Angiotensinergic Neurotransmissions in the Medial Amygdala Nucleus Modulate Behavioral Changes in the Forced Swimming Test Evoked by Acute Restraint Stress in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgery Procedure
2.3. Drug Microinjection into the Brain
2.4. Restraint Stress
2.5. Forced Swimming Test (FST)
2.6. Drugs and Solutions
2.7. Experimental Design
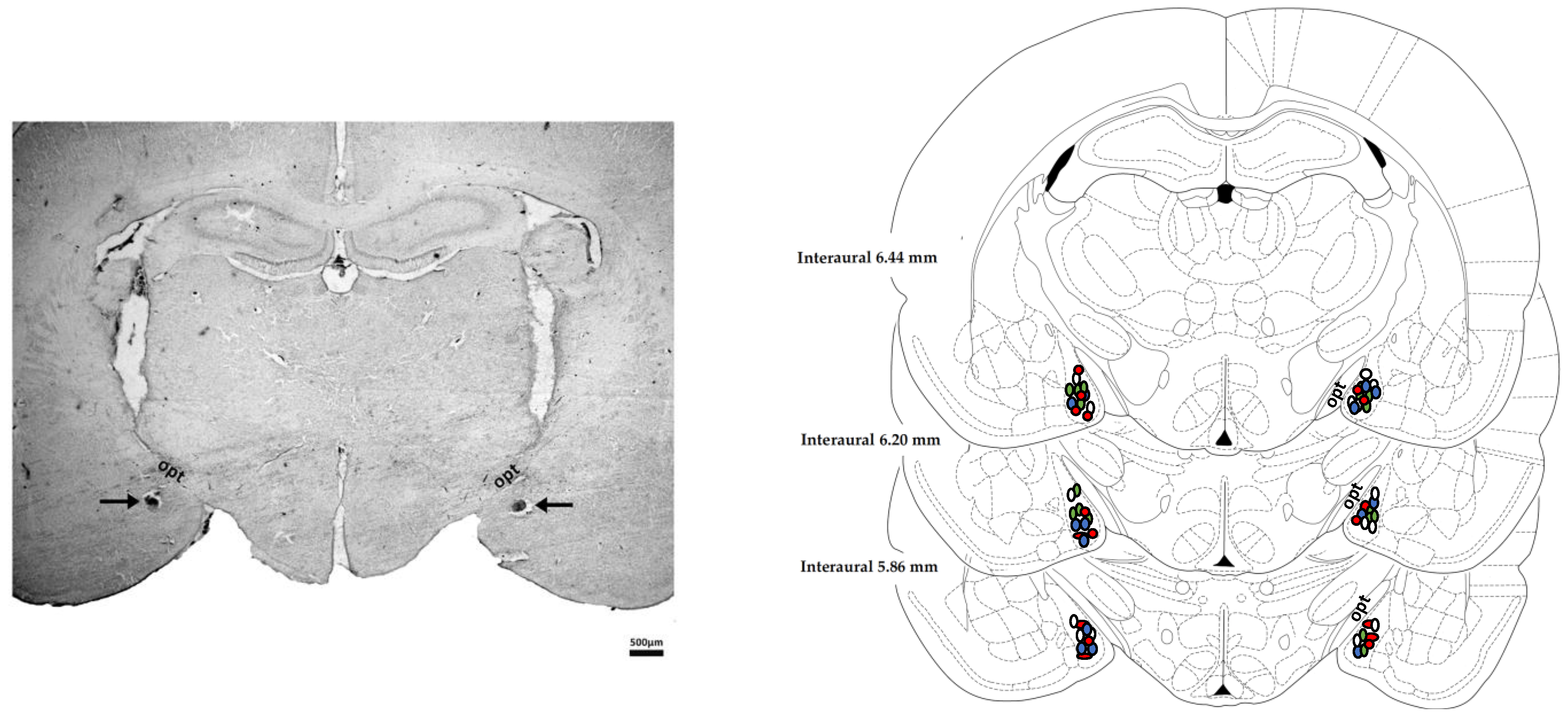
2.8. Histological Determination of the Microinjection Sites
2.9. Data Analysis
3. Results
3.1. Determination of the Microinjection Sites
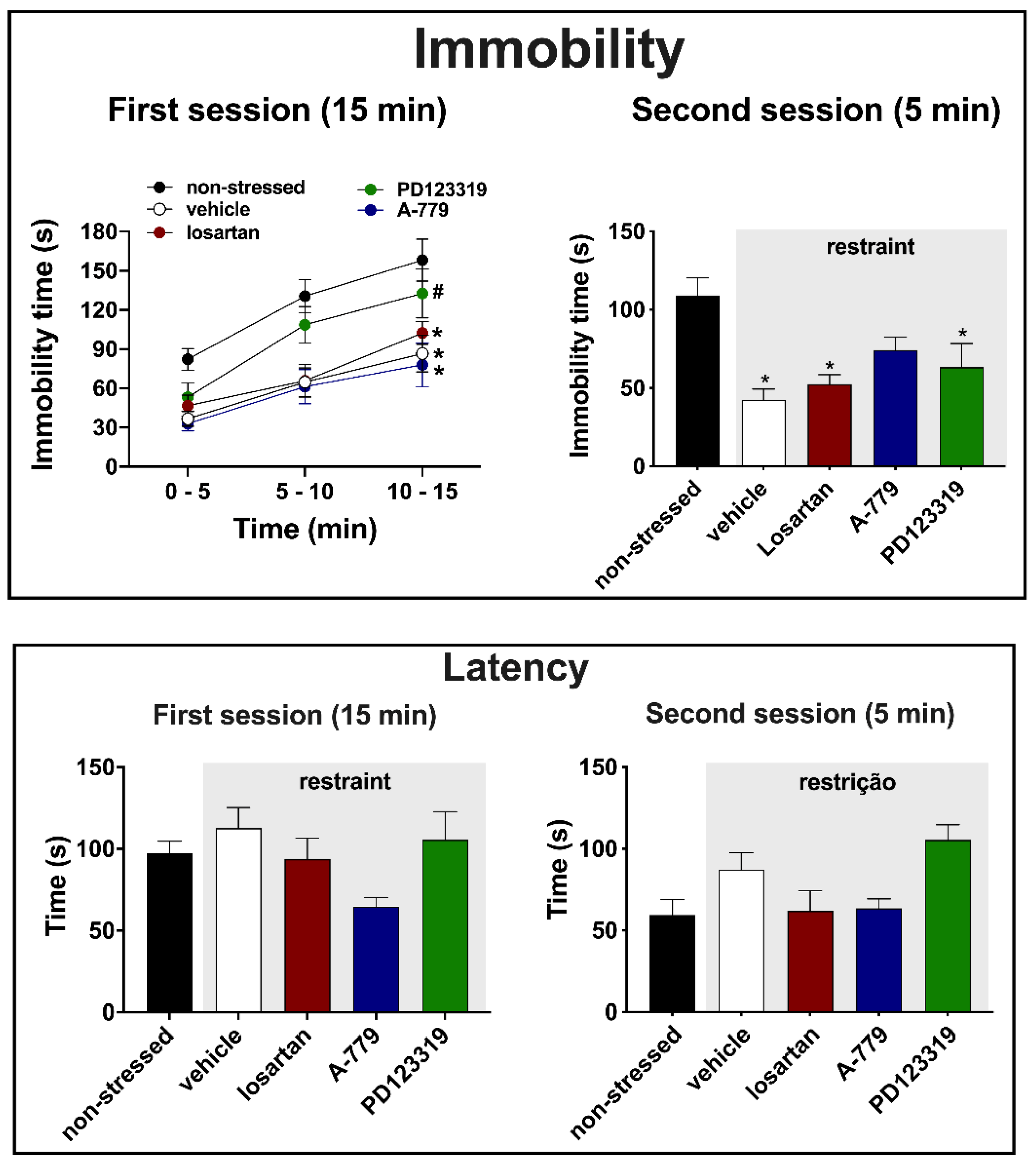
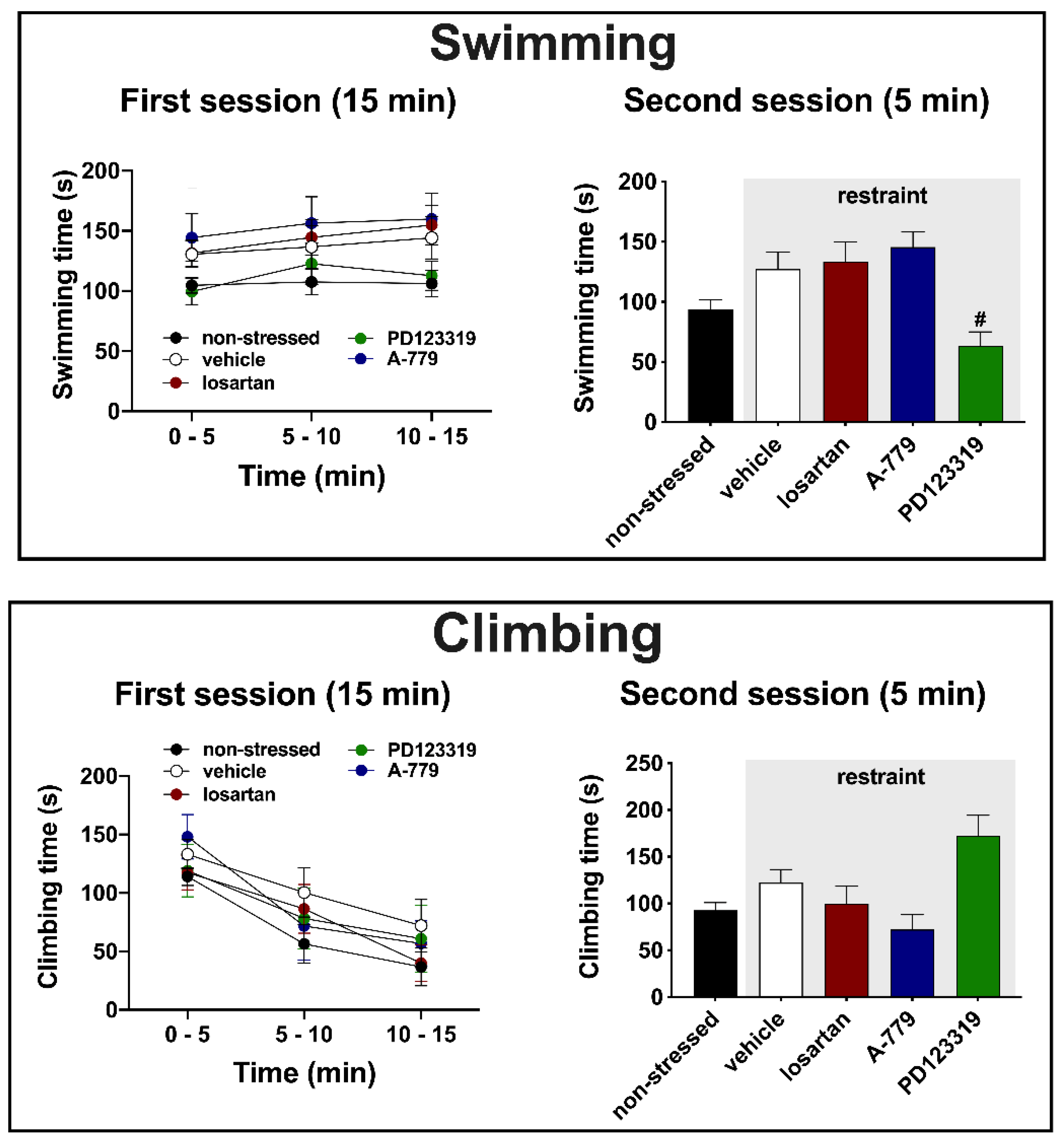
3.2. Effects of MeA Treatment with Angiotensinergic Antagonists on Behavioral Changes in the Forced Swimming Test Evoked by Acute Restraint Stress
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vian, J.; Pereira, C.; Chavarria, V.; Köhler, C.; Stubbs, B.; Quevedo, J.; Kim, S.-W.; Carvalho, A.F.; Berk, M.; Fernandes, B.S. The renin–angiotensin system: A possible new target for depression. BMC Med. 2017, 15, 144. [Google Scholar] [CrossRef]
- Jakubovski, E.; Bloch, M.H. Prognostic Subgroups for Citalopram Response in the STAR*D Trial. J. Clin. Psychiatry 2014, 75, 738–747. [Google Scholar] [CrossRef]
- Papaioannou, A.; Gerozissis, K.; Prokopiou, A.; Bolaris, S.; Stylianopoulou, F. Sex differences in the effects of neonatal handling on the animal’s response to stress and the vulnerability for depressive behaviour. Horm. Behav. 2002, 129, 131–139. [Google Scholar] [CrossRef]
- Armario, A.; Gil, M.; Marti, J.; Pol, O.; Balasch, J. Influence of various acute stressors on the activity of adult male rats in a holeboard and in the forced swim test. Pharmacol. Biochem. Behav. 1991, 39, 373–377. [Google Scholar] [CrossRef]
- Bernal-Morales, B.; Contreras, C.M.; Cueto-Escobedo, J. Acute restraint stress produces behavioral despair in weanling rats in the forced swim test. Behav. Process. 2009, 82, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Sevgi, S.; Ozek, M.; Erogly, L. L-NAME prevents anxiety-like and depression-like behavior in rats exposed to restraint stress. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 95–99. [Google Scholar] [CrossRef]
- Suvrathan, A.; Tomar, A.; Chattarji, S. Effects of chronic and acute stress on rat behaviour in the forced-swim test. Stress 2010, 13, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Sigmund, C.D.; Diz, D.I.; Chappell, M.C. No Brain Renin-Angiotensin System Déjà vu All over Again? Hypertension 2017, 69, 1007–1010. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santo, M.J. The ACE2/Angiotensin-(1-7)/Mas axis of the renin-angiotensin system: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.A.P.; Martins Lima, A.; Santos, R.A.S. Dos Brain angiotensin-(1–7)/Mas axis: A new target to reduce the cardiovascular risk to emotional stress. Neuropeptides 2016, 56, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Gironacci, M.M.; Vicario, A.; Cerezo, G.; Silva, M.G. The depressor axis of the renin–angiotensin system and brain disorders: A translational approach. Clin. Sci. 2018, 132, 1021–1038. [Google Scholar] [CrossRef]
- Karnik, S.S.; Unal, H.; Kemp, J.R.; Tirupula, K.C.; Eguchi, S.; Vanderheyden, P.M.L.; Thomas, W.G. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected]. Pharmacol. Rev. 2015, 67, 754–819. [Google Scholar] [CrossRef] [PubMed]
- Bali, A.; Jaggi, A.S. Angiotensin as stress mediator: Role of its receptor and interrelationships among other stress mediators and receptors. Pharmacol. Res. 2013, 76, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.M. Beneficial effects of Angiotensin II receptor blockers in brain disorders. Pharmacol. Res. 2017, 125, 91–103. [Google Scholar] [CrossRef]
- De Melo, L.A.; Almeida-Santos, A.F. Neuropsychiatric Properties of the ACE2/Ang-(1-7)/Mas Pathway: A Brief Review. Protein Pept. Lett. 2019, 27, 476–483. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Armando, I.; Bregonzio, C.; Juorio, A.; Macova, M.; Pavel, J.; Sanchez-Lemus, E. A Centrally Acting, Anxiolytic Angiotensin II AT1 Receptor Antagonist Prevents the Isolation Stress-Induced Decrease in Cortical CRF1 Receptor and Benzodiazepine Binding. Neuropsychopharmacology 2006, 54, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.M.; Ando, H.; Armando, I.; Baiardi, G.; Bregonzio, C.; Jezova, M.; Zhou, J. Brain Angiotensin II, an Important Stress Hormone: Regulatory Sites and Therapeutic Opportunities. Ann. N. Y. Acad. Sci. 2004, 1018, 76–84. [Google Scholar] [CrossRef]
- Watanabe, T.; Fujioka, T.; Hashimoto, M.; Nakamura, S. Stress and Brain Angiotensin II Receptors. Crit. Rev. Neurobiol. 1998, 12, 305–317. [Google Scholar] [CrossRef]
- Wright, J.W.; Harding, J.W. Brain renin-angiotensin—A new look at an old system. Prog. Neurobiol. 2011, 95, 49–67. [Google Scholar] [CrossRef]
- Ayyub, M.; Najmi, A.; Akhtar, M. Protective Effect of Irbesartan an Angiotensin (AT1) Receptor Antagonist in Unpredictable Chronic Mild Stress Induced Depression in Mice. Drug Res. Stuttg. 2016, 67, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ping, G.; Qian, W.; Song, G.; Zhaochun, S. Valsartan reverses depressive/anxiety-like behavior and induces hippocampal neurogenesis and expression of BDNF protein in unpredictable chronic mild stress mice. Pharmacol. Biochem. Behav. 2014, 124, 5–12. [Google Scholar] [CrossRef] [PubMed]
- VIJAYAPANDI, P.; NAGAPPA, A.N. Biphasic Effects of Losartan Potassium on Immobility in Mice. Yakugaku Zasshi 2005, 125, 653–657. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gard, P.R.; Mandy, A.; Sutcliffe, M.A. Evidence of a possible role of altered angiotensin function in the treatment, but not etiology, of depression. Biol. Psychiatry 1999, 45, 1030–1034. [Google Scholar] [CrossRef]
- Diniz, C.R.A.F.; Casarotto, P.C.; Fred, S.M.; Biojone, C.; Castrén, E.; Joca, S.R.L. Antidepressant-like effect of losartan involves TRKB transactivation from angiotensin receptor type 2 (AGTR2) and recruitment of FYN. Neuropharmacology 2018, 135, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Santos, A.F.; Kangussu, L.M.; Moreira, F.A.; Santos, R.A.S.; Aguiar, D.C.; Campagnole-Santos, M.J. Anxiolytic- and antidepressant-like effects of angiotensin-(1-7) in hypertensive transgenic (mRen2)27 rats. Clin. Sci. 2016, 130, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Kangussu, L.M.; Almeida-Santos, A.F.; Bader, M.; Alenina, N.; Fontes, M.A.Ô.P.; Santos, R.A.S.; Aguiar, D.C.; Campagnole-Santos, M.J. Angiotensin-(1-7) attenuates the anxiety and depression-like behaviors in transgenic rats with low brain angiotensinogen. Behav. Brain Res. 2013. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, J.M.; Armando, I. Angiotensin II AT2 Receptors Contribute to Regulate the Sympathoadrenal and Hormonal Reaction to Stress Stimuli. Cell. Mol. Neurobiol. 2018, 38, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, W.E.; Herman, J.P.; Battaglia, D.F.; Akil, H.; Watson, S.J. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 1995, 64, 477–505. [Google Scholar] [CrossRef]
- Furlong, T.M.; McDowall, L.M.; Horiuchi, J.; Polson, J.W.; Dampney, R.A.L. The effect of air puff stress on c-Fos expression in rat hypothalamus and brainstem: Central circuitry mediating sympathoexcitation and baroreflex resetting. Eur. J. Neurosci. 2014, 39, 1429–1438. [Google Scholar] [CrossRef]
- Davern, P.J.; Head, G.A. Role of the medial amygdala in mediating responses to aversive stimuli leading to hypertension. Clin. Exp. Pharmacol. Physiol. 2011, 38, 136–143. [Google Scholar] [CrossRef]
- Estrada, C.M.; Ghisays, V.; Nguyen, E.T.; Caldwell, J.L.; Streicher, J.; Solomon, M.B. Estrogen signaling in the medial amygdala decreases emotional stress responses and obesity in ovariectomized rats. Horm. Behav. 2018, 98, 33–44. [Google Scholar] [CrossRef]
- Salomé, N.; Stemmelin, J.; Cohen, C.; Griebel, G. Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacol. Berl. 2006, 187, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kazuaki, K.; Hiroaki, A.; Hironaka, A.; Susumu, O. Effects of Minaprine and Sulpiride Injected into the Amygdaloid Nucleus on the Duration of Immobility in Rats Forced to Swim. Jpn. J. Pharmacol. 1990, 53, 411–413. [Google Scholar] [CrossRef]
- Araki, H.; Kawashima, K.; Uchiyma, Y.; Aihara, H. Involvement of amygdaloid catecholaminergic mechanism in suppressive effects of desipramine and imipramine on duration of immobility in rats forced to swim. Eur. J. Pharmacol. 1985, 113, 313–318. [Google Scholar] [CrossRef]
- Kazuaki Kawashima; Hiroaki Araki; Yoshimi Uchiyama; Hironaka Aihara Amygdaloid catecholaminergic mechanisms involved in suppressive effects of electroconvulsive shock on duration of immobility in rat forced to swim. Eur. J. Pharmacol. 1987, 141, 1–6. [CrossRef]
- Lenkei, Z.; Palkovits, M.; Corvol, P.; Llorens-Cortès, C. Expression of Angiotensin Type-1 (AT1) and Type-2 (AT2) Receptor mRNAs in the Adult Rat Brain: A Functional Neuroanatomical Review. Front. Neuroendocrinol. 1997, 18, 383–439. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.R.; Hawelu-Johnson, C.L.; Guyenet, P.G. Localization of brain angiotensinogen mRNA by hybridization histochemistry. Brain Res. 1987, 388, 149–158. [Google Scholar] [CrossRef]
- Von Bohlen und Halbach, O. The renin-angiotensin system in the mammalian central nervous system. Curr. Protein Pept. Sci. 2005, 6, 355–371. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, A.D.; Wang, L.; Ludin, J.A.; Smith, J.A.; Pioquinto, D.J.; Hiller, H.; Steckelings, U.M.; Scheuer, D.A.; Sumners, C.; Krause, E.G. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct. Funct. 2016, 221, 891–912. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 3rd ed.; Acdemic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Fortaleza, E.A.T.; Tavares, R.F.; Corrêa, F.M.A. The medial amygdaloid nucleus modulates cardiovascular responses to acute restraint in rats. Neuroscience 2009, 159, 717–726. [Google Scholar] [CrossRef]
- Moreno-Santos, B.; Marchi-Coelho, C.; Costa-Ferreira, W.; Crestani, C.C. Angiotensinergic receptors in the medial amygdaloid nucleus differently modulate behavioral responses in the elevated plus-maze and forced swimming test in rats. Behav. Brain Res. 2021, 397, 112947. [Google Scholar] [CrossRef] [PubMed]
- Fortaleza, E.A.T.; Ferreira-Junior, N.C.; Lagatta, D.C.; Resstel, L.B.M.; Corrêa, F.M.A. The medial amygdaloid nucleus modulates the baroreflex activity in conscious rats. Auton. Neurosci. 2015, 193, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology Berl. 2005, 177, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Duarte, J.O.; Oliveira, L.A.; Crestani, C.C. Effects of nitric oxide synthesis inhibitor or fluoxetine treatment on depression-like state and cardiovascular changes induced by chronic variable stress in rats. Stress 2015, 18, 462–474. [Google Scholar] [CrossRef] [PubMed]
- PORSOLT, R.D.; LE PICHON, M.; JALFRE, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Abelaira, H.M.; Reus, G.Z.; Quevedo, J. Animal models as tools to study the pathophysiology of depression. Rev. Bras. Psiquiatr. 2013, 35, S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Detke, M.J.; Rickels, M.; Lucki, I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacol. Berl. 1995, 121, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef]
- Oscar, C.G.; Müller-Ribeiro, F.C.D.F.; de Castro, L.G.; Martins Lima, A.; Campagnole-Santos, M.J.; Santos, R.A.S.; Xavier, C.H.; Fontes, M.A.P. Angiotensin-(1–7) in the basolateral amygdala attenuates the cardiovascular response evoked by acute emotional stress. Brain Res. 2015, 1594, 183–189. [Google Scholar] [CrossRef]
- Costa-Ferreira, W.; Morais-Silva, G.; Gomes-de-Souza, L.; Marin, M.T.; Crestani, C.C. The AT1 Receptor Antagonist Losartan Does Not Affect Depressive-Like State and Memory Impairment Evoked by Chronic Stressors in Rats. Front. Pharmacol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Costa-Ferreira, W.; Gomes-de-Souza, L.; Crestani, C.C. Role of angiotensin receptors in the medial amygdaloid nucleus in autonomic, baroreflex and cardiovascular changes evoked by chronic stress in rats. Eur. J. Neurosci. 2021, 53, 763–777. [Google Scholar] [CrossRef]
- Prince, C.R.; Anisman, H. Acute and chronic stress effects on performance in a forced-swim task. Behav. Neural Biol. 1984, 42, 99–119. [Google Scholar] [CrossRef]
- Platt, J.E.; Stone, E. A Chronic restraint stress elicits a positive antidepressant response on the forced swim test. Eur. J. Pharmacol. 1982, 82, 179–181. [Google Scholar] [CrossRef]
- Weiss, J.A.Y.M.; Goodman, P.A.; Charry, J.M.; Bailey, W.H.; Losito, G.; Corrigan, S. BEHAVIORAL DEPRESSION PRODUCED BY AN UNCONTROLLABLE STRESSOR: RELATIONSHIP TO NOREPINEPHRINE, DOPAMINE, AND SEROTONIN LEVELS IN VARIOUS REGIONS OF RAT BRAIN. Brain Res. Rev. 1981, 3, 167–205. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Freitas, A.E.; Neis, V.B.; Santos, D.B.; Ribeiro, C.M.; Rosa, P.B.; Farina, M.; Rodrigues, A.L.S. Guanosine prevents behavioral alterations in the forced swimming test and hippocampal oxidative damage induced by acute restraint stress. Pharmacol. Biochem. Behav. 2014, 127, 7–14. [Google Scholar] [CrossRef]
- Moretti, M.; Budni, J.; dos Santos, D.B.; Antunes, A.; Daufenbach, J.F.; Manosso, L.M.; Farina, M.; Rodrigues, A.L.S. Protective Effects of Ascorbic Acid on Behavior and Oxidative Status of Restraint-Stressed Mice. J. Mol. Neurosci. 2013, 49, 68–79. [Google Scholar] [CrossRef]
- Campos, A.C.; Ferreira, F.R.; Guimarães, F.S.; Lemos, J.I. Facilitation of endocannabinoid effects in the ventral hippocampus modulates anxiety-like behaviors depending on previous stress experience. Neuroscience 2010, 167, 238–246. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Reis, F.M.C.V.; Campos, A.C.; Guimarães, F.S. Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior: Involvement of 5HT1A receptors and previous stressful experience. Eur. Neuropsychopharmacol. 2014, 24, 410–419. [Google Scholar] [CrossRef]
- Gomes-de-Souza, L.; Bianchi, P.C.; Costa-Ferreira, W.; Tomeo, R.A.; Cruz, F.C.; Crestani, C.C. CB1 and CB2 receptors in the bed nucleus of the stria terminalis differently modulate anxiety-like behaviors in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 110, 110284. [Google Scholar] [CrossRef]
- Carlezon, W.A.; Mague, S.D.; Andersen, S.L. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol. Psychiatry 2003, 54, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Camarena, E.; Fernández-Guasti, A.; López-Rubalcava, C. Antidepressant-Like Effect of Different Estrogenic Compounds in the Forced Swimming Test. Neuropsychopharmacology 2003, 28, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Mague, S.D.; Pliakas, A.M.; Todtenkopf, M.S.; Tomasiewicz, H.C.; Zhang, Y.; Stevens, W.C.; Jones, R.M.; Portoghese, P.S.; Carlezon, W.A. Antidepressant-Like Effects of κ-Opioid Receptor Antagonists in the Forced Swim Test in Rats. J. Pharmacol. Exp. Ther. 2003, 305, 323–330. [Google Scholar] [CrossRef]
- Slattery, D.A.; Desrayaud, S.; Cryan, J.F. GABA B Receptor Antagonist-Mediated Antidepressant-Like Behavior Is Serotonin-Dependent. J. Pharmacol. Exp. Ther. 2005, 312, 290–296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchi-Coelho, C.; Costa-Ferreira, W.; Reis-Silva, L.L.; Crestani, C.C. Angiotensinergic Neurotransmissions in the Medial Amygdala Nucleus Modulate Behavioral Changes in the Forced Swimming Test Evoked by Acute Restraint Stress in Rats. Cells 2021, 10, 1217. https://doi.org/10.3390/cells10051217
Marchi-Coelho C, Costa-Ferreira W, Reis-Silva LL, Crestani CC. Angiotensinergic Neurotransmissions in the Medial Amygdala Nucleus Modulate Behavioral Changes in the Forced Swimming Test Evoked by Acute Restraint Stress in Rats. Cells. 2021; 10(5):1217. https://doi.org/10.3390/cells10051217
Chicago/Turabian StyleMarchi-Coelho, Camila, Willian Costa-Ferreira, Lilian L. Reis-Silva, and Carlos C. Crestani. 2021. "Angiotensinergic Neurotransmissions in the Medial Amygdala Nucleus Modulate Behavioral Changes in the Forced Swimming Test Evoked by Acute Restraint Stress in Rats" Cells 10, no. 5: 1217. https://doi.org/10.3390/cells10051217
APA StyleMarchi-Coelho, C., Costa-Ferreira, W., Reis-Silva, L. L., & Crestani, C. C. (2021). Angiotensinergic Neurotransmissions in the Medial Amygdala Nucleus Modulate Behavioral Changes in the Forced Swimming Test Evoked by Acute Restraint Stress in Rats. Cells, 10(5), 1217. https://doi.org/10.3390/cells10051217






