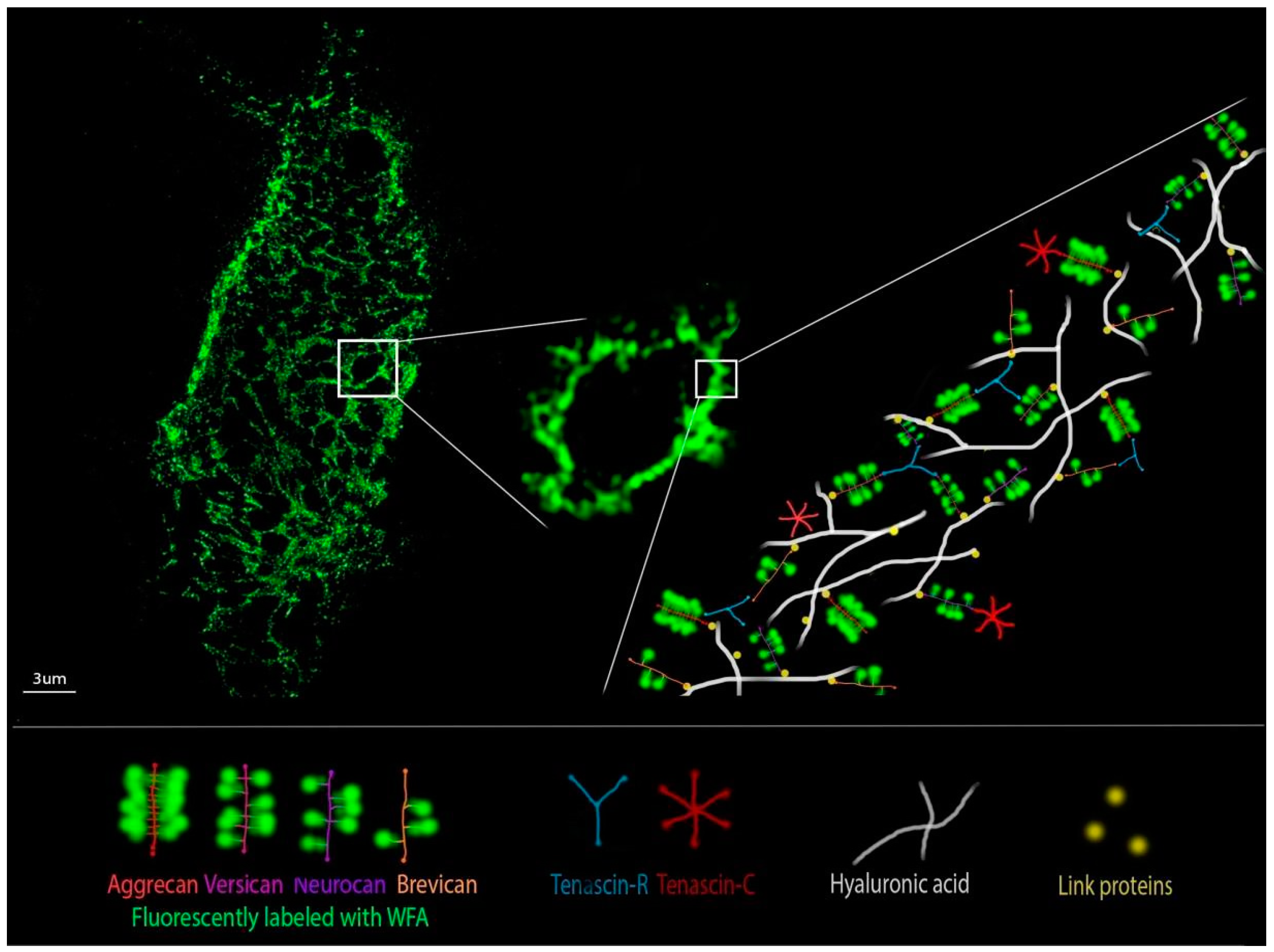
| Microscopy Technique | PNN Characteristics | PNN Labeling | Reference |
|---|---|---|---|
| Fluorescent microscopy | Ability to bind WFA | WFA | [19] |
| Light microscopy | Distribution patterns | WFA, Vicia villosa agglutinin, colloidal iron hydroxide staining | [125] |
| Immunoelectron microscopy | Brevican-TnR colocalization | Brevican TnR | [53] |
| Confocal laser scanning microscope, electron microscopy | Staining intensity in TnR KO mice | TnR Hyaluronic acid Phosphacan Neurocan Brevican | [45] |
| Confocal fluorescence microscopy | Morphological alteration in TnR/PV KO mice | Phosphacan Neurocan WFA | [52] |
| Nuclear microscopy (iPIXE) | Accumulation of metal ions | Colloidal iron | [10] |
| Confocal laser scanning microscope | Expression patterns in aggrecan KO | WFA Hyaluronic acid HAPLP1 TnR Brevican | [26] |
| Confocal laser scanning microscope and epifluorescent microscope | Organization into geometrical patterns | WFA | [128] |
| Super-resolution structural illumination microscopy (SR-SIM) | Ultrastructure using topography measurements | WFA Aggrecan | [126] |
| Stochastic optical reconstruction microscopy (STORM) | Surface intensity and perforating synapses | WFA | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakovljević, A.; Tucić, M.; Blažiková, M.; Korenić, A.; Missirlis, Y.; Stamenković, V.; Andjus, P. Structural and Functional Modulation of Perineuronal Nets: In Search of Important Players with Highlight on Tenascins. Cells 2021, 10, 1345. https://doi.org/10.3390/cells10061345
Jakovljević A, Tucić M, Blažiková M, Korenić A, Missirlis Y, Stamenković V, Andjus P. Structural and Functional Modulation of Perineuronal Nets: In Search of Important Players with Highlight on Tenascins. Cells. 2021; 10(6):1345. https://doi.org/10.3390/cells10061345
Chicago/Turabian StyleJakovljević, Ana, Milena Tucić, Michaela Blažiková, Andrej Korenić, Yannis Missirlis, Vera Stamenković, and Pavle Andjus. 2021. "Structural and Functional Modulation of Perineuronal Nets: In Search of Important Players with Highlight on Tenascins" Cells 10, no. 6: 1345. https://doi.org/10.3390/cells10061345
APA StyleJakovljević, A., Tucić, M., Blažiková, M., Korenić, A., Missirlis, Y., Stamenković, V., & Andjus, P. (2021). Structural and Functional Modulation of Perineuronal Nets: In Search of Important Players with Highlight on Tenascins. Cells, 10(6), 1345. https://doi.org/10.3390/cells10061345






