The Power of Stress: The Telo-Hormesis Hypothesis
Abstract
1. Introduction
2. Telomeres: Basic Molecular Mechanisms in an Evolutionary Context
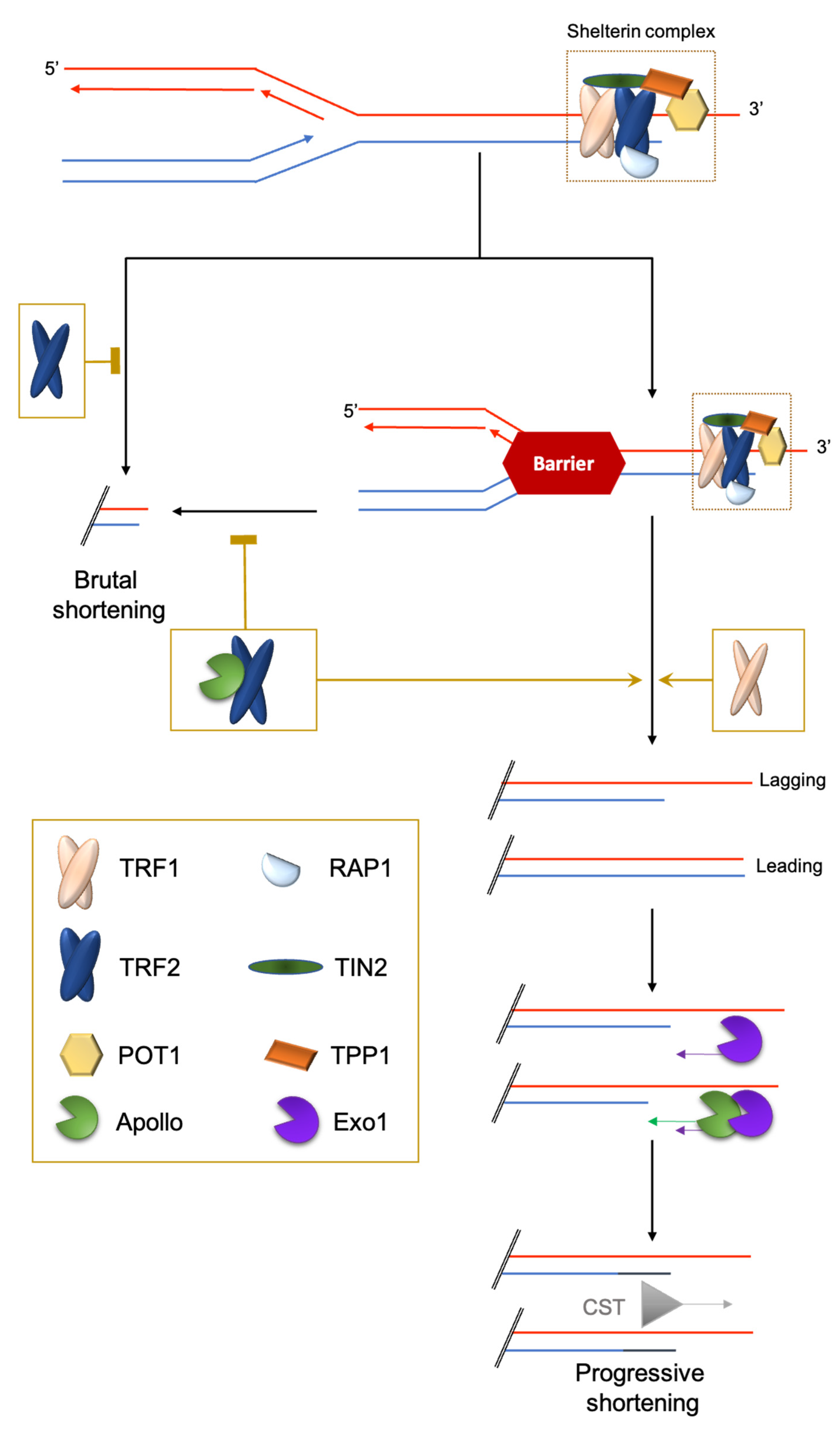
2.1. Challenge 1: The End-Stability Problem
2.2. Challenge 2: End Replication Problem
2.3. Challenge 3: The Hard-to-Replicate Problem
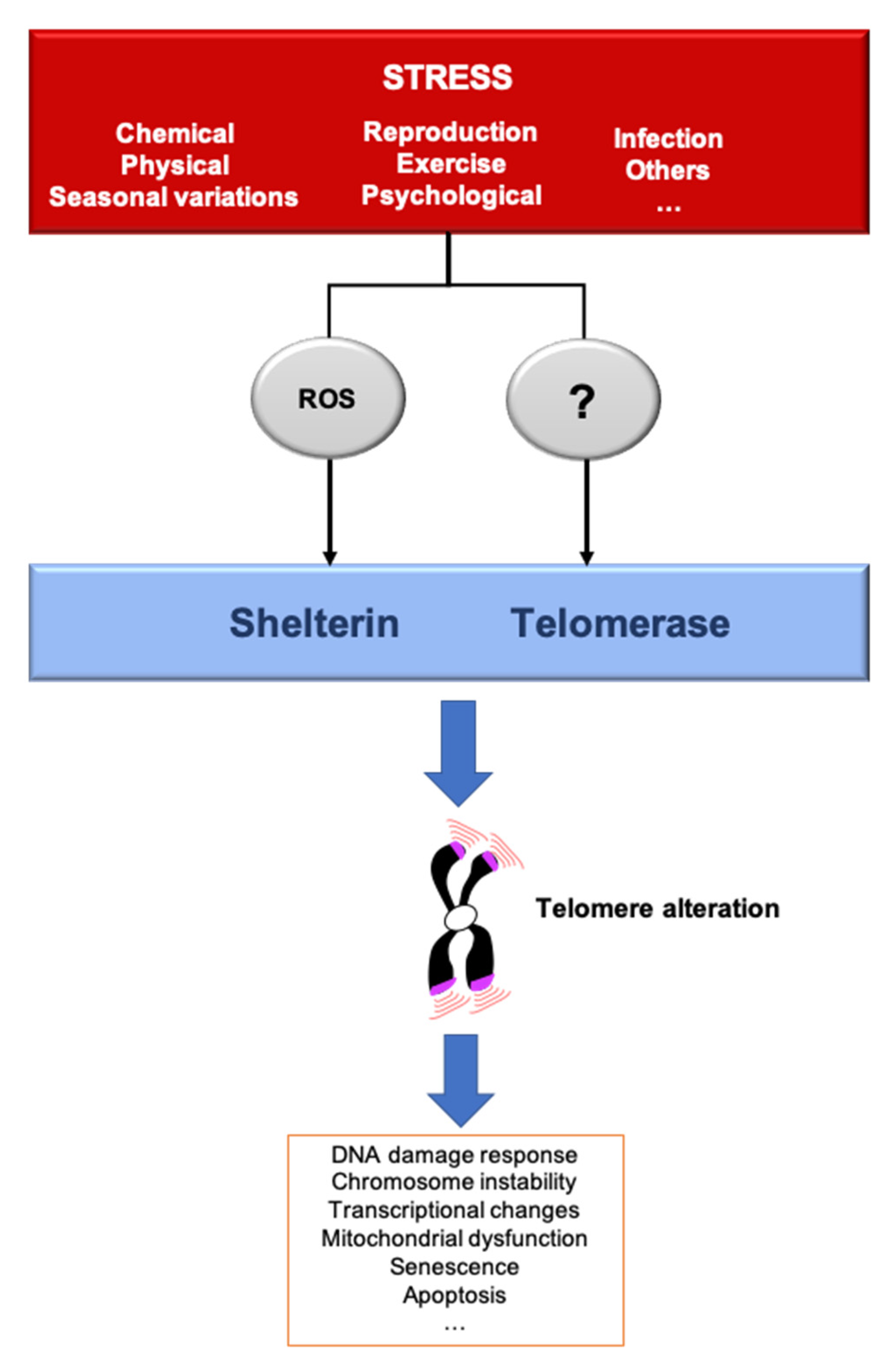
3. Telomere Response to Stress
3.1. Inflammation
3.2. Life Factors
3.3. Chemical Stress
3.4. Physical Stress
4. Telomere and Life-History Trade-Off
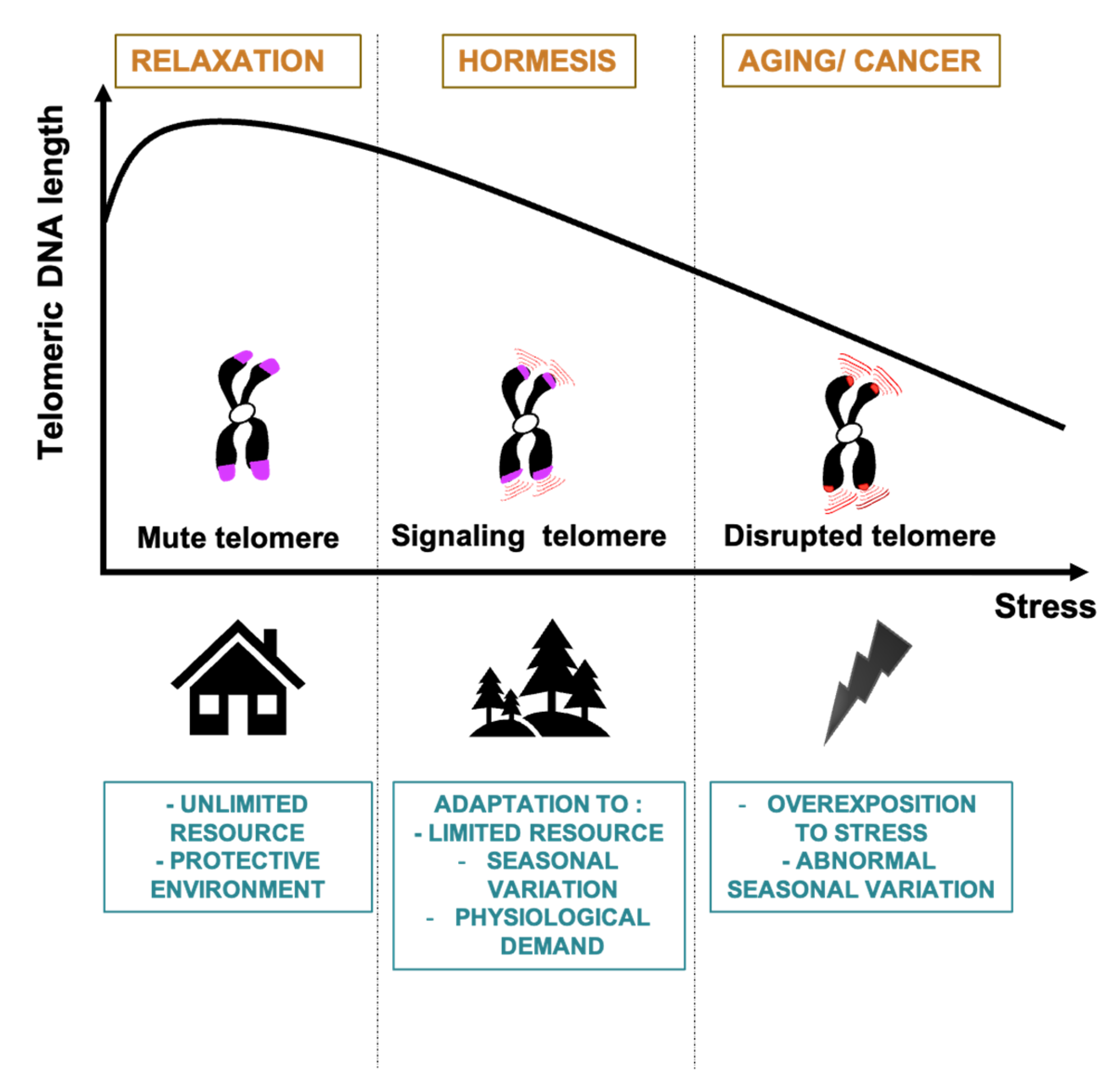
5. The Telomere Hormesis Hypothesis
5.1. Telomere Changes to Prevent Tumor Formation
5.2. A Hormetic Effect of the Telomere Position Effect
5.3. Telomeres and Mito-Hormesis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Radman, M. SOS Repair Hypothesis: Phenomenology of an Inducible DNA Repair Which is Accompanied by Mutagenesis. In Molecular Mechanisms for Repair of DNA: Part A; Basic Life Sciences; Hanawalt, P.C., Setlow, R.B., Eds.; Springer: Boston, MA, USA, 1975; pp. 355–367. ISBN 978-1-4684-2895-7. [Google Scholar]
- Giraud, A.; Matic, I.; Tenaillon, O.; Clara, A.; Radman, M.; Fons, M.; Taddei, F. Costs and Benefits of High Mutation Rates: Adaptive Evolution of Bacteria in the Mouse Gut. Science 2001, 291, 2606–2608. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Panis, M.-J.; Pisano, S.; Poulet, A.; Le Du, M.-H.; Gilson, E. Structural Identity of Telomeric Complexes. FEBS Lett. 2010, 584. [Google Scholar] [CrossRef] [PubMed]
- Moyzis, R.K.; Buckingham, J.M.; Cram, L.S.; Dani, M.; Deaven, L.L.; Jones, M.D.; Meyne, J.; Ratliff, R.L.; Wu, J.R. A Highly Conserved Repetitive DNA Sequence, (TTAGGG)n, Present at the Telomeres of Human Chromosomes. Proc. Natl. Acad. Sci. USA 1988, 85, 6622–6626. [Google Scholar] [CrossRef]
- Meyne, J.; Ratliff, R.L.; Moyzis, R.K. Conservation of the Human Telomere Sequence (TTAGGG)n among Vertebrates. Proc. Natl. Acad. Sci. USA 1989, 86, 7049–7053. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.M.V.; Ryder, O.A.; Houck, M.L.; Charter, S.J.; Walker, W.; Forsyth, N.R.; Austad, S.N.; Venditti, C.; Pagel, M.; Shay, J.W.; et al. Comparative Biology of Mammalian Telomeres: Hypotheses on Ancestral States and the Roles of Telomeres in Longevity Determination. Aging Cell 2011, 10, 761–768. [Google Scholar] [CrossRef]
- Benarroch-Popivker, D.; Pisano, S.; Mendez-Bermudez, A.; Lototska, L.; Kaur, P.; Bauwens, S.; Djerbi, N.; Latrick, C.M.; Fraisier, V.; Pei, B.; et al. TRF2-Mediated Control of Telomere DNA Topology as a Mechanism for Chromosome-End Protection. Mol. Cell 2016, 61, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, I.; Zhang, K.; Zhang, J.; Vorontsov, E.; Shibuya, H. Telomeric Double-Strand DNA-Binding Proteins DTN-1 and DTN-2 Ensure Germline Immortality in Caenorhabditis Elegans. eLife 2021, 10, e64104. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Frydrychova, R.C.; Biessmann, H. Drosophila Telomeres: An Exception Providing New Insights. BioEssays 2008, 30, 25–37. [Google Scholar] [CrossRef]
- Cacchione, S.; Cenci, G.; Raffa, G.D. Silence at the End: How Drosophila Regulates Expression and Transposition of Telomeric Retroelements. J. Mol. Biol. 2020, 432, 4305–4321. [Google Scholar] [CrossRef]
- Chang, M.; Arneric, M.; Lingner, J. Telomerase Repeat Addition Processivity Is Increased at Critically Short Telomeres in a Tel1-Dependent Manner in Saccharomyces Cerevisiae. Genes Dev. 2007, 21, 2485–2494. [Google Scholar] [CrossRef]
- Ancelin, K.; Brunori, M.; Bauwens, S.; Koering, C.-E.; Brun, C.; Ricoul, M.; Pommier, J.-P.; Sabatier, L.; Gilson, E. Targeting Assay To Study the Cis Functions of Human Telomeric Proteins: Evidence for Inhibition of Telomerase by TRF1 and for Activation of Telomere Degradation by TRF2. Mol. Cell. Biol. 2002, 22, 3474–3487. [Google Scholar] [CrossRef] [PubMed]
- Pickett, H.A.; Cesare, A.J.; Johnston, R.L.; Neumann, A.A.; Reddel, R.R. Control of Telomere Length by a Trimming Mechanism That Involves Generation of T-Circles. EMBO J. 2009, 28, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.C.; Smogorzewska, A.; de Lange, T. Homologous Recombination Generates T-Loop-Sized Deletions at Human Telomeres. Cell 2004, 119, 355–368. [Google Scholar] [CrossRef]
- Saint-Léger, A.; Koelblen, M.; Civitelli, L.; Bah, A.; Djerbi, N.; Giraud-Panis, M.-J.; Londoño-Vallejo, A.; Ascenzioni, F.; Gilson, E. The Basic N-Terminal Domain of TRF2 Limits Recombination Endonuclease Action at Human Telomeres. Cell Cycle 2014, 13, 2469–2474. [Google Scholar] [CrossRef]
- Miller, K.M.; Rog, O.; Cooper, J.P. Semi-Conservative DNA Replication through Telomeres Requires Taz1. Nature 2006, 440, 824–828. [Google Scholar] [CrossRef]
- Sfeir, A.; Kosiyatrakul, S.T.; Hockemeyer, D.; MacRae, S.L.; Karlseder, J.; Schildkraut, C.L.; de Lange, T. Mammalian Telomeres Resemble Fragile Sites and Require TRF1 for Efficient Replication. Cell 2009, 138, 90–103. [Google Scholar] [CrossRef]
- Ye, J.; Lenain, C.; Bauwens, S.; Rizzo, A.; Saint-Léger, A.; Poulet, A.; Benarroch, D.; Magdinier, F.; Morere, J.; Amiard, S.; et al. TRF2 and Apollo Cooperate with Topoisomerase 2alpha to Protect Human Telomeres from Replicative Damage. Cell 2010, 142, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.D. Origin of Concatemeric T7DNA. Nat. New Biol. 1972, 239, 197–201. [Google Scholar] [CrossRef]
- Olovnikov, A.M. A Theory of Marginotomy. The Incomplete Copying of Template Margin in Enzymic Synthesis of Polynucleotides and Biological Significance of the Phenomenon. J. Theor. Biol. 1973, 41, 181–190. [Google Scholar] [CrossRef]
- Lingner, J.; Cooper, J.P.; Cech, T.R. Telomerase and DNA End Replication: No Longer a Lagging Strand Problem? Science 1995, 269, 1533–1534. [Google Scholar] [CrossRef]
- Gilson, E.; Géli, V. How Telomeres Are Replicated. Nat. Rev. Mol. Cell. Biol. 2007, 8, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Takai, H.; de Lange, T. Telomeric 3′ Overhangs Derive from Resection by Exo1 and Apollo and Fill-in by POT1b-Associated CST. Cell 2012, 150, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Marcand, S.; Brevet, V.; Gilson, E. Progressive Cis-Inhibition of Telomerase upon Telomere Elongation. EMBO J. 1999, 18, 3509–3519. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase Activity in Human Germline and Embryonic Tissues and Cells. Dev. Genet. 1996, 173–179. [Google Scholar] [CrossRef]
- Haussmann, M.F.; Winkler, D.W.; Huntington, C.E.; Nisbet, I.C.T.; Vleck, C.M. Telomerase Activity Is Maintained throughout the Lifespan of Long-Lived Birds. Exp. Gerontol. 2007, 42, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Roake, C.M.; Artandi, S.E. Regulation of Human Telomerase in Homeostasis and Disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 384–397. [Google Scholar] [CrossRef]
- Foley, N.M.; Hughes, G.M.; Huang, Z.; Clarke, M.; Jebb, D.; Whelan, C.V.; Petit, E.J.; Touzalin, F.; Farcy, O.; Jones, G.; et al. Growing Old, yet Staying Young: The Role of Telomeres in Bats’ Exceptional Longevity. Sci. Adv. 2018, 4, eaao0926. [Google Scholar] [CrossRef]
- Whittemore, K.; Vera, E.; Martínez-Nevado, E.; Sanpera, C.; Blasco, M.A. Telomere Shortening Rate Predicts Species Life Span. Proc. Natl. Acad. Sci. USA 2019, 116, 15122–15127. [Google Scholar] [CrossRef]
- Bryan, T.M.; Englezou, A.; Dalla-Pozza, L.; Dunham, M.A.; Reddel, R.R. Evidence for an Alternative Mechanism for Maintaining Telomere Length in Human Tumors and Tumor-Derived Cell Lines. Nat. Med. 1997, 3, 1271–1274. [Google Scholar] [CrossRef]
- Neumann, A.A.; Watson, C.M.; Noble, J.R.; Pickett, H.A.; Tam, P.P.L.; Reddel, R.R. Alternative Lengthening of Telomeres in Normal Mammalian Somatic Cells. Genes Dev. 2013, 27, 18–23. [Google Scholar] [CrossRef]
- Role of Alternative Telomere Lengthening Unmasked in Telomerase Knock-Out Mutant Plants | SpringerLink. Available online: https://link.springer.com/article/10.1007/s11103-008-9295-7 (accessed on 28 April 2021).
- Kim, C.; Sung, S.; Kim, J.-S.; Lee, H.; Jung, Y.; Shin, S.; Kim, E.; Seo, J.J.; Kim, J.; Kim, D.; et al. Telomeres Reforged with Non-Telomeric Sequences in Mouse Embryonic Stem Cells. Nat. Commun. 2021, 12, 1097. [Google Scholar] [CrossRef]
- Ohki, R.; Ishikawa, F. Telomere-Bound TRF1 and TRF2 Stall the Replication Fork at Telomeric Repeats. Nucleic Acids Res. 2004, 32, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Touzot, F.; Callebaut, I.; Soulier, J.; Gaillard, L.; Azerrad, C.; Durandy, A.; Fischer, A.; de Villartay, J.-P.; Revy, P. Function of Apollo (SNM1B) at Telomere Highlighted by a Splice Variant Identified in a Patient with Hoyeraal–Hreidarsson Syndrome. Proc. Natl. Acad. Sci. USA 2010, 107, 10097–10102. [Google Scholar] [CrossRef] [PubMed]
- Quesada, V.; Freitas-Rodríguez, S.; Miller, J.; Pérez-Silva, J.G.; Jiang, Z.-F.; Tapia, W.; Santiago-Fernández, O.; Campos-Iglesias, D.; Kuderna, L.F.K.; Quinzin, M.; et al. Giant Tortoise Genomes Provide Insights into Longevity and Age-Related Disease. Nat. Ecol. Evol. 2019, 3, 87–95. [Google Scholar] [CrossRef]
- Aviv, A. Telomeres and Human Somatic Fitness. J. Gerontol. Ser. A 2006, 61, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Heidinger, B.J.; Blount, J.D.; Boner, W.; Griffiths, K.; Metcalfe, N.B.; Monaghan, P. Telomere Length in Early Life Predicts Lifespan. Proc. Natl. Acad. Sci. USA 2012, 109, 1743–1748. [Google Scholar] [CrossRef]
- Aviv, A.; Shay, J.W. Reflections on Telomere Dynamics and Ageing-Related Diseases in Humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef]
- The Telomeres Mendelian Randomization Collaboration; Haycock, P.C.; Burgess, S.; Nounu, A.; Zheng, J.; Okoli, G.N.; Bowden, J.; Wade, K.H.; Timpson, N.J.; Evans, D.M.; et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017, 3, 636. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Passos, J.F. Stress, Cell Senescence and Organismal Ageing. Mech. Ageing Dev. 2018, 170, 2–9. [Google Scholar] [CrossRef]
- von Zglinicki, T.; Saretzki, G.; Döcke, W.; Lotze, C. Mild Hyperoxia Shortens Telomeres and Inhibits Proliferation of Fibroblasts: A Model for Senescence? Exp. Cell Res. 1995, 220, 186–193. [Google Scholar] [CrossRef]
- Jacome Burbano, M.S.; Cherfils-Vicini, J.; Gilson, E. Neutrophils: Mediating TelOxidation and Senescence. EMBO J. 2021, 40, e108164. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.-Y.; Campisi, J. Role of Immune Cells in the Removal of Deleterious Senescent Cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef]
- Aikata, H.; Takaishi, H.; Kawakami, Y.; Takahashi, S.; Kitamoto, M.; Nakanishi, T.; Nakamura, Y.; Shimamoto, F.; Kajiyama, G.; Ide, T. Telomere Reduction in Human Liver Tissues with Age and Chronic Inflammation. Exp. Cell Res. 2000, 256, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Ilmonen, P.; Kotrschal, A.; Penn, D.J. Telomere Attrition Due to Infection. PLoS ONE 2008, 3, e2143. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, D.; Hu, B.; Mao, X.; Rashid, A.; Li, J.; Li, J.; Liao, W.; Whitley, E.M.; Dey, P.; Hou, P.; et al. Telomere Dysfunction Activates YAP1 to Drive Tissue Inflammation. Nat. Commun. 2020, 11, 4766. [Google Scholar] [CrossRef]
- Maekawa, T.; Liu, B.; Nakai, D.; Yoshida, K.; Nakamura, K.-I.; Yasukawa, M.; Koike, M.; Takubo, K.; Chatton, B.; Ishikawa, F.; et al. ATF7 Mediates TNF-α-Induced Telomere Shortening. Nucleic Acids Res. 2018, 46, 4487–4504. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Martínez-A, C.; Blasco, M.A. Impaired Germinal Center Reaction in Mice with Short Telomeres. EMBO J. 2000, 19, 472–481. [Google Scholar] [CrossRef]
- Lagnado, A.; Leslie, J.; Ruchaud-Sparagano, M.-H.; Victorelli, S.; Hirsova, P.; Ogrodnik, M.; Collins, A.L.; Vizioli, M.G.; Habiballa, L.; Saretzki, G.; et al. Neutrophils Induce Paracrine Telomere Dysfunction and Senescence in ROS-Dependent Manner. EMBO J. 2021, 40, e106048. [Google Scholar] [CrossRef]
- Saretzki, G.; Murphy, M.P.; Zglinicki, T.V. MitoQ Counteracts Telomere Shortening and Elongates Lifespan of Fibroblasts under Mild Oxidative Stress. Aging Cell 2003, 2, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The Impact of Oxidative DNA Damage and Stress on Telomere Homeostasis. Mech Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Monaghan, P.; Spencer, K.A. Stress and Life History. Curr. Biol. 2014, 24, R408–R412. [Google Scholar] [CrossRef] [PubMed]
- Haussmann, M.F.; Marchetto, N.M. Telomeres: Linking Stress and Survival, Ecology and Evolution. Curr. Zool. 2010, 56, 714–727. [Google Scholar] [CrossRef]
- Shalev, I.; Moffitt, T.E.; Sugden, K.; Williams, B.; Houts, R.M.; Danese, A.; Mill, J.; Arseneault, L.; Caspi, A. Exposure to Violence during Childhood Is Associated with Telomere Erosion from 5 to 10 Years of Age: A Longitudinal Study. Mol. Psychiatry 2013, 18, 576–581. [Google Scholar] [CrossRef]
- Epel, E.S.; Lin, J.; Dhabhar, F.S.; Wolkowitz, O.M.; Puterman, E.; Karan, L.; Blackburn, E.H. Dynamics of Telomerase Activity in Response to Acute Psychological Stress. Brain Behav. Immun. 2010, 24, 531–539. [Google Scholar] [CrossRef]
- Beery, A.K.; Lin, J.; Biddle, J.S.; Francis, D.D.; Blackburn, E.H.; Epel, E.S. Chronic Stress Elevates Telomerase Activity in Rats. Biol. Lett. 2012, 8, 1063–1066. [Google Scholar] [CrossRef]
- Angelier, F.; Costantini, D.; Blévin, P.; Chastel, O. Do Glucocorticoids Mediate the Link between Environmental Conditions and Telomere Dynamics in Wild Vertebrates? A Review. Gen. Comp. Endocrinol. 2018, 256, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D.; Marasco, V.; Møller, A.P. A Meta-Analysis of Glucocorticoids as Modulators of Oxidative Stress in Vertebrates. J. Comp. Physiol. B 2011, 181, 447–456. [Google Scholar] [CrossRef]
- Casagrande, S.; Stier, A.; Monaghan, P.; Loveland, J.L.; Boner, W.; Lupi, S.; Trevisi, R.; Hau, M. Increased Glucocorticoid Concentrations in Early Life Cause Mitochondrial Inefficiency and Short Telomeres. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated Telomere Shortening in Response to Life Stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.M.; Hu, F.B.; Vivo, I.D. Mediterranean Diet and Telomere Length in Nurses’ Health Study: Population Based Cohort Study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef]
- Liu, J.J.; Crous-Bou, M.; Giovannucci, E.; De Vivo, I. Coffee Consumption Is Positively Associated with Longer Leukocyte Telomere Length in the Nurses’ Health Study. J. Nutr. 2016, 146, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Shi, Q.; Fan, X.; Qi, K. Association of Telomere Length and Telomerase Methylation with N-3 Fatty Acids in Preschool Children with Obesity. BMC Pediatrics 2021, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical Activity and Telomere Length: Impact of Aging and Potential Mechanisms of Action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Diman, A.; Boros, J.; Poulain, F.; Rodriguez, J.; Purnelle, M.; Episkopou, H.; Bertrand, L.; Francaux, M.; Deldicque, L.; Decottignies, A. Nuclear Respiratory Factor 1 and Endurance Exercise Promote Human Telomere Transcription. Sci. Adv. 2016, 2, e1600031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, S.; Funk, W.E.; Hou, L. Republished: Environmental and Occupational Exposure to Chemicals and Telomere Length in Human Studies. Postgrad. Med. J. 2013, 89, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, T.; Abumock, H.; Beery, E.; Edel, Y.; Lahav, M.; Rozovski, U.; Uziel, O. The Effect of Ethanol on Telomere Dynamics and Regulation in Human Cells. Cells 2018, 7, 169. [Google Scholar] [CrossRef]
- Romano, G.H.; Harari, Y.; Yehuda, T.; Podhorzer, A.; Rubinstein, L.; Shamir, R.; Gottlieb, A.; Silberberg, Y.; Pe’er, D.; Ruppin, E.; et al. Environmental Stresses Disrupt Telomere Length Homeostasis. PLoS Genet. 2013, 9, e1003721. [Google Scholar] [CrossRef]
- Haorah, J.; Ramirez, S.H.; Floreani, N.; Gorantla, S.; Morsey, B.; Persidsky, Y. Mechanism of Alcohol-Induced Oxidative Stress and Neuronal Injury. Free Radic. Biol. Med. 2008, 45, 1542–1550. [Google Scholar] [CrossRef]
- Saulnier, A.; Bleu, J.; Boos, A.; El Masoudi, I.; Ronot, P.; Zahn, S.; Del Nero, M.; Massemin, S. Consequences of Trace Metal Cocktail Exposure in Zebra Finch (Taeniopygia Guttata) and Effect of Calcium Supplementation. Ecotoxicol. Environ. Saf. 2020, 193, 110357. [Google Scholar] [CrossRef]
- Rochette, P.J.; Brash, D.E. Human Telomeres Are Hypersensitive to UV-Induced DNA Damage and Refractory to Repair. PLoS Genet. 2010, 6, e1000926. [Google Scholar] [CrossRef]
- Fumagalli, M.; Rossiello, F.; Clerici, M.; Barozzi, S.; Cittaro, D.; Kaplunov, J.M.; Bucci, G.; Dobreva, M.; Matti, V.; Beausejour, C.M.; et al. Telomeric DNA Damage Is Irreparable and Causes Persistent DNA-Damage-Response Activation. Nat. Cell Biol. 2012, 14, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Fouquerel, E.; Barnes, R.P.; Wang, H.; Opresko, P.L. Measuring UV Photoproduct Repair in Isolated Telomeres and Bulk Genomic DNA. Methods Mol. Biol. 2019, 1999, 295–306. [Google Scholar] [CrossRef]
- Ma, H.-M.; Liu, W.; Zhang, P.; Yuan, X.-Y. Human Skin Fibroblast Telomeres Are Shortened after Ultraviolet Irradiation. J. Int. Med. Res. 2012, 40, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Aida, J.; Hatamochi, A.; Hamasaki, Y.; Izumiyama-Shimomura, N.; Nakamura, K.; Ishikawa, N.; Poon, S.S.; Fujiwara, M.; Tomita, K.; et al. Quantitative Fluorescence in Situ Hybridization Measurement of Telomere Length in Skin with/without Sun Exposure or Actinic Keratosis. Hum. Pathol. 2014, 45, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Stout, G.J.; Blasco, M.A. Telomere Length and Telomerase Activity Impact the UV Sensitivity Syndrome Xeroderma Pigmentosum C. Cancer Res. 2013, 73, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Parikh, D.; Fouquerel, E.; Murphy, C.T.; Wang, H.; Opresko, P.L. Telomeres Are Partly Shielded from Ultraviolet-Induced Damage and Proficient for Nucleotide Excision Repair of Photoproducts. Nat. Commun. 2015, 6, 8214. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A Multidimensional Analysis of a Year-Long Human Spaceflight. Science 2019, 364. [Google Scholar] [CrossRef]
- Luxton, J.J.; McKenna, M.J.; Taylor, L.E.; George, K.A.; Zwart, S.R.; Crucian, B.E.; Drel, V.R.; Garrett-Bakelman, F.E.; Mackay, M.J.; Butler, D.; et al. Temporal Telomere and DNA Damage Responses in the Space Radiation Environment. Cell Rep. 2020, 33, 108435. [Google Scholar] [CrossRef]
- Fouquerel, E.; Barnes, R.P.; Uttam, S.; Watkins, S.C.; Bruchez, M.P.; Opresko, P.L. Targeted and Persistent 8-Oxoguanine Base Damage at Telomeres Promotes Telomere Loss and Crisis. Mol. Cell 2019, 75, 117–130.e6. [Google Scholar] [CrossRef]
- O’Sullivan, R.J.; Arnoult, N.; Lackner, D.H.; Oganesian, L.; Haggblom, C.; Corpet, A.; Almouzni, G.; Karlseder, J. Rapid Induction of Alternative Lengthening of Telomeres by Depletion of the Histone Chaperone ASF1. Nat. Struct. Mol. Biol. 2014, 21, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.V.; Velichko, A.K.; Kantidze, O.L.; Razin, S.V. Heat Shock-induced Dissociation of TRF2 from Telomeres Does Not Initiate a Telomere-dependent DNA Damage Response. Cell Biol. Int. 2014, 38, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Koskas, S.; Decottignies, A.; Dufour, S.; Pezet, M.; André, V.; Vourc’h, C.; Faure, V. Heat Shock Factor 1 Promotes TERRA Transcription and Telomere Protection upon Heat Stress. NAR 2017, 45, 6321–6333. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wu, S.; Xue, Y.; Tao, J.; Li, F.; Chen, Y.; Liu, H.; Ma, W.; Huang, J.; Zhao, Y. Preferential Extension of Short Telomeres Induced by Low Extracellular PH. Nucleic Acids Res. 2016, 44, 8086–8096. [Google Scholar] [CrossRef] [PubMed]
- Hemann, M.T.; Greider, C.W. Wild-Derived Inbred Mouse Strains Have Short Telomeres. Nucleic Acids Res. 2000, 28, 4474–4478. [Google Scholar] [CrossRef]
- Cherif, H.; Tarry, J.L.; Ozanne, S.E.; Hales, C.N. Ageing and Telomeres: A Study into Organ- and Gender-specific Telomere Shortening. Nucleic Acids Res. 2003, 31, 1576–1583. [Google Scholar] [CrossRef]
- Pepke, M.L.; Eisenberg, D.T.A. On the Comparative Biology of Mammalian Telomeres: Telomere Length Co-Evolves with Body Mass, Lifespan and Cancer Risk. Mol. Ecol. 2021. [Google Scholar] [CrossRef]
- Kotrschal, A.; Ilmonen, P.; Penn, D.J. Stress Impacts Telomere Dynamics. Biol. Lett. 2007, 3, 128–130. [Google Scholar] [CrossRef]
- Plot, V.; Criscuolo, F.; Zahn, S.; Georges, J.-Y. Telomeres, Age and Reproduction in a Long-Lived Reptile. PLoS ONE 2012, 7, e40855. [Google Scholar] [CrossRef]
- Bauch, C.; Becker, P.H.; Verhulst, S. Telomere Length Reflects Phenotypic Quality and Costs of Reproduction in a Long-Lived Seabird. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122540. [Google Scholar] [CrossRef]
- Gao, J.; Munch, S.B. Does Reproductive Investment Decrease Telomere Length in Menidia Menidia? PLoS ONE 2015, 10, e0125674. [Google Scholar] [CrossRef]
- Bauch, C.; Riechert, J.; Verhulst, S.; Becker, P.H. Telomere Length Reflects Reproductive Effort Indicated by Corticosterone Levels in a Long-lived Seabird. Mol. Ecol. 2016, 25, 5785–5794. [Google Scholar] [CrossRef]
- Graham, J.L.; Bauer, C.M.; Heidinger, B.J.; Ketterson, E.D.; Greives, T.J. Early-breeding Females Experience Greater Telomere Loss. Mol. Ecol. 2019, 28. [Google Scholar] [CrossRef] [PubMed]
- Sudyka, J. Does Reproduction Shorten Telomeres? Towards Integrating Individual Quality with Life-History Strategies in Telomere Biology. BioEssays 2019, 41, 1900095. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.F.; Biessmann, M.R.; Benitez, C.; Török, T.; Mason, J.M.; Biessmann, H. Effects of Telomere Length in Drosophila Melanogaster on Life Span, Fecundity and Fertility. Chromosoma 2007, 116, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, R.G.; Kirkwood, T.B. Human Longevity at the Cost of Reproductive Success. Nature 1998, 396, 743–746. [Google Scholar] [CrossRef]
- Hsin, H.; Kenyon, C. Signals from the Reproductive System Regulate the Lifespan of C. Elegans. Nature 1999, 399, 362–366. [Google Scholar] [CrossRef]
- Koubová, J.; Jehlík, T.; Kodrík, D.; Sábová, M.; Šima, P.; Sehadová, H.; Závodská, R.; Frydrychová, R.Č. Telomerase Activity Is Upregulated in the Fat Bodies of Pre-Diapause Bumblebee Queens (Bombus Terrestris). Insect. Biochem. Mol. Biol. 2019, 115, 103241. [Google Scholar] [CrossRef]
- Hammers, M.; Kingma, S.A.; Spurgin, L.G.; Bebbington, K.; Dugdale, H.L.; Burke, T.; Komdeur, J.; Richardson, D.S. Breeders That Receive Help Age More Slowly in a Cooperatively Breeding Bird. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Young, A.J. The Role of Telomeres in the Mechanisms and Evolution of Life-History Trade-Offs and Ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef]
- Bauch, C.; Gatt, M.C.; Granadeiro, J.P.; Verhulst, S.; Catry, P. Sex-Specific Telomere Length and Dynamics in Relation to Age and Reproductive Success in Cory’s Shearwaters. Mol. Ecol. 2020, 29, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological Stress Response Terminology: Integrating the Concepts of Adaptive Response and Preconditioning Stress within a Hormetic Dose-Response Framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, S.; Furumoto, K.; Hiyama, E.; Miwa, N. Slow-down of Age-Dependent Telomere Shortening Is Executed in Human Skin Keratinocytes by Hormesis-like-Effects of Trace Hydrogen Peroxide or by Anti-Oxidative Effects of pro-Vitamin C in Common Concurrently with Reduction of Intracellular Oxidative Stress. J. Cell Biochem. 2004, 93, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz-Potoczny, M.; Lydall, D. Costs, Benefits and Redundant Mechanisms of Adaption to Chronic Low-Dose Stress in Yeast. Cell Cycle 2016, 15, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Suram, A.; Kaplunov, J.; Patel, P.L.; Ruan, H.; Cerutti, A.; Boccardi, V.; Fumagalli, M.; Di Micco, R.; Mirani, N.; Gurung, R.L.; et al. Oncogene-Induced Telomere Dysfunction Enforces Cellular Senescence in Human Cancer Precursor Lesions. EMBO J. 2012, 31, 2839–2851. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.L.; Suram, A.; Mirani, N.; Bischof, O.; Herbig, U. Derepression of HTERT Gene Expression Promotes Escape from Oncogene-Induced Cellular Senescence. Proc. Natl. Acad. Sci. USA 2016, 113, E5024–E5033. [Google Scholar] [CrossRef]
- González-Suárez, E.; Samper, E.; Flores, J.M.; Blasco, M.A. Telomerase-Deficient Mice with Short Telomeres Are Resistant to Skin Tumorigenesis. Nat. Genet. 2000, 26, 114–117. [Google Scholar] [CrossRef]
- Zhang, C.; Doherty, J.A.; Burgess, S.; Hung, R.J.; Lindström, S.; Kraft, P.; Gong, J.; Amos, C.I.; Sellers, T.A.; Monteiro, A.N.A.; et al. Genetic Determinants of Telomere Length and Risk of Common Cancers: A Mendelian Randomization Study. Hum. Mol. Genet. 2015, 24, 5356–5366. [Google Scholar] [CrossRef]
- Hansen, M.E.B.; Hunt, S.C.; Stone, R.C.; Horvath, K.; Herbig, U.; Ranciaro, A.; Hirbo, J.; Beggs, W.; Reiner, A.P.; Wilson, J.G.; et al. Shorter Telomere Length in Europeans than in Africans Due to Polygenetic Adaptation. Hum. Mol. Genet. 2016, 25, 2324–2330. [Google Scholar] [CrossRef]
- Gao, R.; Price, D.K.; Sissung, T.; Reed, E.; Figg, W.D. Ethnic Disparities in Americans of European Descent versus Americans of African Descent Related to Polymorphic ERCC1, ERCC2, XRCC1, and PARP1. Mol. Cancer Ther. 2008, 7, 1246–1250. [Google Scholar] [CrossRef]
- González-Suárez, E.; Samper, E.; Ramírez, A.; Flores, J.M.; Martín-Caballero, J.; Jorcano, J.L.; Blasco, M.A. Increased Epidermal Tumors and Increased Skin Wound Healing in Transgenic Mice Overexpressing the Catalytic Subunit of Telomerase, MTERT, in Basal Keratinocytes. EMBO J. 2001, 20, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.A.; Chin, L.; Femino, A.; Lee, K.H.; Gottlieb, G.J.; Singer, R.H.; Greider, C.W.; DePinho, R.A. Short Dysfunctional Telomeres Impair Tumorigenesis in the INK4a(Delta2/3) Cancer-Prone Mouse. Cell 1999, 97, 515–525. [Google Scholar] [CrossRef]
- Rudolph, K.L.; Millard, M.; Bosenberg, M.W.; DePinho, R.A. Telomere Dysfunction and Evolution of Intestinal Carcinoma in Mice and Humans. Nat. Genet. 2001, 28, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Feldser, D.M.; Greider, C.W. Short Telomeres Limit Tumor Progression in Vivo by Inducing Senescence. Cancer Cell 2007, 11, 461–469. [Google Scholar] [CrossRef]
- Khoo, C.M.; Carrasco, D.R.; Bosenberg, M.W.; Paik, J.-H.; DePinho, R.A. Ink4a/Arf Tumor Suppressor Does Not Modulate the Degenerative Conditions or Tumor Spectrum of the Telomerase-Deficient Mouse. Proc. Natl. Acad. Sci. USA 2007, 104, 3931–3936. [Google Scholar] [CrossRef]
- Jaskelioff, M.; Song, W.; Xia, J.; Liu, C.; Kramer, J.; Koido, S.; Gendler, S.J.; Calderwood, S.K.; Gong, J. Telomerase Deficiency and Telomere Dysfunction Inhibit Mammary Tumors Induced by Polyomavirus Middle T Oncogene. Oncogene 2009, 28, 4225–4236. [Google Scholar] [CrossRef][Green Version]
- Muñoz-Lorente, M.A.; Cano-Martin, A.C.; Blasco, M.A. Mice with Hyper-Long Telomeres Show Less Metabolic Aging and Longer Lifespans. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.Y.; Campisi, J. Senescent Fibroblasts Promote Epithelial Cell Growth and Tumorigenesis: A Link between Cancer and Aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef]
- Lex, K.; Gil, M.M.; Lopes-Bastos, B.; Figueira, M.; Marzullo, M.; Giannetti, K.; Carvalho, T.; Ferreira, M.G. Telomere Shortening Produces an Inflammatory Environment That Increases Tumor Incidence in Zebrafish. Proc. Natl. Acad. Sci. USA 2020, 117, 15066–15074. [Google Scholar] [CrossRef]
- Akincilar, S.C.; Chan, C.H.T.; Ng, Q.F.; Fidan, K.; Tergaonkar, V. Non-Canonical Roles of Canonical Telomere Binding Proteins in Cancers. Cell. Mol. Life Sci. 2021. [Google Scholar] [CrossRef]
- Robles-Espinoza, C.D.; Harland, M.; Ramsay, A.J.; Aoude, L.G.; Quesada, V.; Ding, Z.; Pooley, K.A.; Pritchard, A.L.; Tiffen, J.C.; Petljak, M.; et al. POT1 Loss-of-Function Variants Predispose to Familial Melanoma. Nat. Genet. 2014, 46, 478–481. [Google Scholar] [CrossRef]
- Shi, J.; Yang, X.R.; Ballew, B.; Rotunno, M.; Calista, D.; Fargnoli, M.C.; Ghiorzo, P.; Bressac-de Paillerets, B.; Nagore, E.; Avril, M.F.; et al. Rare Missense Variants in POT1 Predispose to Familial Cutaneous Malignant Melanoma. Nat. Genet. 2014, 46, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Calvete, O.; Martinez, P.; Garcia-Pavia, P.; Benitez-Buelga, C.; Paumard-Hernández, B.; Fernandez, V.; Dominguez, F.; Salas, C.; Romero-Laorden, N.; Garcia-Donas, J.; et al. A Mutation in the POT1 Gene Is Responsible for Cardiac Angiosarcoma in TP53 -Negative Li–Fraumeni-like Families. Nat. Commun. 2015, 6, 8383. [Google Scholar] [CrossRef]
- Calvete, O.; Garcia-Pavia, P.; Domínguez, F.; Bougeard, G.; Kunze, K.; Braeuninger, A.; Teule, A.; Lasa, A.; Ramón y Cajal, T.; Llort, G.; et al. The Wide Spectrum of POT1 Gene Variants Correlates with Multiple Cancer Types. Eur. J. Hum. Genet. 2017, 25, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Stock, A.J.; Liu, Y. The Enigma of Excessively Long Telomeres in Cancer: Lessons Learned from Rare Human POT1 Variants. Curr. Opin. Genet. Dev. 2020, 60, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Biroccio, A.; Cherfils-Vicini, J.; Augereau, A.; Pinte, S.; Bauwens, S.; Ye, J.; Simonet, T.; Horard, B.; Jamet, K.; Cervera, L.; et al. TRF2 Inhibits a Cell-Extrinsic Pathway through Which Natural Killer Cells Eliminate Cancer Cells. Nat. Cell. Biol. 2013, 15, 818–828. [Google Scholar] [CrossRef] [PubMed]
- El Maï, M.; Wagner, K.-D.; Michiels, J.-F.; Ambrosetti, D.; Borderie, A.; Destree, S.; Renault, V.; Djerbi, N.; Giraud-Panis, M.-J.; Gilson, E.; et al. The Telomeric Protein TRF2 Regulates Angiogenesis by Binding and Activating the PDGFRβ Promoter. Cell Rep. 2014, 9, 1047–1060. [Google Scholar] [CrossRef]
- Maï, M.E.; Wagner, K.-D.; Michiels, J.-F.; Gilson, E.; Wagner, N. TRF2 Acts as a Transcriptional Regulator in Tumor Angiogenesis. Mol. Cell. Oncol. 2015, 2. [Google Scholar] [CrossRef]
- Cherfils-Vicini, J.; Iltis, C.; Cervera, L.; Pisano, S.; Croce, O.; Sadouni, N.; Győrffy, B.; Collet, R.; Renault, V.M.; Rey-Millet, M.; et al. Cancer Cells Induce Immune Escape via Glycocalyx Changes Controlled by the Telomeric Protein TRF2. EMBO J. 2019, 38. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Renault, V.M.; Jamet, K.; Gilson, E. Transcriptional Outcome of Telomere Signalling. Nat. Rev. Genet. 2014, 15, 491–503. [Google Scholar] [CrossRef]
- Kueng, S.; Oppikofer, M.; Gasser, S.M. SIR Proteins and the Assembly of Silent Chromatin in Budding Yeast. Ann. Rev. Genet. 2013, 47, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Fourel, G.; Revardel, E.; Koering, C.E.; Gilson, É. Cohabitation of Insulators and Silencing Elements in Yeast Subtelomeric Regions. EMBO J. 1999, 18, 2522. [Google Scholar] [CrossRef] [PubMed]
- Ofir, R.; Wong, A.C.C.; McDermid, H.E.; Skorecki, K.L.; Selig, S. Position Effect of Human Telomeric Repeats on Replication Timing. Proc. Natl. Acad. Sci. USA 1999, 96, 11434–11439. [Google Scholar] [CrossRef] [PubMed]
- Riethman, H.C.; Xiang, Z.; Paul, S.; Morse, E.; Hu, X.L.; Flint, J.; Chi, H.C.; Grady, D.L.; Moyzis, R.K. Integration of Telomere Sequences with the Draft Human Genome Sequence. Nature 2001, 409, 948–951. [Google Scholar] [CrossRef][Green Version]
- Baur, J.A.; Zou, Y.; Shay, J.W.; Wright, W.E. Telomere Position Effect in Human Cells. Science 2001, 292, 2075–2077. [Google Scholar] [CrossRef]
- Koering, C.E.; Pollice, A.; Zibella, M.P.; Bauwens, S.; Puisieux, A.; Brunori, M.; Brun, C.; Martins, L.; Sabatier, L.; Pulitzer, J.F.; et al. Human Telomeric Position Effect Is Determined by Chromosomal Context and Telomeric Chromatin Integrity. EMBO Rep. 2002, 3, 1055–1061. [Google Scholar] [CrossRef]
- Mason, J.M.; Konev, A.Y.; Biessmann, H. Telomeric Position Effect in Drosophila Melanogaster Reflects a Telomere Length Control Mechanism. Genetica 2003, 117, 319–325. [Google Scholar] [CrossRef]
- Elgin, S.C.R.; Reuter, G. Position-Effect Variegation, Heterochromatin Formation, and Gene Silencing in Drosophila. Cold Spring Harb. Perspect. Biol. 2013, 5, a017780. [Google Scholar] [CrossRef]
- Glover, L.; Horn, D. Repression of Polymerase I-Mediated Gene Expression at Trypanosoma Brucei Telomeres. EMBO Rep. 2006, 7, 93–99. [Google Scholar] [CrossRef]
- Robin, J.D.; Ludlow, A.T.; Batten, K.; Magdinier, F.; Stadler, G.; Wagner, K.R.; Shay, J.W.; Wright, W.E. Telomere Position Effect: Regulation of Gene Expression with Progressive Telomere Shortening over Long Distances. Genes Dev. 2014, 28, 2464–2476. [Google Scholar] [CrossRef]
- Robin, J.D.; Ludlow, A.T.; Batten, K.; Gaillard, M.-C.; Stadler, G.; Magdinier, F.; Wright, W.E.; Shay, J.W. SORBS2 Transcription Is Activated by Telomere Position Effect–over Long Distance upon Telomere Shortening in Muscle Cells from Patients with Facioscapulohumeral Dystrophy. Genome Res. 2015, 25, 1781–1790. [Google Scholar] [CrossRef]
- Kim, W.; Ludlow, A.T.; Min, J.; Robin, J.D.; Stadler, G.; Mender, I.; Lai, T.-P.; Zhang, N.; Wright, W.E.; Shay, J.W. Regulation of the Human Telomerase Gene TERT by Telomere Position Effect—Over Long Distances (TPE-OLD): Implications for Aging and Cancer. PLoS Biol. 2016, 14, e2000016. [Google Scholar] [CrossRef]
- Robin, J.D.; Jacome Burbano, M.S.; Peng, H.; Croce, O.; Thomas, J.L.; Laberthonniere, C.; Renault, V.; Lototska, L.; Pousse, M.; Tessier, F.; et al. Mitochondrial Function in Skeletal Myofibers Is Controlled by a TRF2-SIRT3 Axis over Lifetime. Aging Cell 2020, 19. [Google Scholar] [CrossRef]
- de Bruin, D.; Zaman, Z.; Liberatore, R.A.; Ptashne, M. Telomere Looping Permits Gene Activation by a Downstream UAS in Yeast. Nature 2001, 409, 109–113. [Google Scholar] [CrossRef]
- Zaman, Z.; Heid, C.; Ptashne, M. Telomere Looping Permits Repression “at a Distance” in Yeast. Curr. Biol. 2002, 12, 930–933. [Google Scholar] [CrossRef][Green Version]
- Lebrun, E.; Fourel, G.; Defossez, P.-A.; Gilson, E. A Methyltransferase Targeting Assay Reveals Silencer-Telomere Interactions in Budding Yeast. Mol. Cell. Biol. 2003, 23, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Bystricky, K.; Laroche, T.; van Houwe, G.; Blaszczyk, M.; Gasser, S.M. Chromosome Looping in Yeast: Telomere Pairing and Coordinated Movement Reflect Anchoring Efficiency and Territorial Organization. J. Cell Biol. 2005, 168, 375–387. [Google Scholar] [CrossRef]
- Miele, A.; Bystricky, K.; Dekker, J. Yeast Silent Mating Type Loci Form Heterochromatic Clusters through Silencer Protein-Dependent Long-Range Interactions. PLoS Genet. 2009, 5, e1000478. [Google Scholar] [CrossRef] [PubMed]
- Vannier, J.-B.; Depeiges, A.; White, C.; Gallego, M.E. ERCC1/XPF Protects Short Telomeres from Homologous Recombination in Arabidopsis Thaliana. PLoS Genet. 2009, 5, e1000380. [Google Scholar] [CrossRef] [PubMed]
- Pryde, F.E.; Louis, E.J. Limitations of Silencing at Native Yeast Telomeres. EMBO J. 1999, 18, 2538–2550. [Google Scholar] [CrossRef]
- Wyrick, J.J.; Holstege, F.C.; Jennings, E.G.; Causton, H.C.; Shore, D.; Grunstein, M.; Lander, E.S.; Young, R.A. Chromosomal Landscape of Nucleosome-Dependent Gene Expression and Silencing in Yeast. Nature 1999, 402, 418–421. [Google Scholar] [CrossRef]
- Wood, A.M.; Danielsen, J.M.R.; Lucas, C.A.; Rice, E.L.; Scalzo, D.; Shimi, T.; Goldman, R.D.; Smith, E.D.; Le Beau, M.M.; Kosak, S.T. TRF2 and Lamin A/C Interact to Facilitate the Functional Organization of Chromosome Ends. Nat. Commun. 2014, 5, 5467. [Google Scholar] [CrossRef] [PubMed]
- Maillet, L.; Boscheron, C.; Gotta, M.; Marcand, S.; Gilson, E.; Gasser, S.M. Evidence for Silencing Compartments within the Yeast Nucleus: A Role for Telomere Proximity and Sir Protein Concentration in Silencer-Mediated Repression. Genes Dev. 1996, 10, 1796–1811. [Google Scholar] [CrossRef]
- Platt, J.M.; Ryvkin, P.; Wanat, J.J.; Donahue, G.; Ricketts, M.D.; Barrett, S.P.; Waters, H.J.; Song, S.; Chavez, A.; Abdallah, K.O.; et al. Rap1 Relocalization Contributes to the Chromatin-Mediated Gene Expression Profile and Pace of Cell Senescence. Genes Dev. 2013, 27, 1406–1420. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Gómez-López, G.; Pisano, D.G.; Flores, J.M.; Blasco, M.A. A Genetic Interaction between RAP1 and Telomerase Reveals an Unanticipated Role for RAP1 in Telomere Maintenance. Aging Cell 2016, 15, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Bertram, P.G.; Tsang, C.K.; Chan, T.-F.; Zheng, X.F.S. Regulation of Subtelomeric Silencing during Stress Response. Mol. Cell 2002, 10, 1295–1305. [Google Scholar] [CrossRef]
- Robyr, D.; Suka, Y.; Xenarios, I.; Kurdistani, S.K.; Wang, A.; Suka, N.; Grunstein, M. Microarray Deacetylation Maps Determine Genome-Wide Functions for Yeast Histone Deacetylases. Cell 2002, 109, 437–446. [Google Scholar] [CrossRef]
- Bruhn, C.; Ajazi, A.; Ferrari, E.; Lanz, M.C.; Batrin, R.; Choudhary, R.; Walvekar, A.; Laxman, S.; Longhese, M.P.; Fabre, E.; et al. The Rad53 CHK1/CHK2 -Spt21 NPAT and Tel1 ATM Axes Couple Glucose Tolerance to Histone Dosage and Subtelomeric Silencing. Nat. Commun. 2020, 11, 4154. [Google Scholar] [CrossRef]
- Schroeder, E.A.; Raimundo, N.; Shadel, G.S. Epigenetic Silencing Mediates Mitochondria Stress-Induced Longevity. Cell Metab. 2013, 17, 954–964. [Google Scholar] [CrossRef]
- Lou, Z.; Wei, J.; Riethman, H.; Baur, J.A.; Voglauer, R.; Shay, J.W.; Wright, W.E. Telomere Length Regulates ISG15 Expression in Human Cells. Aging 2009, 1, 608–621. [Google Scholar] [CrossRef]
- Trask, B.J.; Friedman, C.; Martin-Gallardo, A.; Rowen, L.; Akinbami, C.; Blankenship, J.; Collins, C.; Giorgi, D.; Iadonato, S.; Johnson, F.; et al. Members of the Olfactory Receptor Gene Family Are Contained in Large Blocks of DNA Duplicated Polymorphically near the Ends of Human Chromosomes. Hum. Mol. Genet. 1998, 7, 13–26. [Google Scholar] [CrossRef] [PubMed]
- De Las Peñas, A.; Pan, S.-J.; Castaño, I.; Alder, J.; Cregg, R.; Cormack, B.P. Virulence-Related Surface Glycoproteins in the Yeast Pathogen Candida Glabrata Are Encoded in Subtelomeric Clusters and Subject to RAP1- and SIR-Dependent Transcriptional Silencing. Genes Dev. 2003, 17, 2245–2258. [Google Scholar] [CrossRef]
- Bárcena, C.; Mayoral, P.; Quirós, P.M. Mitohormesis, an Antiaging Paradigm. Int. Rev. Cell. Mol. Biol. 2018, 340, 35–77. [Google Scholar] [CrossRef]
- von Zglinicki, T. Oxidative Stress Shortens Telomeres. Trends Biochem. Sci. 2002, 27, 339–344. [Google Scholar] [CrossRef]
- Serra, V.; von Zglinicki, T.; Lorenz, M.; Saretzki, G. Extracellular Superoxide Dismutase Is a Major Antioxidant in Human Fibroblasts and Slows Telomere Shortening*. J. Biol. Chem. 2003, 278, 6824–6830. [Google Scholar] [CrossRef]
- Stauffer, J.; Panda, B.; Ilmonen, P. Telomere Length, Sibling Competition and Development of Antioxidant Defense in Wild House Mice. Mech. Ageing Dev. 2018, 169, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Aeby, E.; Ahmed, W.; Redon, S.; Simanis, V.; Lingner, J. Peroxiredoxin 1 Protects Telomeres from Oxidative Damage and Preserves Telomeric DNA for Extension by Telomerase. Cell Rep. 2016, 17, 3107–3114. [Google Scholar] [CrossRef]
- Ahmed, W.; Lingner, J. PRDX1 and MTH1 Cooperate to Prevent ROS-Mediated Inhibition of Telomerase. Genes Dev. 2018, 32, 658–669. [Google Scholar] [CrossRef]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase Does Not Counteract Telomere Shortening but Protects Mitochondrial Function under Oxidative Stress. J. Cell Sci. 2008, 121, 1046–1053. [Google Scholar] [CrossRef]
- Haendeler, J.; Dröse, S.; Büchner, N.; Jakob, S.; Altschmied, J.; Goy, C.; Spyridopoulos, I.; Zeiher, A.M.; Brandt, U.; Dimmeler, S. Mitochondrial Telomerase Reverse Transcriptase Binds to and Protects Mitochondrial DNA and Function from Damage. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Spilsbury, A.; Miwa, S.; Attems, J.; Saretzki, G. The Role of Telomerase Protein TERT in Alzheimer’s Disease and in Tau-Related Pathology in Vitro. J. Neurosci. 2015, 35, 1659–1674. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Czapiewski, R.; Wan, T.; Bell, A.; Hill, K.N.; von Zglinicki, T.; Saretzki, G. Decreased MTOR Signalling Reduces Mitochondrial ROS in Brain via Accumulation of the Telomerase Protein TERT within Mitochondria. Aging (Albany NY) 2016, 8, 2551–2567. [Google Scholar] [CrossRef]
- Zheng, Q.; Huang, J.; Wang, G. Mitochondria, Telomeres and Telomerase Subunits. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, P.; Zheng, Q.; Gao, G.; Yuan, J.; Wang, P.; Huang, J.; Xie, L.; Lu, X.; Tong, T.; et al. Mitochondrial Trafficking and Processing of Telomerase RNA TERC. Cell Rep. 2018, 24, 2589–2595. [Google Scholar] [CrossRef]
- Kim, H.; Li, F.; He, Q.; Deng, T.; Xu, J.; Jin, F.; Coarfa, C.; Putluri, N.; Liu, D.; Songyang, Z. Systematic Analysis of Human Telomeric Dysfunction Using Inducible Telosome/Shelterin CRISPR/Cas9 Knockout Cells. Cell Discov. 2017, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Zhang, Y.; Zhang, Q.; Li, H.; Luo, Z.; Fang, H.; Kim, S.H.; Qin, L.; Yotnda, P.; Xu, J.; et al. Mitochondrial Localization of Telomeric Protein TIN2 Links Telomere Regulation to Metabolic Control. Mol. Cell 2012, 47, 839–850. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacome Burbano, M.S.; Gilson, E. The Power of Stress: The Telo-Hormesis Hypothesis. Cells 2021, 10, 1156. https://doi.org/10.3390/cells10051156
Jacome Burbano MS, Gilson E. The Power of Stress: The Telo-Hormesis Hypothesis. Cells. 2021; 10(5):1156. https://doi.org/10.3390/cells10051156
Chicago/Turabian StyleJacome Burbano, Maria Sol, and Eric Gilson. 2021. "The Power of Stress: The Telo-Hormesis Hypothesis" Cells 10, no. 5: 1156. https://doi.org/10.3390/cells10051156
APA StyleJacome Burbano, M. S., & Gilson, E. (2021). The Power of Stress: The Telo-Hormesis Hypothesis. Cells, 10(5), 1156. https://doi.org/10.3390/cells10051156





