Ablation of TRPV1 Abolishes Salicylate-Induced Sympathetic Activity Suppression and Exacerbates Salicylate-Induced Renal Dysfunction in Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Telemetry Blood Pressure Assay
2.3. Glucose and Insulin Tolerance Testing and Serum Leptin and Insulin Measurement
2.4. Recording of Afferent Renal Nerve Activity
2.5. Determination of Baroreflex Function
2.6. Renal Blood Flow Recording
2.7. Renal NF-κB p65 Activity Assay
2.8. Plasma and Urine Analysis
2.9. Statistical Analysis
3. Results
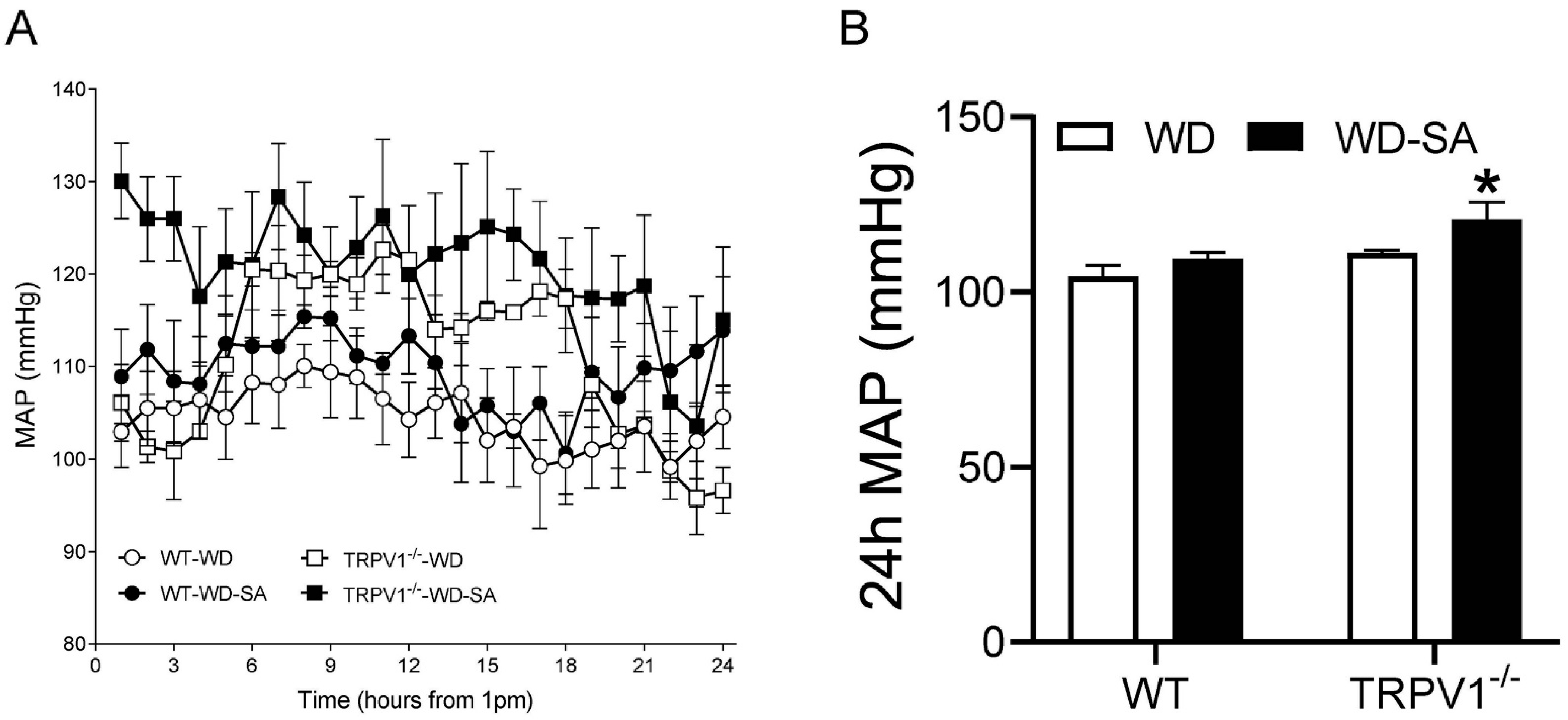
3.1. Effects of SA on Blood Pressure
3.2. Effects of SA on Glucose Tolerance and Insulin Resistance
3.3. Effects of SA on Autonomic Nerve Activity
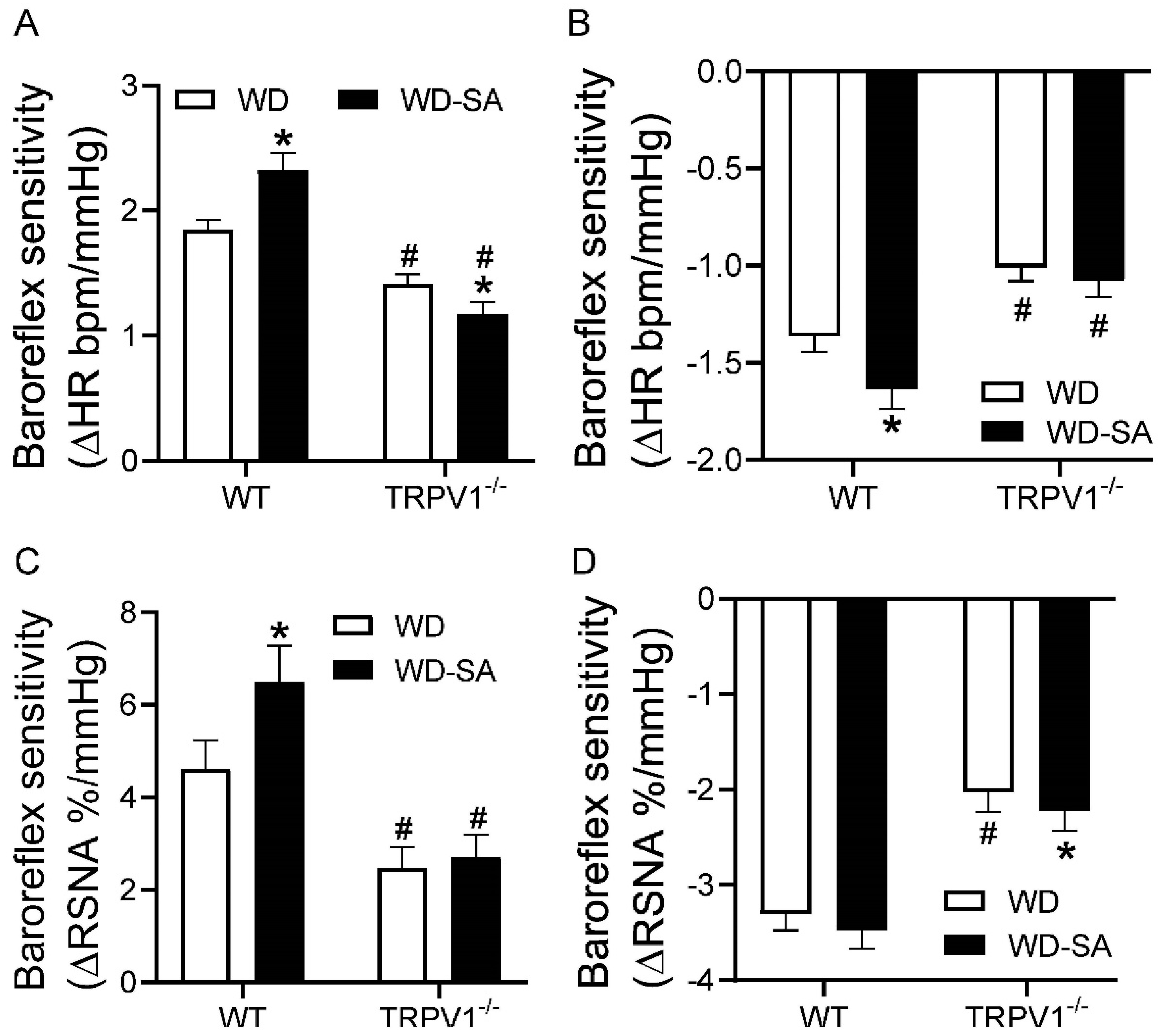
3.4. Effects of SA Treatment on Baroreflex Sensitivity
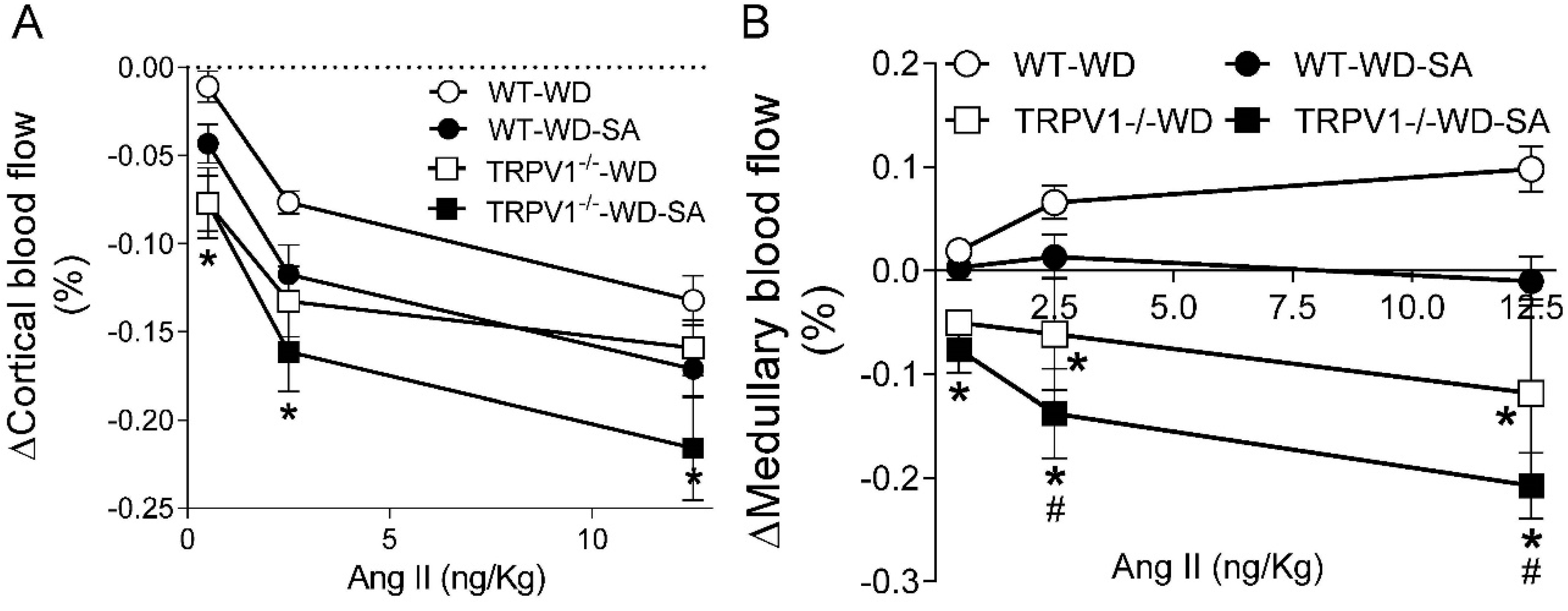
3.5. Effects of SA on Renal Blood Flow
3.6. Effects of SA on Renal Inflammatory Markers
3.7. Effects of SA on Renal Function
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Danneskiold-Samsoe, N.B.; Sonne, S.B.; Larsen, J.M.; Hansen, A.N.; Fjaere, E.; Isidor, M.S.; Petersen, S.; Henningsen, J.; Severi, I.; Sartini, L.; et al. Overexpression of cyclooxygenase-2 in adipocytes reduces fat accumulation in inguinal white adipose tissue and hepatic steatosis in high-fat fed mice. Sci. Rep. 2019, 9, 8979. [Google Scholar] [CrossRef] [PubMed]
- Davidge, S.T. Prostaglandin H synthase and vascular function. Circ. Res. 2001, 89, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; McKanna, J.A.; Akai, Y.; Jacobson, H.R.; Dubois, R.N.; Breyer, M.D. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J. Clin. Investig. 1994, 94, 2504–2510. [Google Scholar] [CrossRef] [PubMed]
- Helmersson, J.; Vessby, B.; Larsson, A.; Basu, S. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation 2004, 109, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Quilley, J.; Santos, M.; Pedraza, P. Renal protective effect of chronic inhibition of COX-2 with SC-58236 in streptozotocin-diabetic rats. Am. J. Physiol. 2011, 300, H2316–H2322. [Google Scholar] [CrossRef]
- Gullner, H.G.; Nicolaou, K.C.; Bartter, F.C.; Kelly, G. Effect of prostacyclin (PGI2) on renal function and renin secretion in hypophysectomized dogs. Nephron 1980, 25, 283–287. [Google Scholar] [CrossRef]
- Villa, E.; Garcia-Robles, R.; Haas, J.; Romero, J.C. Comparative effect of PGE2 and PGI2 on renal function. Hypertension 1997, 30, 664–666. [Google Scholar] [CrossRef]
- Morera, S.; Santoro, F.M.; Roson, M.I.; de la Riva, I.J. Prostacyclin (PGI2) synthesis in the vascular wall of rats with bilateral renal artery stenosis. Hypertension 1983, 5, V38–V42. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Wang, D.H. Diuresis and natriuresis caused by activation of VR1-positive sensory nerves in renal pelvis of rats. Hypertension 2005, 46, 992–997. [Google Scholar] [CrossRef]
- Xie, C.; Sachs, J.R.; Wang, D.H. Interdependent regulation of afferent renal nerve activity and renal function: Role of transient receptor potential vanilloid type 1, neurokinin 1, and calcitonin gene-related peptide receptors. J. Pharm. Exp. Ther. 2008, 325, 751–757. [Google Scholar] [CrossRef]
- Kopp, U.C.; Jones, S.Y.; DiBona, G.F. Afferent renal denervation impairs baroreflex control of efferent renal sympathetic nerve activity. Am. J. Physiol Regul Integr Comp. Physiol. 2008, 295, R1882–R1890. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wang, D.H. Ablation of transient receptor potential vanilloid 1 abolishes endothelin-induced increases in afferent renal nerve activity: Mechanisms and functional significance. Hypertension 2009, 54, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Ditting, T.; Freisinger, W.; Siegel, K.; Fiedler, C.; Small, L.; Neuhuber, W.; Heinlein, S.; Reeh, P.W.; Schmieder, R.E.; Veelken, R. Tonic postganglionic sympathetic inhibition induced by afferent renal nerves? Hypertension 2012, 59, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Haddock, R.E.; Hill, C.E. Sympathetic overdrive in obesity involves purinergic hyperactivity in the resistance vasculature. J. Physiol. 2011, 589, 3289–3307. [Google Scholar] [CrossRef]
- Alvarez, G.E.; Beske, S.D.; Ballard, T.P.; Davy, K.P. Sympathetic neural activation in visceral obesity. Circulation 2002, 106, 2533–2536. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, N.; Liatis, S.; Katsilambros, N. Sympathetic system activity in obesity and metabolic syndrome. Ann. N. Y. Acad. Sci. 2006, 1083, 129–152. [Google Scholar] [CrossRef]
- Skrapari, I.; Tentolouris, N.; Perrea, D.; Bakoyiannis, C.; Papazafiropoulou, A.; Katsilambros, N. Baroreflex sensitivity in obesity: Relationship with cardiac autonomic nervous system activity. Obesity 2007, 15, 1685–1693. [Google Scholar] [CrossRef]
- Lee, E.; Jung, D.Y.; Kim, J.H.; Patel, P.R.; Hu, X.; Lee, Y.; Azuma, Y.; Wang, H.F.; Tsitsilianos, N.; Shafiq, U.; et al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015, 29, 3182–3192. [Google Scholar] [CrossRef]
- Li, T.; Wang, G.; Hui, V.C.C.; Saad, D.; de Sousa Valente, J.; La Montanara, P.; Nagy, I. TRPV1 feed-forward sensitisation depends on COX2 upregulation in primary sensory neurons. Sci. Rep. 2021, 11, 3514. [Google Scholar] [CrossRef]
- Kopp, U.C.; Cicha, M.Z.; Nakamura, K.; Nusing, R.M.; Smith, L.A.; Hokfelt, T. Activation of EP4 receptors contributes to prostaglandin E2-mediated stimulation of renal sensory nerves. Am. J. Physiol Renal Physiol. 2004, 287, F1269–F1282. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, C.; Wang, D.H. TRPV1-mediated diuresis and natriuresis induced by hypertonic saline perfusion of the renal pelvis. Am. J. Nephrol. 2007, 27, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, D.P.; Chen, S.R.; Hittelman, W.N.; Pan, H.L. Sensing of blood pressure increase by transient receptor potential vanilloid 1 receptors on baroreceptors. J. Pharmacol. Exp. Ther.. 2009, 331, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Patschan, D.; Epstein, T.; Goligorsky, M.S.; Winaver, J. Delayed recovery of renal regional blood flow in diabetic mice subjected to acute ischemic kidney injury. Am. J. Physiol. 2007, 293, F1512–F1517. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.J.; Mattson, D.L.; Cowley, A.W., Jr. Measurement of regional blood flow in the kidney using laser-Doppler flowmetry. Methods Mol. Med. 2001, 51, 407–426. [Google Scholar]
- Dunn, S.R.; Qi, Z.; Bottinger, E.P.; Breyer, M.D.; Sharma, K. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int. 2004, 65, 1959–1967. [Google Scholar] [CrossRef]
- Fardin, N.M.; Oyama, L.M.; Campos, R.R. Changes in baroreflex control of renal sympathetic nerve activity in high-fat-fed rats as a predictor of hypertension. Obesity 2012, 20, 1591–1597. [Google Scholar] [CrossRef]
- Kopp, U.C.; Cicha, M.Z.; Yorek, M.A. Impaired responsiveness of renal sensory nerves in streptozotocin-treated rats and obese Zucker diabetic fatty rats: Role of angiotensin. Am. J. Physiol Regul Integr Comp. Physiol. 2008, 294, R858–R866. [Google Scholar] [CrossRef]
- Marfella, R.; Nappo, F.; Marfella, M.A.; Giugliano, D. Acute hyperglycemia and autonomic function. Diabetes Care. 2001, 24, 2016–2017. [Google Scholar] [CrossRef]
- de Lima, D.C.; Silveira, S.A.; Haibara, A.S.; Coimbra, C.C. The enhanced hyperglycemic response to hemorrhage hypotension in obese rats is related to an impaired baroreflex. Metab. Brain Dis. 2008, 23, 361–373. [Google Scholar] [CrossRef]
- Arnold, A.C.; Shaltout, H.A.; Gallagher, P.E.; Diz, D.I. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension 2009, 54, 1001–1008. [Google Scholar] [CrossRef]
- McKernan, A.M.; Calaresu, F.R. Insulin microinjection into the nucleus tractus solitarii of the rat attenuates the baroreceptor reflex. J. Auton. Nerv. Syst. 1996, 61, 128–138. [Google Scholar] [CrossRef]
- Rahmouni, K.; Haynes, W.G. Leptin and the cardiovascular system. Recent Prog. Horm. Res. 2004, 59, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Sanya, E.O.; Brown, C.M.; Dutsch, M.; Zikeli, U.; Neundorfer, B.; Hilz, M.J. Impaired cardiovagal and vasomotor responses to baroreceptor stimulation in type II diabetes mellitus. Eur. J. Clin. Investig. 2003, 33, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Monbaron, D.; Parati, G.; Perret, S.; Haesler, E.; Danzeisen, C.; Hayoz, D. Diabetic neuropathy is a more important determinant of baroreflex sensitivity than carotid elasticity in type 2 diabetes. Hypertension 2005, 46, 162–167. [Google Scholar] [CrossRef]
- Hajduczok, G.; Chapleau, M.W.; Johnson, S.L.; Abboud, F.M. Increase in sympathetic activity with age. I. Role of impairment of arterial baroreflexes. Am. J. Physiol. 1991, 260, H1113–H1120. [Google Scholar] [CrossRef]
- Ying, Z.; Giachini, F.R.; Tostes, R.C.; Webb, R.C. Salicylates dilate blood vessels through inhibiting PYK2-mediated RhoA/Rho-kinase activation. Cardiovasc Res. 2009, 83, 155–162. [Google Scholar] [CrossRef]
- Imig, J.D. Eicosanoids and renal vascular function in diseases. Clin. Sci. 2006, 111, 21–34. [Google Scholar] [CrossRef]
- Premkumar, L.S.; Raisinghani, M. Nociceptors in cardiovascular functions: Complex interplay as a result of cyclooxygenase inhibition. Mol. Pain 2006, 2, 26. [Google Scholar] [CrossRef]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef]
- Kumagai, K.; Suzuki, H.; Ichikawa, M.; Jimbo, M.; Murakami, M.; Ryuzaki, M.; Saruta, T. Nitric oxide increases renal blood flow by interacting with the sympathetic nervous system. Hypertension 1994, 24, 220–226. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, B.; Ma, S.; Wang, D.H. Ablation of TRPV1 Abolishes Salicylate-Induced Sympathetic Activity Suppression and Exacerbates Salicylate-Induced Renal Dysfunction in Diet-Induced Obesity. Cells 2021, 10, 1234. https://doi.org/10.3390/cells10051234
Zhong B, Ma S, Wang DH. Ablation of TRPV1 Abolishes Salicylate-Induced Sympathetic Activity Suppression and Exacerbates Salicylate-Induced Renal Dysfunction in Diet-Induced Obesity. Cells. 2021; 10(5):1234. https://doi.org/10.3390/cells10051234
Chicago/Turabian StyleZhong, Beihua, Shuangtao Ma, and Donna H. Wang. 2021. "Ablation of TRPV1 Abolishes Salicylate-Induced Sympathetic Activity Suppression and Exacerbates Salicylate-Induced Renal Dysfunction in Diet-Induced Obesity" Cells 10, no. 5: 1234. https://doi.org/10.3390/cells10051234
APA StyleZhong, B., Ma, S., & Wang, D. H. (2021). Ablation of TRPV1 Abolishes Salicylate-Induced Sympathetic Activity Suppression and Exacerbates Salicylate-Induced Renal Dysfunction in Diet-Induced Obesity. Cells, 10(5), 1234. https://doi.org/10.3390/cells10051234




