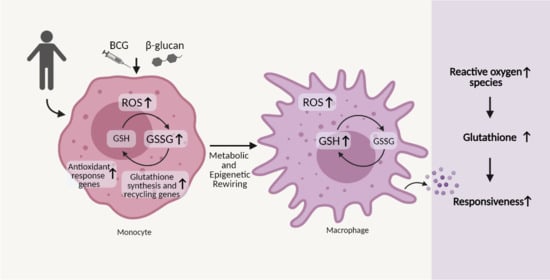
Glutathione Metabolism Contributes to the Induction of Trained Immunity
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Human Peripheral Blood Mononuclear Cells (PBMC) and Monocytes
2.2. In Vitro Trained Immunity Model
2.3. Reactive Oxygen Species (ROS) Quantification
2.4. RNA Sequencing Data of Human Monocytes Stimulated In Vitro
2.5. Glutathione Quantification
2.6. In Vitro and In Vivo Trained Immunity Models for Genetic and Metabolic Analysis
2.7. Genetic Analysis
2.8. Metabolomics Analysis
2.9. Cytokine Quantification and Analysis
3. Results
3.1. β-Glucan- and BCG-Induced Trained Immunity Trigger a Sustained Reactive Oxygen Species Production
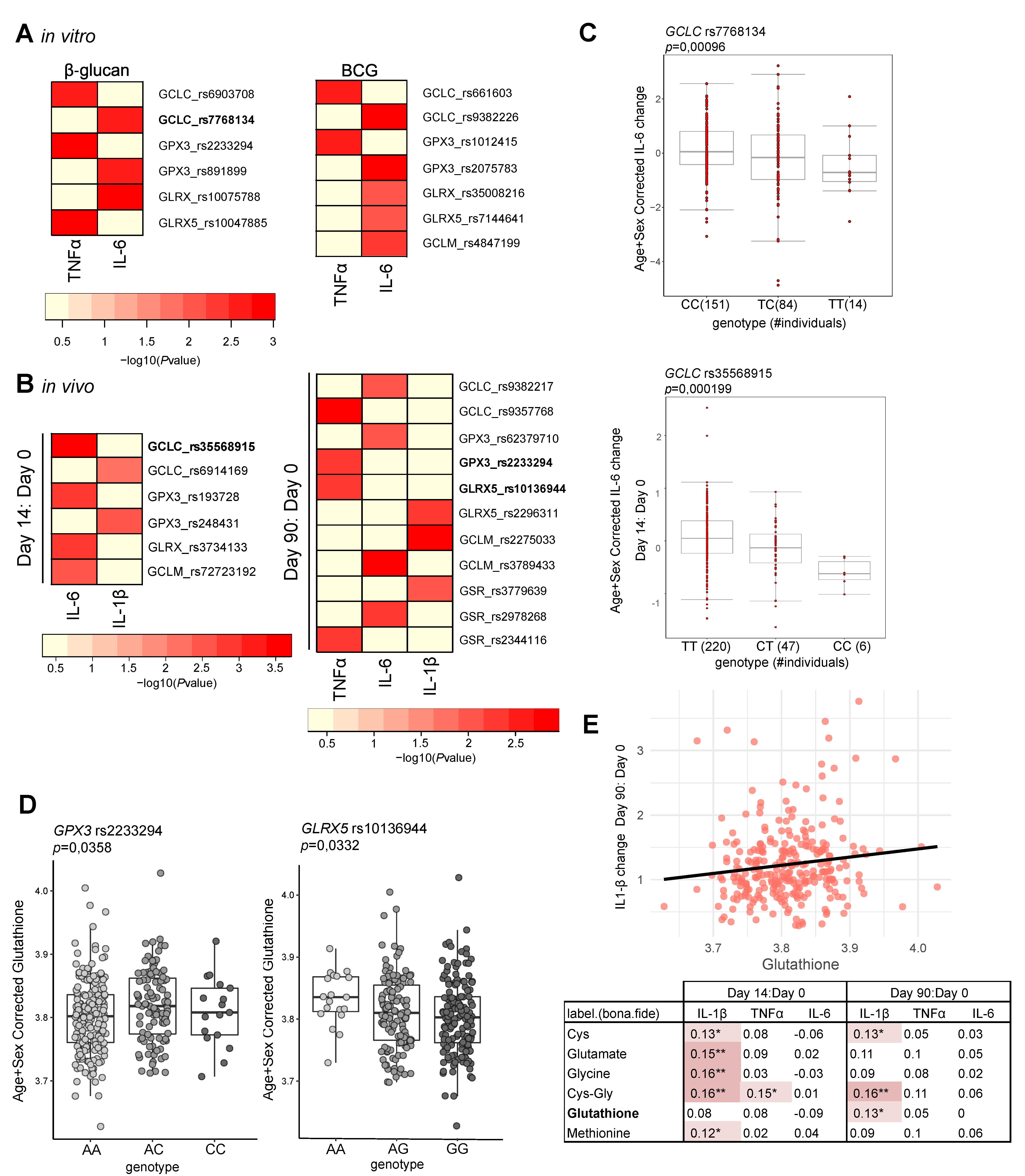
3.2. Glutathione Metabolism Influences Trained Immunity Responses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.J.; Wijmenga, C.; et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; Van Der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. Arter. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A Small Jab-A Big Effect: Nonspecific Immunomodulation By Vaccines. Trends Immunol. 2013, 34, 431–439. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Novakovic, B.; ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.C.; Wang, S.Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.; Groh, L.; van der Heijden, C.; Rodriguez, H.; Dos Santos, J.; Fanucchi, S.; Okabe, J.; Kn, H.; van Puffelen, J.; Helder, L.; et al. The Set7 Lysine Methyltransferase Regulates Plasticity in Oxidative Phosphorylation Necessary for Trained Immunity Induced by Beta-Glucan. Cell Rep. 2020, 31, 107548. [Google Scholar] [CrossRef] [PubMed]
- García-Giménez, J.L.; Romá-Mateo, C.; Pérez-Machado, G.; Peiró-Chova, L.; Pallardó, F.V. Role of glutathione in the regulation of epigenetic mechanisms in disease. Free Radic. Biol. Med. 2017, 112, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.B.; Riksen, N.P.; Van Crevel, R.; Netea, M.G. In Vitro Experimental Model of Trained Innate Immunity in Human. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Lagache, S.M.M.; Schnack, L.; Godfrey, R.; Kahles, F.; Bruemmer, D.; Waltenberger, J.; Findeisen, H.M. mTOR-Dependent Oxidative Stress Regulates oxLDL-Induced Trained Innate Immunity in Human Monocytes. Front. Immunol. 2019, 9, 3155. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, C.D.C.C.; Keating, S.T.; Groh, L.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P. Aldosterone induces trained immunity: The role of fatty acid synthesis. Cardiovasc. Res. 2020, 116, 317–328. [Google Scholar] [CrossRef]
- Domínguez-Andrés, J.; Arts, R.J.W.; Bekkering, S.; Bahrar, H.; Blok, B.A.; de Bree, L.C.J.; Bruno, M.; Bulut, Ö.; Debisarun, P.A.; Dijkstra, H.; et al. In vitro induction of trained immunity in adherent human monocytes. STAR Protoc. 2021, 2, 100365. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, B.; Habibi, E.; Wang, S.-Y.; Arts, R.J.W.; Davar, R.; Megchelenbrink, W.; Kim, B.; Kuznetsova, T.; Kox, M.; Zwaag, J.; et al. β-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 2016, 167, 1354–1368.e14. [Google Scholar] [CrossRef]
- Kostidis, S.; Addie, R.D.; Morreau, H.; Mayboroda, O.A.; Giera, M. Quantitative NMR analysis of intra- and extracellular metabolism of mammalian cells: A tutorial. Anal. Chim. Acta 2017, 980, 1–24. [Google Scholar] [CrossRef]
- Koeken, V.A.C.M.; de Bree, L.C.J.; Mourits, V.P.; Moorlag, S.J.C.F.M.; Walk, J.; Cirovic, B.; Arts, R.J.W.; Jaeger, M.; Dijkstra, H.; Lemmers, H.; et al. BCG vaccination in humans inhibits systemic inflammation in a sex-dependent manner. J. Clin. Investig. 2020, 130, 5591–5602. [Google Scholar] [CrossRef]
- Human Functional Genomcis Project. Available online: http://www.humanfunctionalgenomics.org/site/ (accessed on 19 April 2021).
- Shah, T.S.; Liu, J.Z.; Floyd, J.A.B.; Morris, J.A.; Wirth, N.; Barrett, J.C.; Anderson, C.A. OptiCall: A robust genotype-calling algorithm for rare, low-frequency and common variants. Bioinformatics 2012, 28, 1598–1603. [Google Scholar] [CrossRef]
- Deelen, P.; Bonder, M.J.; Van Der Velde, K.J.; Westra, H.J.; Winder, E.; Hendriksen, D.; Franke, L.; Swertz, M.A. Genotype harmonizer: Automatic strand alignment and format conversion for genotype data integration. BMC Res. Notes 2014, 7. [Google Scholar] [CrossRef]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar] [CrossRef]
- Shabalin, A.A. Matrix eQTL: Ultra fast eQTL analysis via large matrix operations. Bioinformatics 2012, 28, 1353–1358. [Google Scholar] [CrossRef]
- Fuhrer, T.; Heer, D.; Begemann, B.; Zamboni, N. High-throughput, accurate mass metabolome profiling of cellular extracts by flow injection-time-of-flight mass spectrometry. Anal. Chem. 2011, 83, 7074–7080. [Google Scholar] [CrossRef]
- Kalafati, L.; Kourtzelis, I.; Schulte-schrepping, J.; Verginis, P.; Mitroulis, I. Innate Immune Training of Granulopoiesis Promotes Anti-tumor Activity. Cell 2020, 183, 771–785.e12. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.A.; Rao, N.A.; Aghajanirefah, A.; et al. MTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Brennan, M.S.; Matos, M.F.; Li, B.; Hronowski, X.; Gao, B.; Juhasz, P.; Rhodes, K.J.; Scannevin, R.H. Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS ONE 2015, 10, e0120254. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.-C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.V.; Koeken, V.A.C.M.; Matzaraki, V.; Kostidis, S.; Alarcon-Barrera, J.C.; de Bree, L.C.J.; Moorlag, S.J.C.F.M.; Mourits, V.P.; Novakovic, B.; Giera, M.A.; et al. Glutathione Metabolism Contributes to the Induction of Trained Immunity. Cells 2021, 10, 971. https://doi.org/10.3390/cells10050971
Ferreira AV, Koeken VACM, Matzaraki V, Kostidis S, Alarcon-Barrera JC, de Bree LCJ, Moorlag SJCFM, Mourits VP, Novakovic B, Giera MA, et al. Glutathione Metabolism Contributes to the Induction of Trained Immunity. Cells. 2021; 10(5):971. https://doi.org/10.3390/cells10050971
Chicago/Turabian StyleFerreira, Anaisa V., Valerie A. C. M. Koeken, Vasiliki Matzaraki, Sarantos Kostidis, Juan Carlos Alarcon-Barrera, L. Charlotte J. de Bree, Simone J. C. F. M. Moorlag, Vera P. Mourits, Boris Novakovic, Martin A. Giera, and et al. 2021. "Glutathione Metabolism Contributes to the Induction of Trained Immunity" Cells 10, no. 5: 971. https://doi.org/10.3390/cells10050971
APA StyleFerreira, A. V., Koeken, V. A. C. M., Matzaraki, V., Kostidis, S., Alarcon-Barrera, J. C., de Bree, L. C. J., Moorlag, S. J. C. F. M., Mourits, V. P., Novakovic, B., Giera, M. A., Netea, M. G., & Domínguez-Andrés, J. (2021). Glutathione Metabolism Contributes to the Induction of Trained Immunity. Cells, 10(5), 971. https://doi.org/10.3390/cells10050971