Can Sexual Selection Drive the Evolution of Sperm Cell Structure?
Abstract
1. Introduction
2. Materials and Methods
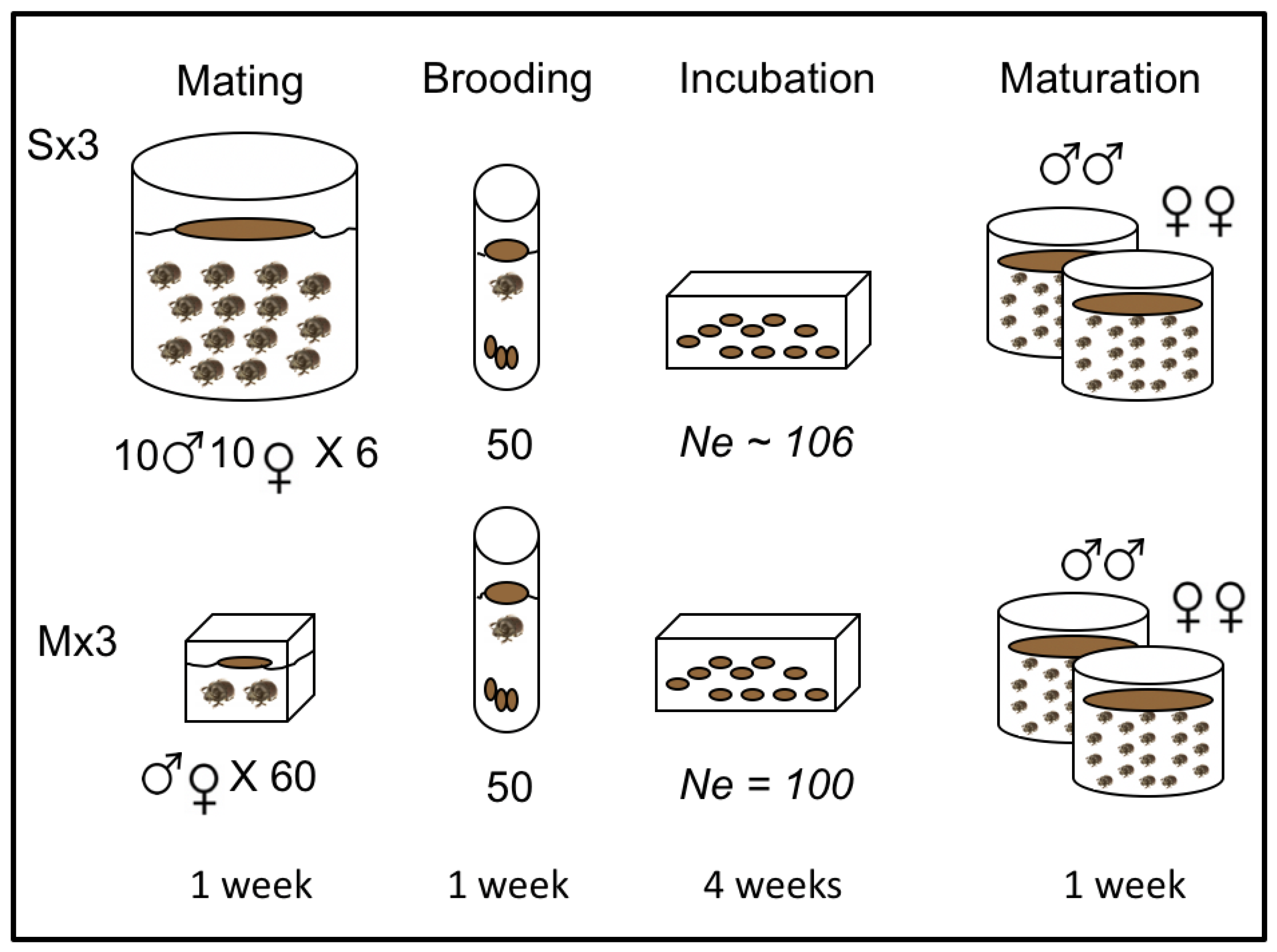
2.1. Experimental Evolution
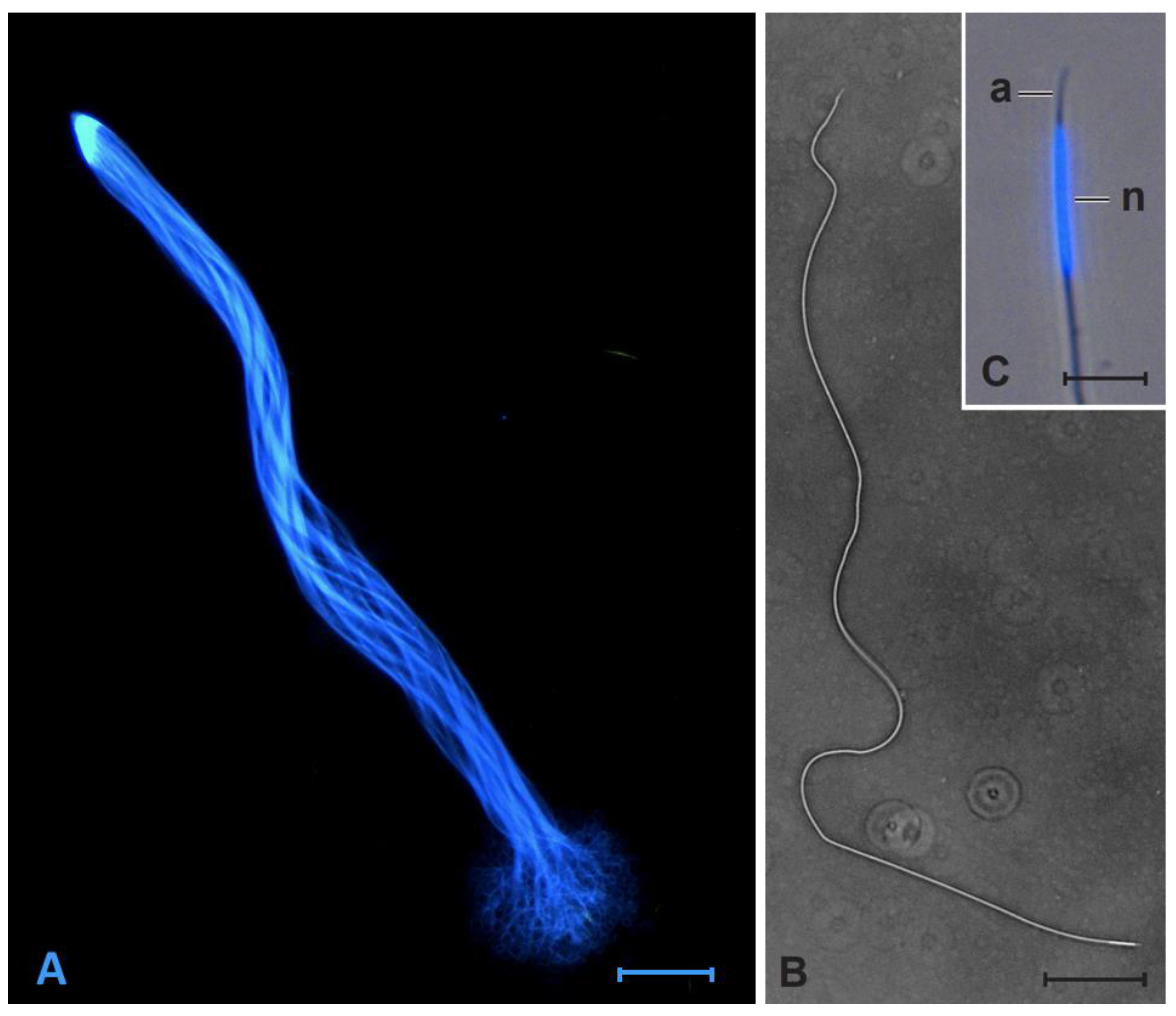
2.2. Sperm Cell Measurement
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitnick, S.; Hosken, D.J.; Birkhead, T.R. Sperm morphological diversity. In Sperm Biology: An Evolutionary Perspective; Birkhead, T.R., Hosken, D.J., Pitnick, S., Eds.; Academic Press: Burlington, MA, USA, 2009; pp. 69–149. [Google Scholar]
- Jamieson, B.G.M. Fish Evolution and Systematics: Evidence from Spermatozoa; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Jamieson, B.G.M. Spermatozoal phylogeny of the vertebrata. In The Male Gamete From Basic Science to Clinical Applications; Gagnon, C., Ed.; Cache River Press: Vienna, Austria, 1999; pp. 304–331. [Google Scholar]
- Jamieson, B.G.M.; Dallai, R.; Afzelius, B.A. Insects: Their Spermatozoa and Phylogeny; Science Publishers, Inc.: Enfield, NH, USA, 1999. [Google Scholar]
- Darwin, C. The Descent of Man and Selection in Relation to Sex; John Murray: London, UK, 1871. [Google Scholar]
- West-Eberhard, M.J. Sexual selection, social competition, and speciation. Quart. Rev. Biol. 1983, 58, 155–183. [Google Scholar] [CrossRef]
- Andersson, M. Sexual Selection; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Emlen, D.J. The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 387–413. [Google Scholar] [CrossRef]
- Simmons, L.W.; Fitzpatrick, J.L. Sperm competition and the coevolution of pre- and post-copulatory traits: Weapons evolve faster than testes among onthophagine dung beetles. Evolution 2016, 70, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Reuland, C.; Simmons, L.W.; Lüpold, S.; Fitzpatrick, J.L. Weapons evolve faster than sperm in even-toed ungulates. Cells 2021, 10, 1062. [Google Scholar] [CrossRef]
- Parker, G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970, 45, 525–567. [Google Scholar] [CrossRef]
- Parker, G.A. How soon hath time… A history of two “seminal” publications. Cells 2021, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A.; Baker, R.R.; Smith, V.G.F. The origin and evolution of gamete dimorphism and the male-female phenomenon. J. Theor. Biol. 1972, 36, 529–553. [Google Scholar] [CrossRef]
- Simmons, L.W.; Wedell, N. Fifty years of sperm competition: The structure of a scientific revolution. Philos. Trans. R. Soc. B 2020, 375, 20200060. [Google Scholar] [CrossRef]
- Thornhill, R. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am. Nat. 1983, 122, 765–788. [Google Scholar] [CrossRef]
- Eberhard, W.G. Female Control: Sexual Selection by Cryptic Female Choice; Princeton University Press: Princeton, NJ, USA, 1996. [Google Scholar]
- Firman, R.C.; Gasparini, C.; Manier, M.K.; Pizzari, T. Postmating female control: 20 years of cryptic female choice. Trends Ecol. Evol. 2017, 32, 368–382. [Google Scholar] [CrossRef]
- Sivinski, J. Sexual selection and insect sperm. Fla. Ent. 1979, 63, 99–111. [Google Scholar] [CrossRef]
- Sivinski, J. Sperm in competition. In Sperm Competition and the Evolution of Animal Mating Systems; Smith, R.L., Ed.; Academic Press: London, UK, 1984; pp. 86–115. [Google Scholar]
- Lüpold, S.; Pitnick, S. Sperm form and function: What do we know about the role of sexual selection? Reproduction 2018, 155, R229–R243. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.W.; Fitzpatrick, J.L. Sperm wars and the evolution of male fertility. Reproduction 2012, 144, 519–534. [Google Scholar] [CrossRef]
- Lüpold, S.; de Boer, R.A.; Evans, J.P.; Tomkins, J.L.; Fitzpatrick, J.L. How sperm competition shapes the evolution of testes and sperm: A meta-analysis. Phil. Trans. R. Soc. B 2020, 375, 2020064. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.A. Sperm competition games: Sperm size and sperm number under adult control. Proc. R. Soc. Lond. B 1993, 253, 245–254. [Google Scholar]
- Parker, G.A.; Immler, S.; Pitnick, S.; Birkhead, T.R. Sperm competition games: Sperm size (mass) and number under raffle and displacement and the evolution of P2. J. Theor. Biol. 2010, 264, 1003–1023. [Google Scholar] [CrossRef]
- Pitnick, S.; Wolfner, M.F.; Suarez, S.S. Ejaculate-female and sperm-female interactions. In Sperm Biology An Evolutionary Approach; Birkhead, T.R., Hosken, D.J., Pitnick, S., Eds.; Academic Press: London, UK, 2009; pp. 247–301. [Google Scholar]
- Dybas, L.K.; Dybas, H.S. Coadaptation and taxanomic differentiation of sperm and spermathecae in featherwing beetles. Evolution 1981, 35, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Pitnick, S.; Markow, T.A.; Spicer, G.S. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution 1999, 53, 1804–1822. [Google Scholar] [CrossRef]
- Presgraves, D.C.; Baker, R.H.; Wilkinson, G.S. Coevolution of sperm and female reproductive tract morphology in stalk-eyed flies. Proc. R. Soc. Lond. B 1999, 266, 1041–1047. [Google Scholar] [CrossRef]
- Minder, A.M.; Hosken, D.J.; Ward, P.I. Co-evolution of male and female reproductive characters across the Scathophagidae (Diptera). J. Evol. Biol. 2005, 18, 60–69. [Google Scholar] [CrossRef]
- Morrow, E.H.; Gage, M.J.G. The evolution of sperm length in moths. Proc. R. Soc. Lond. B 2000, 267, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Higginson, D.M.; Miller, K.B.; Segraves, K.A.; Pitnick, S. Female reproductive tract form drives the evolution of complex sperm morphology. Proc. Natl. Acad. Sci. USA 2012, 109, 4538–4543. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.T.; Pitnick, S. Sperm-female coevolution in Drosophila. Science 2002, 298, 1230–1233. [Google Scholar] [CrossRef]
- Lüpold, S.; Manier, M.K.; Berben, K.S.; Smith, K.J.; Daley, B.D.; Buckley, S.H.; Belote, J.M.; Pitnick, S. How multivariate ejaculate traits determine competitive fertilization success in Drosophila Melanogaster. Curr. Biol. 2012, 22, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Lüpold, S.; Pitnick, S.; Berben, K.S.; Blengini, C.S.; Belote, J.M.; Manier, M.K. Female mediation of competitive fertilization success in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2013, 110, 10693–10698. [Google Scholar] [CrossRef]
- Lüpold, S.; Manier, M.K.; Puniamoorthy, N.; Schoff, C.; Starmer, W.T.; Luepold, S.H.B.; Belote, J.M.; Pitnick, S. How sexual selection can drive the evolution of costly sperm ornamentation. Nature 2016, 533, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Humphries, S.; Evans, J.P.; Simmons, L.W. Sperm competition: Linking form to function. BMC Evol. Biol. 2008, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Garland, T., Jr.; Rose, M.R. Experimental Evolution; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 2009. [Google Scholar]
- Kawecki, T.J.; Lenski, R.E.; Ebert, D.; Hollis, B.; Olivieri, I.; Whitlock, M.C. Experimental evolution. Trends Ecol. Evol. 2012, 27, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Linklater, J.R.; Wertheim, B.; Wigby, S.; Chapman, T. Ejaculate depletion patterns evolve in response to experimental manipulation of sex ratio in Drosophila melanogaster. Evolution 2007, 61, 2027–2034. [Google Scholar] [CrossRef]
- Holland, B.; Rice, W.R. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl. Acad. Sci. USA 1999, 96, 5083–5088. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, L.; Millard, A.L.; Martin, O.Y.; Lumley, A.J.; Emerson, B.C.; Gage, M.J.G. Experimental evolution exposes female and male responses to sexual selection and conflict in Tribolium castaneum. Evolution 2011, 65, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Hosken, D.J.; Ward, P.I. Experimental evidence for testis size evolution via sperm competition. Ecol. Let. 2001, 4, 10–13. [Google Scholar] [CrossRef]
- Firman, R.C.; Simmons, L.W. Experimental evolution of sperm quality via postcopulatory sexual selection in house mice. Evolution 2010, 64, 1245–1256. [Google Scholar] [CrossRef]
- Simmons, L.W.; Garcia-Gonzalez, F. Experimental coevolution of male and female genital morphology. Nat. Commun. 2011, 2, 374. [Google Scholar] [CrossRef] [PubMed]
- Firman, R.C.; Gomendio, M.; Roldan, E.R.S.; Simmons, L.W. The coevolution of ova defensiveness with sperm competitiveness in house mice. Am. Nat. 2014, 183, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.L.; Vasudeva, R.; Michalczyk, Ł.; Martin, O.Y.; Lumley, A.J.; Chapman, T.; Gage, M.J.G. Experimental evolution reveals that sperm competition intensity selects for longer, more costly sperm. Evol. Lett. 2017, 1, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.T.; Starmer, W.T.; Pitnick, S. Quantitative genetics of seminal receptacle length in Drosophila melanogaster. Heredity 2001, 87, 25–32. [Google Scholar] [CrossRef][Green Version]
- Gay, L.; Hosken, D.J.; Vasudev, R.; Tregenza, T.; Eady, P.E. Sperm competition and maternal effects differentially influence testis and sperm size in Callosobruchus maculatus. J. Evol. Biol. 2009, 22, 1143–1150. [Google Scholar] [CrossRef]
- Simmons, L.W.; Ridsdill-Smith, T.J. Ecology and Evolution of Dung Beetles; Blackwell Publishing Ltd: Oxford, UK, 2011. [Google Scholar]
- McCullough, E.; Buzatto, B.A.; Simmons, L.W. Benefits of polyandry: Molecular evidence from field-caught dung beetles. Mol. Ecol. 2017, 26, 3546–3555. [Google Scholar] [CrossRef]
- Simmons, L.W.; Beveridge, M.; Krauss, S. Genetic analysis of parentage within experimental populations of a male dimorphic beetle, Onthophagus taurus, using amplified fragment length polymorphism. Behav. Ecol. Sociobiol. 2004, 57, 164–173. [Google Scholar] [CrossRef]
- Tomkins, J.L.; Simmons, L.W. Sperm competition games played by dimorphic male beetles: Fertilisation gains with equal mating access. Proc. R. Soc. Lond. B 2000, 267, 1547–1553. [Google Scholar] [CrossRef]
- Parker, G.A.; Pizzari, T. Sperm competition and ejaculate economics. Biol. Rev. 2010, 85, 897–934. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.W.; Emlen, D.J.; Tomkins, J.L. Sperm competition games between sneaks and guards: A comparative analysis using dimorphic male beetles. Evolution 2007, 61, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.W.; García-González, F. Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung beetles. Evolution 2008, 62, 2580–2591. [Google Scholar] [CrossRef]
- García-González, F.; Simmons, L.W. Shorter sperm confer higher competitive fertilization success. Evolution 2007, 61, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Simmons, L.W. Ultrastructure of spermatozoa of Onthophagus taurus (Coleoptera, Scarabaeidae) exhibits heritable variation. Naturewissenschaften 2011, 98, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, M.A.; Nakagawa, S.; Schielzeth, H. rptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 2017, 8, 1639–1644. [Google Scholar] [CrossRef]
- Simmons, L.W.; Kotiaho, J.S. Quantitative genetic correlation between trait and preference supports a sexually selected sperm process. Proc. Natnl. Acad. Sci. USA 2007, 104, 16604–16608. [Google Scholar] [CrossRef]
- Simmons, L.W.; Kotiaho, J.S. Evolution of ejaculates: Patterns of phenotypic and genotypic variation and condition dependence in sperm competition traits. Evolution 2002, 56, 1622–1631. [Google Scholar] [CrossRef]
- Simmons, L.W.; House, C.M.; Hunt, J.; García-González, F. Evolutionary response to sexual selection in male genital morphology. Curr. Biol. 2009, 19, 1442–1446. [Google Scholar] [CrossRef]
- Pitnick, S.; Miller, G.T.; Reagan, J.; Holland, B. Males’ evolutionary responses to experimental removal of sexual selection. Proc. R. Soc. Lond. B 2001, 268, 1071–1080. [Google Scholar] [CrossRef]
- Karr, T.L.; Swanson, W.J.; Snook, R.R. The evolutionary significance of variation in sperm-egg interactions. In Sperm Biology; Birkhead, T.R., Hosken, D.J., Pitnick, S., Eds.; Academic Press: London, UK, 2009; pp. 305–365. [Google Scholar]
- Malo, A.F.; Gomendio, M.; Garde, J.; Lang-Lenton, B.; Soler, A.J.; Roldan, E.R.S. Sperm design and sperm function. Biol. Lett. 2006, 2, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Støstad, H.N.; Johnsen, A.; Lifjeld, J.T.; Rowe, M. Sperm head morphology is associated with sperm swimming speed: A comparative study of songbirds using electron microscopy. Evolution 2018, 72, 1918–1932. [Google Scholar] [CrossRef] [PubMed]
- Janicke, T.; Sandner, P.; Ramm, S.A.; Vizoso, D.B.; Schärer, L. Experimentally evolved and phenotypically plastic responses to enforced monogamy in a hermaphroditic flatworm. J. Evol. Biol. 2016, 29, 1713–1727. [Google Scholar] [CrossRef] [PubMed]
- Schärer, L.; Littlewood, D.T.J.; Waeschenbach, A.; Yoshida, W.; Vizoso, D.B. Mating behavior and the evolution of sperm design. Proc. Natl. Acad. Sci. USA 2011, 108, 1490–1495. [Google Scholar] [CrossRef]
- Friesen, C.R.; Kahrl, A.F.; Olsson, M. Sperm competition in squamate reptiles. Phil. Trans. R. Soc. B 2020, 375, 20200079. [Google Scholar] [CrossRef] [PubMed]
- Kahrl, A.F.; Johnson, M.A.; Cox, R.M. Rapid evolution of testis size relative to sperm morphology suggests that post-copulatory selection targets sperm number in Anolis lizards. J. Evol. Biol. 2019, 32, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.L.; Bridge, C.D.; Snook, R.R. Repeated evidence that the accelerated evolution of sperm is associated with their fertilization function. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201286. [Google Scholar] [CrossRef]
- Avidor-Reiss, T. Rapid evolution of sperm produces diverse centriole structures that reveal the most rudimentary structure needed for function. Cells 2018, 7, 67. [Google Scholar] [CrossRef]
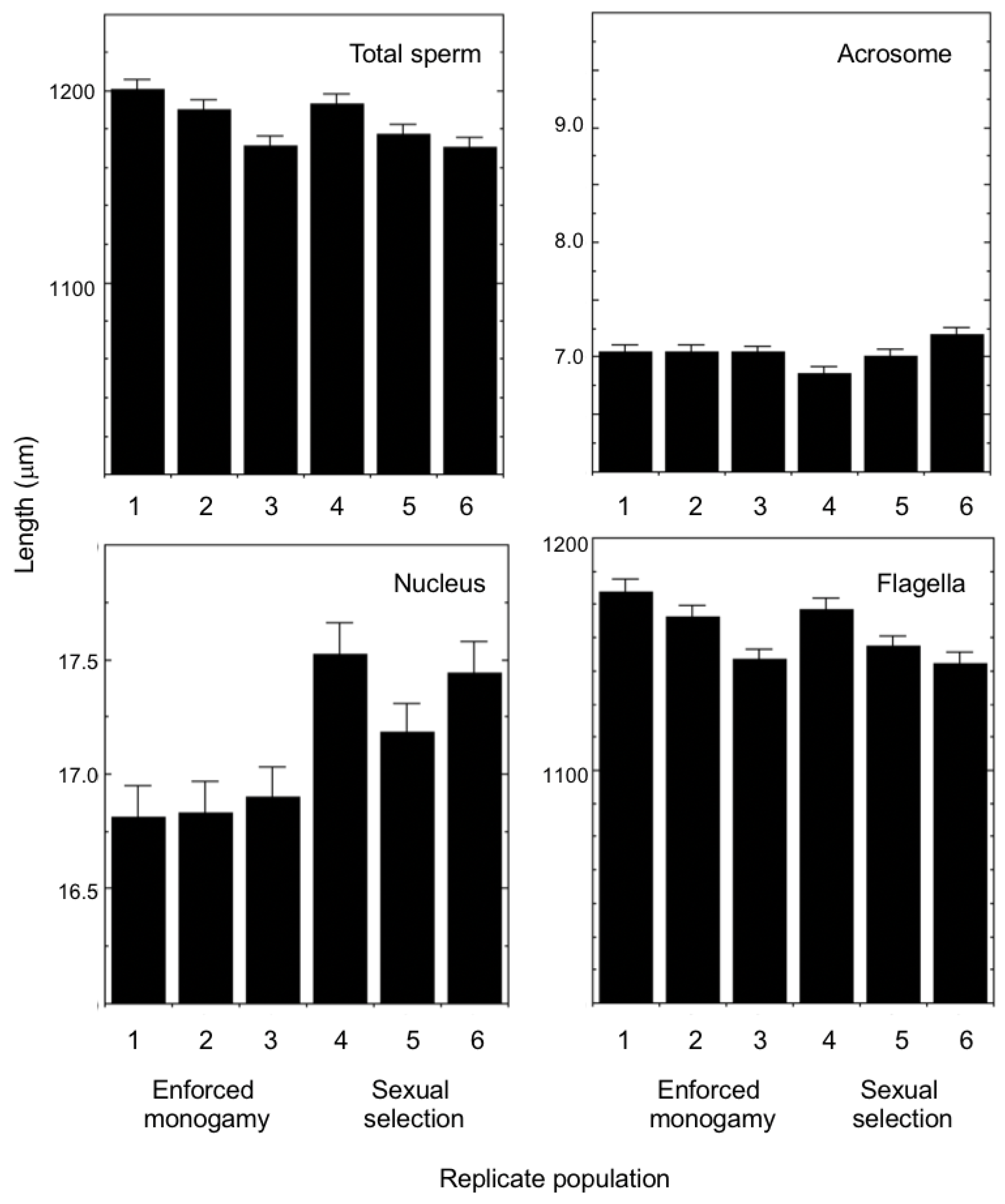
| Measure Effect | MS | df | F | P |
|---|---|---|---|---|
| Total sperm length | ||||
| Selection history | 1321.28 | 1 | 0.41 | 0.555 |
| Replicate [Selection history] | 3197.65 | 4 | 6.87 | <0.001 |
| Error | 47,492.59 | 102 | ||
| Acrosome length | ||||
| Selection history | 0.02 | 1 | 0.06 | 0.820 |
| Replicate [Selection history] | 0.25 | 4 | 3.74 | 0.007 |
| Error | 0.07 | 102 | ||
| Nucleus length | ||||
| Selection history | 7.59 | 1 | 25.24 | 0.007 |
| Replicate [Selection history] | 0.30 | 4 | 0.90 | 0.466 |
| Error | 0.33 | 102 | ||
| Flagella length | ||||
| Selection history | 3223.65 | 1 | 0.47 | 0.531 |
| Replicate [Selection history] | 3231.33 | 4 | 6.96 | <0.001 |
| Error | 47,351.24 | 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simmons, L.W.; Garcia-Gonzalez, F. Can Sexual Selection Drive the Evolution of Sperm Cell Structure? Cells 2021, 10, 1227. https://doi.org/10.3390/cells10051227
Simmons LW, Garcia-Gonzalez F. Can Sexual Selection Drive the Evolution of Sperm Cell Structure? Cells. 2021; 10(5):1227. https://doi.org/10.3390/cells10051227
Chicago/Turabian StyleSimmons, Leigh W., and Francisco Garcia-Gonzalez. 2021. "Can Sexual Selection Drive the Evolution of Sperm Cell Structure?" Cells 10, no. 5: 1227. https://doi.org/10.3390/cells10051227
APA StyleSimmons, L. W., & Garcia-Gonzalez, F. (2021). Can Sexual Selection Drive the Evolution of Sperm Cell Structure? Cells, 10(5), 1227. https://doi.org/10.3390/cells10051227







