OPALS: A New Osimertinib Adjunctive Treatment of Lung Adenocarcinoma or Glioblastoma Using Five Repurposed Drugs
Abstract
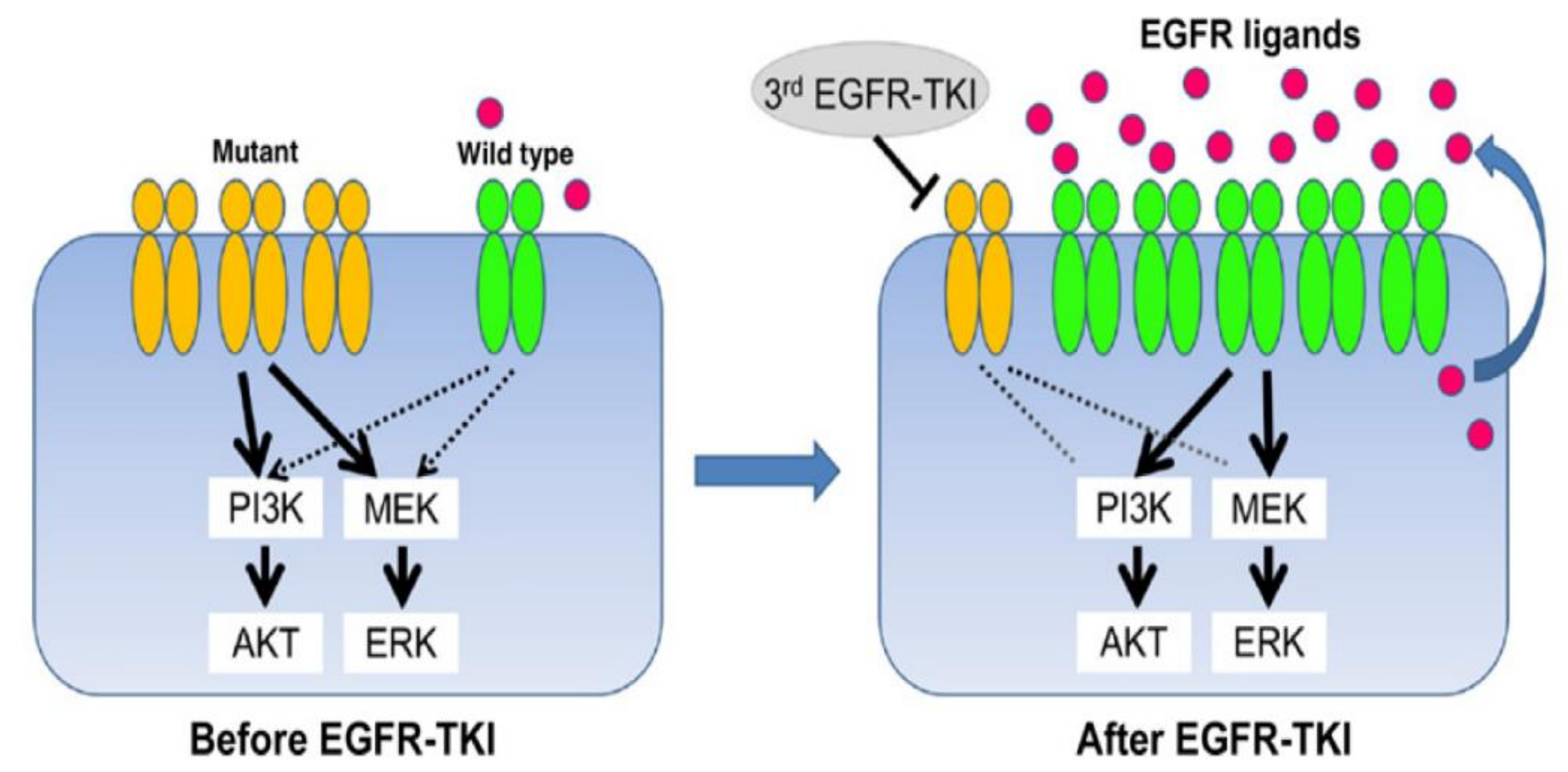
1. Introduction
2. Osimertinib
3. The OPALS Medicines
3.1. Cyproheptadine
3.2. Azithromycin
3.3. Pyrimethamine
3.4. Loratadine
3.5. Spironolactone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSC | cancer stem cells |
| DHFR | dihydrofolate reductase |
| GB | glioblastoma |
| EGFR | epidermal growth factor receptor |
| eIF4E | eukaryotic translation initiation factor 4E |
| HCC | hepatocellular carcinoma |
| MMP-9 | matrix metalloproteinase-9 |
| MOA | mode of action |
| MR | mineralocorticoid receptor |
| MTX | methotrexate |
| NSCLC | non-small cell carcinoma |
References
- Halatsch, M.-E.; Kast, R.E.; Dwucet, A.; Hlavac, M.; Heiland, T.; Westhoff, M.-A.; Debatin, K.-M.; Wirtz, C.R.; Siegelin, M.D.; Karpel-Massler, G. Bcl-2/Bcl-xL inhibition predominantly synergistically enhances the anti-neoplastic activity of a low-dose CUSP9 repurposed drug regime against glioblastoma. Br. J. Pharmacol. 2019, 176, 3681–3694. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Karpel-Massler, G.; Halatsch, M.-E. CUSP9* treatment protocol for recurrent glioblastoma: Aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide. Oncotarget 2014, 5, 8052–8082. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Boockvar, J.A.; Brüning, A.; Cappello, F.; Chang, W.-W.; Cvek, B.; Dou, Q.P.; Duenas-Gonzalez, A.; Efferth, T.; Focosi, D.; et al. A conceptually new treatment approach for relapsed glioblastoma: Coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget 2013, 4, 502–530. [Google Scholar] [CrossRef] [PubMed]
- Serafin, M.B.; Bottega, A.; Da Rosa, T.F.; Machado, C.S.; Foletto, V.S.; Coelho, S.S.; Da Mota, A.D.; Hörner, R. Drug Repositioning in Oncology. Am. J. Ther. 2021, 28, e111–e117. [Google Scholar] [CrossRef]
- Palmer, A.C.; Chidley, C.; Sorger, P.K. A curative combination cancer therapy achieves high fractional cell killing through low cross-resistance and drug additivity. eLife 2019, 8. [Google Scholar] [CrossRef]
- Grassi, G.; Grassi, M. Drug Repurposing in Human Cancers. Curr. Med. Chem. 2020, 27, 7213. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in UntreatedEGFR-Mutated Advanced Non—Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Lamb, Y.N.; Scott, L.J. Osimertinib: A Review in T790M-Positive Advanced Non-Small Cell Lung Cancer. Target. Oncol. 2017, 12, 555–562. [Google Scholar] [CrossRef]
- Brown, H.; Vansteenkiste, J.; Nakagawa, K.; Cobo, M.; John, T.; Barker, C.; Kohlmann, A.; Todd, A.; Saggese, M.; Chmielecki, J.; et al. Programmed Cell Death Ligand 1 Expression in Untreated EGFR Mutated Advanced NSCLC and Response to Osimertinib Versus Comparator in FLAURA. J. Thorac. Oncol. 2020, 15, 138–143. [Google Scholar] [CrossRef]
- Niessen, S.; Dix, M.M.; Barbas, S.; Potter, Z.E.; Lu, S.; Brodsky, O.; Planken, S.; Behenna, D.; Almaden, C.; Gajiwala, K.S.; et al. Proteome-wide Map of Targets of T790M-EGFR-Directed Covalent Inhibitors. Cell Chem. Biol. 2017, 24, 1388–1400.e7. [Google Scholar] [CrossRef]
- Ballard, P.; Yates, J.W.; Yang, Z.; Kim, D.-W.; Yang, J.C.-H.; Cantarini, M.; Pickup, K.; Jordan, A.; Hickey, M.; Grist, M.; et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin. Cancer Res. 2016, 22, 5130–5140. [Google Scholar] [CrossRef]
- Makhlin, I.; Salinas, R.D.; Zhang, D.; Jacob, F.; Ming, G.-L.; Song, H.; Saxena, D.; Dorsey, J.F.; Nasrallah, M.P.; Morrissette, J.J.; et al. Clinical activity of the EGFR tyrosine kinase inhibitor osimertinib in EGFR-mutant glioblastoma. CNS Oncol. 2019, 8, CNS43. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Westhoff, M.A.; Kast, R.E.; Wirtz, C.R.; Halatsch, M.-E. Erlotinib in Glioblastoma—Lost in Translation? Anti-Cancer Agents Med. Chem. 2011, 11, 748–755. [Google Scholar] [CrossRef]
- Chagoya, G.; Kwatra, S.G.; Nanni, C.W.; Roberts, C.M.; Phillips, S.M.; Nullmeyergh, S.; Gilmore, S.P.; Spasojevic, I.; Corcoran, D.L.; Young, C.C.; et al. Efficacy of osimertinib against EGFRvIII+ glioblastoma. Oncotarget 2020, 11, 2074–2082. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, N.; Ou, Q.; Xiang, Y.; Jiang, T.; Wu, X.; Bao, H.; Tong, X.; Wang, X.; Shao, Y.W.; et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non—Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 3097–3107. [Google Scholar] [CrossRef]
- Chaib, I.; Karachaliou, N.; Pilotto, S.; Servat, J.C.; Cai, X.; Li, X.; Drozdowskyj, A.; Servat, C.C.; Yang, J.; Hu, C.; et al. Co-activation of STAT3 and YES-Associated Protein 1 (YAP1) Pathway in EGFR-Mutant NSCLC. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Karachaliou, N.; Chaib, I.; Cardona, A.F.; Berenguer, J.; Bracht, J.W.P.; Yang, J.; Cai, X.; Wang, Z.; Hu, C.; Drozdowskyj, A.; et al. Common Co-activation of AXL and CDCP1 in EGFR-mutation-positive Non-Small Cell Lung Cancer Associated With Poor Prognosis. EBioMedicine 2018, 29, 112–127. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Chan, J.M.; Kubota, D.; Sato, H.; Rizvi, H.; Daneshbod, Y.; Chang, J.C.; Paik, P.K.; Offin, M.; Arcila, M.E.; et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin. Cancer Res. 2020, 26, 2654–2663. [Google Scholar] [CrossRef]
- Verusingam, N.D.; Chen, Y.-C.; Lin, H.-F.; Liu, C.-Y.; Lee, M.-C.; Lu, K.-H.; Cheong, S.-K.; Ong, A.H.-K.; Chiou, S.-H.; Wang, M.-L. Generation of osimertinib-resistant cells from epidermal growth factor receptor L858R/T790M mutant non-small cell lung carcinoma cell line. J. Chin. Med. Assoc. 2021, 84, 248–254. [Google Scholar] [CrossRef]
- Piotrowska, Z.; Isozaki, H.; Lennerz, J.K.; Gainor, J.F.; Lennes, I.T.; Zhu, V.W.; Marcoux, N.; Banwait, M.K.; Digumarthy, S.R.; Su, W.; et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion. Cancer Discov. 2018, 8, 1529–1539. [Google Scholar] [CrossRef]
- Nukaga, S.; Yasuda, H.; Tsuchihara, K.; Hamamoto, J.; Masuzawa, K.; Kawada, I.; Naoki, K.; Matsumoto, S.; Mimaki, S.; Ikemura, S.; et al. Amplification of EGFR Wild-Type Alleles in Non–Small Cell Lung Cancer Cells Confers Acquired Resistance to Mutation-Selective EGFR Tyrosine Kinase Inhibitors. Cancer Res. 2017, 77, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Y.; Xu, Z.; Chen, L.-K.; Zhang, X.; To, K.K.W.; Zhao, H.; Wang, F.; Xia, Z.; Chen, X.; et al. Osimertinib (AZD9291) Enhanced the Efficacy of Chemotherapeutic Agents in ABCB1- and ABCG2-Overexpressing Cells In Vitro, In Vivo, and Ex Vivo. Mol. Cancer Ther. 2016, 15, 1845–1858. [Google Scholar] [CrossRef] [PubMed]
- De Gooijer, M.C.; de Vries, N.A.; Buckle, T.; Buil, L.C.; Beijnen, J.H.; Boogerd, W.; van Tellingen, O. Improved Brain Penetration and Antitumor Efficacy of Temozolomide by Inhibition of ABCB1 and ABCG2. Neoplasia 2018, 20, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cheng, C.-D.; Wu, H.; Wang, Z.-W.; Wang, L.; Jiang, Z.-R.; Wang, A.-L.; Hu, C.; Dong, Y.-F.; Niu, W.-X.; et al. Osimertinib successfully combats EGFR-negative glioblastoma cells by inhibiting the MAPK pathway. Acta Pharmacol. Sin. 2021, 42, 108–114. [Google Scholar] [CrossRef]
- Roth, B.L.; Driscol, J. Cyproheptadine. “PDSP Ki Database”. Psychoactive Drug Screening Program (PDSP); University of North Carolina at Chapel Hill: Chapel Hill, NC, USA, 2017. [Google Scholar]
- Hogervorst, C.O.V.W.; Koppeschaar, H.P.; Zelissen, P.M.; Lips, C.J.; Garcia, B.M. Cortisol secretory patterns in Cushing’s disease and response to cyproheptadine treatment. J. Clin. Endocrinol. Metab. 1996, 81, 652–655. [Google Scholar] [CrossRef]
- Harrison, M.E.; Norris, M.L.; Robinson, A.; Spettigue, W.; Morrissey, M.; Isserlin, L. Use of cyproheptadine to stimulate appetite and body weight gain: A systematic review. Appetite 2019, 137, 62–72. [Google Scholar] [CrossRef]
- Feng, Y.-M.; Feng, C.-W.; Chen, S.-Y.; Hsieh, H.-Y.; Chen, Y.-H.; Hsu, C.-D. Cyproheptadine, an antihistaminic drug, inhibits proliferation of hepatocellular carcinoma cells by blocking cell cycle progression through the activation of P38 MAP kinase. BMC Cancer 2015, 15, 1–13. [Google Scholar] [CrossRef]
- Feng, Y.-M.; Feng, C.-W.; Chen, S.C.-C.; Hsu, C.-D. Unexpected remission of hepatocellular carcinoma (HCC) with lung metastasis to the combination therapy of thalidomide and cyproheptadine: Report of two cases and a preliminary HCC cell line study. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Hung, S.-K.; Chiou, W.-Y.; Lee, M.-S.; Shen, B.-J.; Chen, L.-C.; Liu, D.-W.; Tsai, W.-T.; Lin, P.-H.; Shih, Y.-T.; et al. Significant symptoms alleviation and tumor volume reduction after combined simultaneously integrated inner-escalated boost and volumetric-modulated arc radiotherapy in a patient with unresectable bulky hepatocellular carcinoma. Medicine 2016, 95, e4717. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Lee, W.-H.; Wu, A.T.; Chow, J.-M.; Chang, C.-L.; Yuan, K.S.-P.; Wu, S.-Y. Cyproheptadine use in hepatocellular carcinoma. Am. J. Cancer Res. 2017, 7, 584–602. [Google Scholar]
- Chang, H.-C.; Lin, C.-T.; Lin, W.-Y.; Yu, P.-T. Analysis of the effects of cyproheptadine on bladder cancer through big data. Am. J. Cancer Res. 2020, 10, 2114–2119. [Google Scholar]
- Feng, Y.-M.; Feng, C.-W.; Lu, C.-L.; Lee, M.-Y.; Chen, C.-Y.; Chen, S.C.-C. Cyproheptadine significantly improves the overall and progression-free survival of sorafenib-treated advanced HCC patients. Jpn. J. Clin. Oncol. 2015, 45, 336–342. [Google Scholar] [CrossRef]
- Paoluzzi, L.; Scotto, L.; Marchi, E.; Seshan, V.E.; O’Connor, O.A. The anti-histaminic cyproheptadine synergizes the antineoplastic activity of bortezomib in mantle cell lymphoma through its effects as a histone deacetylase inhibitor. Br. J. Haematol. 2009, 146, 656–659. [Google Scholar] [CrossRef]
- Mao, X.; Liang, S.-B.; Hurren, R.; Gronda, M.; Chow, S.; Xu, G.W.; Wang, X.; Zavareh, R.B.; Jamal, N.; Messner, H.; et al. Cyproheptadine displays preclinical activity in myeloma and leukemia. Blood 2008, 112, 760–769. [Google Scholar] [CrossRef]
- Harris, A.L.; Smith, I.E. Regression of carcinoid tumour with cyproheptadine. BMJ 1982, 285, 475. [Google Scholar] [CrossRef][Green Version]
- Leitner, S.P.; Greenberg, P.; Danieu, L.A.; Michaelson, R.A. Partial Remission of Carcinoid Tumor in Response to Cyproheptadine. Ann. Intern. Med. 1989, 111, 760–761. [Google Scholar] [CrossRef]
- Moertel, C.G.; Kvols, L.K.; Rubin, J. A study of cyproheptadine in the treatment of metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer 1991, 67, 33–36. [Google Scholar] [CrossRef]
- Kacar, S.; Hacioglu, C.; Kar, F.; Sahinturk, V.; Kanbak, G. Cyproheptadine causes apoptosis and decreases inflammation by disrupting thiol/disulfide balance and enhancing the levels of SIRT1 in C6 glioblastoma cells. Toxicol. Vitr. 2021, 73, 105135. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine signaling system in progression of lung cancers. Pharmacol. Ther. 2019, 194, 222–254. [Google Scholar] [CrossRef]
- Spindel, E.R. Cholinergic Targets in Lung Cancer. Curr. Pharm. Des. 2016, 22, 2152–2159. [Google Scholar] [CrossRef]
- Russo, P.; Del Bufalo, A.; Milić, M.; Salinaro, G.; Fini, M.; Cesario, A. Cholinergic Receptors as Target for Cancer Therapy in a Systems Medicine Perspective. Curr. Mol. Med. 2014, 14, 1126–1138. [Google Scholar] [CrossRef]
- Sales, M.E.; Español, A.J.; Salem, A.R.; Pulido, P.M.; Sanchez, Y.; Sanchez, F.; Martínez, P.P. Role of Muscarinic Acetylcholine Receptors in Breast Cancer: Design of Metronomic Chemotherapy. Curr. Clin. Pharmacol. 2019, 14, 91–100. [Google Scholar] [CrossRef]
- Hsieh, H.-Y.; Shen, C.-H.; Lin, R.-I.; Feng, Y.-M.; Huang, S.-Y.; Wang, Y.-H.; Wu, S.-F.; Hsu, C.-D.; Chan, M.W. Cyproheptadine exhibits antitumor activity in urothelial carcinoma cells by targeting GSK3β to suppress mTOR and β-catenin signaling pathways. Cancer Lett. 2016, 370, 56–65. [Google Scholar] [CrossRef]
- Li, J.; Cao, B.; Zhou, S.; Zhu, J.; Zhang, Z.; Hou, T.; Mao, X. Cyproheptadine-induced myeloma cell apoptosis is associated with inhibition of the PI3K/AKT signaling. Eur. J. Haematol. 2013, 91, 514–521. [Google Scholar] [CrossRef]
- Clark, R.B.; Perkins, J.P. Regulation of Adenosine 3′:5′-Cyclic Monophosphate Concentration in Cultured Human Astrocytoma Cells by Catecholamines and Histamine. Proc. Natl. Acad. Sci. USA 1971, 68, 2757–2760. [Google Scholar] [CrossRef]
- Falus, A. Interleukin-6 biosynthesis is increased by histamine in human B-cell and glioblastoma cell lines. Immunology 1993, 78, 193–196. [Google Scholar]
- Skaga, E.; Skaga, I.Ø.; Grieg, Z.; Sandberg, C.J.; Langmoen, I.A.; Vik-Mo, E.O. The efficacy of a coordinated pharmacological blockade in glioblastoma stem cells with nine repurposed drugs using the CUSP9 strategy. J. Cancer Res. Clin. Oncol. 2019, 145, 1495–1507. [Google Scholar] [CrossRef]
- Weydt, P.; Möller, T.; Labrakakis, C.; Patt, S.; Kettenmann, H. Neuroligand-triggered calcium signalling in cultured human glioma cells. Neurosci. Lett. 1997, 228, 91–94. [Google Scholar] [CrossRef]
- Fioretti, B.; Catacuzzeno, L.; Sforna, L.; Aiello, F.; Pagani, F.; Ragozzino, D.; Castigli, E.; Franciolini, F. Histamine hyperpolarizes human glioblastoma cells by activating the intermediate-conductance Ca2+-activated K+ channel. Am. J. Physiology-Cell Physiol. 2009, 297, C102–C110. [Google Scholar] [CrossRef]
- Kast, R. Profound blocking cxcr4 signaling at multiple points using synergy between plerixafor, mirtazapine, and clotrimazole as a new glioblastoma treatment adjunct. Turk. Neurosurg. 2010, 20, 425–429. [Google Scholar] [CrossRef][Green Version]
- Thompson, E.G.; Sontheimer, H. Acetylcholine Receptor Activation as a Modulator of Glioblastoma Invasion. Cells 2019, 8, 1203. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Ji, D.; Qu, X.; Liu, S.; Yang, X.; Wang, G.; Liu, Q.; Du, J. Mining and validating the expression pattern and prognostic value of acetylcholine receptors in non-small cell lung cancer. Medicine 2019, 98, e15555. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, E.; Uddin, M.; Mankuta, D.; Dubinett, S.M.; Levi-Schaffer, F. Mast cells and histamine enhance the proliferation of non-small cell lung cancer cells. Lung Cancer 2012, 75, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, X.; Shi, L.; Chen, B.; Zhao, G.; Yang, H. Integrative genomic analyses of the histamine H1 receptor and its role in cancer prediction. Int. J. Mol. Med. 2014, 33, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Kondratenko, T.Y.; Zacharova, I.V.; Kuzina, N.V.; Katukov, V.Y.; Severin, E.S. The role of histamine H1-receptors in the modula-tion of neurotransmitter receptors activity in human lung cancer. Biochem. Mol. Biol. Int. 1995, 36, 429–437. [Google Scholar] [PubMed]
- Kondratenko, T.Y.; Zacharova, I.V.; Katukov, V.Y.; Kuzina, N.V.; Severin, E.S.; Kornilova, Z.C.; Perelman, M.I. The study of hista-mine H1- and H2-receptors in human lung cancer. Biochem. Mol. Biol. Int. 1993, 31, 399–404. [Google Scholar] [PubMed]
- Chu, D.; Yao, D.; Zhuang, Y.; Hong, Y.; Zhu, X.; Fang, Z.; Yu, J.; Yu, Z. Azithromycin enhances the favorable results of paclitaxel and cisplatin in patients with advanced non-small cell lung cancer. Genet. Mol. Res. 2014, 13, 2796–2805. [Google Scholar] [CrossRef]
- Lagler, H.; Kiesewetter, B.; Dolak, W.; Obermueller, M.; Simonitsch-Klupp, I.; Lukas, J.; Neuper, O.; Lamm, W.W.; Mayerhoefer, M.E.; Raderer, M. Treatment of mucosa associated lymphoid tissue lymphoma with a long-term once-weekly regimen of oral azithromycin: Results from the phase II MALT—A trial. Hematol. Oncol. 2018, 37, 22–26. [Google Scholar] [CrossRef]
- Fassl, A.; Brain, C.; Abu-Remaileh, M.; Stukan, I.; Butter, D.; Stepien, P.; Feit, A.S.; Bergholz, J.; Michowski, W.; Otto, T.; et al. Increased lysosomal biomass is responsible for the resistance of triple-negative breast cancers to CDK4/6 inhibition. Sci. Adv. 2020, 6, eabb2210. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, X.; Shang, Y.; Li, Y.; Chen, S.-Z. Azithromycin enhances anticancer activity of TRAIL by inhibiting autophagy and up-regulating the protein levels of DR4/5 in colon cancer cells In Vitro and in vivo. Cancer Commun. 2018, 38, 1–13. [Google Scholar] [CrossRef]
- Hirasawa, K.; Moriya, S.; Miyahara, K.; Kazama, H.; Hirota, A.; Takemura, J.; Abe, A.; Inazu, M.; Hiramoto, M.; Tsukahara, K.; et al. Macrolide Antibiotics Exhibit Cytotoxic Effect under Amino Acid-Depleted Culture Condition by Blocking Autophagy Flux in Head and Neck Squamous Cell Carcinoma Cell Lines. PLoS ONE 2016, 11, e0164529. [Google Scholar] [CrossRef]
- Moriya, S.; Che, X.-F.; Komatsu, S.; Abe, A.; Kawaguchi, T.; Gotoh, A.; Inazu, M.; Tomoda, A.; Miyazawa, K. Macrolide antibiotics block autophagy flux and sensitize to bortezomib via endoplasmic reticulum stress-mediated CHOP induction in myeloma cells. Int. J. Oncol. 2013, 42, 1541–1550. [Google Scholar] [CrossRef]
- Abbas, S.; Singh, S.K.; Saxena, A.K.; Tiwari, S.; Sharma, L.K.; Tiwari, M. Role of autophagy in regulation of glioma stem cells population during therapeutic stress. J. Stem Cells Regen. Med. 2020, 16, 80–89. [Google Scholar] [CrossRef]
- Li, F.; Shuang, L.; Ji, D.; Meng, Q.; Wang, C.; Chenghong, L.; Wang, X.; Zhu, Z.; Jiang, C.; Shi, C.; et al. Azithromycin effectively inhibits tumor angiogenesis by suppressing vascular endothelial growth factor receptor 2-mediated signaling pathways in lung cancer. Oncol. Lett. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Vandooren, J.; Knoops, S.; Buzzo, J.L.A.; Boon, L.; Martens, E.; Opdenakker, G.; Kolaczkowska, E. Differential inhibition of activity, activation and gene expression of MMP-9 in THP-1 cells by azithromycin and minocycline versus bortezomib: A comparative study. PLoS ONE 2017, 12, e0174853. [Google Scholar] [CrossRef]
- Gong, L.; Wu, D.; Zou, J.; Chen, J.; Chen, L.; Chen, Y.; Ni, C.; Yuan, H. Prognostic impact of serum and tissue MMP-9 in non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 18458–18468. [Google Scholar] [CrossRef]
- Rajesh, Y.; Banerjee, A.; Pal, I.; Biswas, A.; Das, S.; Dey, K.K.; Kapoor, N.; Ghosh, A.K.; Mitra, P.; Mandal, M. Delineation of crosstalk between HSP27 and MMP-2/MMP-9: A synergistic therapeutic avenue for glioblastoma management. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 1196–1209. [Google Scholar] [CrossRef]
- Goethe, E.; Carter, B.Z.; Rao, G.; Pemmaraju, N. Glioblastoma and acute myeloid leukemia: Malignancies with striking similarities. J. Neuro-Oncol. 2017, 136, 223–231. [Google Scholar] [CrossRef]
- Morris, D.L.; De Souza, A.; Jones, J.A.; Morgan, W.E. High and prolonged pulmonary tissue concentrations of azithromycin following a single oral dose. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 859–861. [Google Scholar] [CrossRef]
- Hannewald, P.; Maunit, B.; Muller, J.-F. Screening of DHFR-binding drugs by MALDI-TOFMS. Anal. Bioanal. Chem. 2008, 392, 1335–1344. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Slotky, J. Characterization of a lipophilic antifolate resistance provoked by treatment of mammalian cells with the antiparasitic agent pyrimethamine. J. Biol. Chem. 1993, 268, 4556–4566. [Google Scholar] [CrossRef]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse Event Profile of Pyrimethamine-Based Therapy in Toxoplasmosis: A Systematic Review. Drugs R&D 2017, 17, 523–544. [Google Scholar] [CrossRef]
- De Ven, E.S.-V.; Galama, J.; Vree, T.; Camps, W.; Baars, I.; Eskes, T.; Meuwissen, J.; Melchers, W. Study of treatment of congenital Toxoplasma gondii infection in rhesus monkeys with pyrimethamine and sulfadiazine. Antimicrob. Agents Chemother. 1995, 39, 137–144. [Google Scholar] [CrossRef]
- Jansen, G.; Barr, H.; Kathmann, I.; Bunni, M.A.; Priest, D.G.; Noordhuis, P.; Peters, G.J.; Assaraf, Y.G. Multiple mechanisms of resistance to polyglutamatable and lipophilic antifolates in mammalian cells: Role of increased folylpolyglutamylation, expanded folate pools, and intralysosomal drug sequestration. Mol. Pharmacol. 1999, 55, 761–769. [Google Scholar]
- Sharma, A.; Jyotsana, N.; Lai, C.K.; Chaturvedi, A.; Gabdoulline, R.; Görlich, K.; Murphy, C.; Blanchard, J.E.; Ganser, A.; Brown, E.; et al. Pyrimethamine as a Potent and Selective Inhibitor of Acute Myeloid Leukemia Identified by High-throughput Drug Screening. Curr. Cancer Drug Targets 2016, 16, 818–828. [Google Scholar] [CrossRef]
- Bowcock, S.; Linch, D.; Machin, S.; Stewart, J. Pyrimethamine in the myeloproliferative disorders: A forgotten treatment? Int. J. Lab. Hematol. 1987, 9, 129–136. [Google Scholar] [CrossRef]
- Armata, J.; Cyklis, R.; Borkowski, W. Pyrimethamine in prevention of relapses of meningeal leukemia. Report of two cases. Cancer 1978, 42, 1216–1218. [Google Scholar] [CrossRef]
- Smyth, A.C.; Wiernik, P.H. Combination chemotherapy of adult acute lymphocytic leukemia. Clin. Pharmacol. Ther. 1976, 19, 240–245. [Google Scholar] [CrossRef]
- Wiernik, P.H.; Schimpff, S.C.; Schiffer, C.; Lichtenfeld, J.L.; Aisner, J.; O’Connell, M.J.; Fortner, C. Randomized clinical comparison of daunorubicin (NSC-82151) alone with a combination of daunorubicin, cytosine arabinoside (NSC-63878), 6-thioguanine (NSC-752), and pyrimethamine (NSC-3061) for the treatment of acute nonlymphocytic leukemia. Cancer Treat. Rep. 1976, 60, 41–53. [Google Scholar]
- Fritz, I.; Wagner, P.; Bottai, M.; Eriksson, H.; Ingvar, C.; Krakowski, I.; Nielsen, K.; Olsson, H. Desloratadine and loratadine use associated with improved melanoma survival. Allergy 2020, 75, 2096–2099. [Google Scholar] [CrossRef] [PubMed]
- Fritz, I.; Wagner, P.; Broberg, P.; Einefors, R.; Olsson, H. Desloratadine and loratadine stand out among common H1-antihistamines for association with improved breast cancer survival. Acta Oncol. 2020, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Verdoodt, F.; Dehlendorff, C.; Jäättelä, M.; Strauss, R.; Pottegård, A.; Hallas, J.; Friis, S.; Kjaer, S.K. Antihistamines and Ovarian Cancer Survival: Nationwide Cohort Study and In Vitro Cell Viability Assay. J. Natl. Cancer Inst. 2019, 112, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Liu, B.; Giacobini, J.D.; Maeda, K.; Rohde, M.; Jäättelä, M. Cell Death Induced by Cationic Amphiphilic Drugs Depends on Lysosomal Ca2+ Release and Cyclic AMP. Mol. Cancer Ther. 2019, 18, 1602–1614. [Google Scholar] [CrossRef]
- Ellegaard, A.-M.; Dehlendorff, C.; Vind, A.C.; Anand, A.; Cederkvist, L.; Petersen, N.H.; Nylandsted, J.; Stenvang, J.; Mellemgaard, A.; Østerlind, K.; et al. Repurposing Cationic Amphiphilic Antihistamines for Cancer Treatment. EBioMedicine 2016, 9, 130–139. [Google Scholar] [CrossRef]
- Kölzer, M.; Werth, N.; Sandhoff, K. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine. FEBS Lett. 2004, 559, 96–98. [Google Scholar] [CrossRef]
- Chanas-Larue, A.; Villalpando-Rodriguez, G.E.; Henson, E.S.; Johnston, J.B.; Gibson, S.B. Antihistamines are synergistic with Bruton’s tyrosine kinase inhibiter ibrutinib mediated by lysosome disruption in chronic lymphocytic leukemia (CLL) cells. Leuk. Res. 2020, 96, 106423. [Google Scholar] [CrossRef]
- Ma, J.; Qi, J.; Li, S.; Zhang, C.; Wang, H.; Shao, L.; Yuan, X.; Sha, Q. Desloratadine, a Novel Antigrowth Reagent for Bladder Cancer. Technol. Cancer Res. Treat. 2020, 19. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.S.; Kim, I.S.; Yoon, S. Histamine Receptor Antagonists, Loratadine and Azelastine, Sensitize P-gp-overexpressing Antimitotic Drug-resistant KBV20C Cells Through Different Molecular Mechanisms. Anticancer. Res. 2019, 39, 3767–3775. [Google Scholar] [CrossRef]
- Le Joncour, V.; Filppu, P.; Hyvönen, M.; Holopainen, M.; Turunen, S.P.; Sihto, H.; Burghardt, I.; Joensuu, H.; Tynninen, O.; Jääskeläinen, J.; et al. Vulnerability of invasive glioblastoma cells to lysosomal membrane destabilization. EMBO Mol. Med. 2019, 11, e9034. [Google Scholar] [CrossRef]
- Corvol, P.; Claire, M.; Oblin, M.; Geering, K.; Rossier, B. Mechanism of the antimineralocorticoid effects of spirolactones. Kidney Int. 1981, 20, 1–6. [Google Scholar] [CrossRef]
- Gabbard, R.D.; Hoopes, R.R.; Kemp, M.G. Spironolactone and XPB: An Old Drug with a New Molecular Target. Biomolecules 2020, 10, 756. [Google Scholar] [CrossRef]
- Karim, A. Spironolactone: Disposition, Metabolism, Pharmacodynamics, and Bioavailability. Drug Metab. Rev. 1978, 8, 151–188. [Google Scholar] [CrossRef]
- Reul, J.M.H.M.; De Kloet, E.R.; Van Sluijs, F.J.; Rijnberk, A.; Rothuizen, J. Binding Characteristics of Mineralocorticoid and Glucocorticoid Receptors in Dog Brain and Pituitary. Endocrinology 1990, 127, 907–915. [Google Scholar] [CrossRef]
- Lainscak, M.; Pelliccia, F.; Rosano, G.; Vitale, C.; Schiariti, M.; Greco, C.; Speziale, G.; Gaudio, C. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int. J. Cardiol. 2015, 200, 25–29. [Google Scholar] [CrossRef]
- Krug, A.W.; Grossmann, C.; Schuster, C.; Freudinger, R.; Mildenberger, S.; Govindan, M.V.; Gekle, M. Aldosterone stimulates epider-mal growth factor receptor expression. J. Biol. Chem. 2003, 278, 43060–43066. [Google Scholar] [CrossRef]
- Ruhs, S.; Nolze, A.; Hübschmann, R.; Grossmann, C. 30 Years of the Mineralocorticoid Receptor: Nongenomic effects via the mineralocorticoid receptor. J. Endocrinol. 2017, 234, T107–T124. [Google Scholar] [CrossRef]
- Grossmann, C.; Krug, A.W.; Freudinger, R.; Mildenberger, S.; Voelker, K.; Gekle, M. Aldosterone induced EGFR expression: Interac-tion between the human mineralocorticoid receptor and the human EGFR promoter. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1790–E1800. [Google Scholar] [CrossRef]
- Grossmann, C.; Gekle, M. Nongenotropic aldosterone effects and the EGFR: Interaction and biological relevance. Steroids 2008, 73, 973–978. [Google Scholar] [CrossRef]
- Grossmann, C.; Husse, B.; Mildenberger, S.; Schreier, B.; Schuman, K.; Gekle, M. Colocalization of mineralocorticoid and EGF receptor at the plasma membrane. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1803, 584–590. [Google Scholar] [CrossRef]
- Grossmann, C.; Gekle, M. Interaction between mineralocorticoid receptor and epidermal growth factor receptor signaling. Mol. Cell. Endocrinol. 2012, 350, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Schreier, B.; Rabe, S.; Winter, S.; Ruhs, S.; Mildenberger, S.; Schneider, B.; Sibilia, M.; Gotthardt, M.; Kempe, S.; Mäder, K.; et al. Moderate inappropriately high aldosterone/NaCl constellation in mice: Cardiovascular effects and the role of cardiovascular epidermal growth factor receptor. Sci. Rep. 2014, 4, 7430. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kawai, T.; O’Brien, S.; Thomas, W.; Harris, R.C.; Eguchi, S.; Elliott, K.J. Epidermal Growth Factor Receptor Transactivation: Mechanisms, Pathophysiology, and Potential Therapies in the Cardiovascular System. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 627–653. [Google Scholar] [CrossRef] [PubMed]
- Mitsuishi, Y.; Shibata, H.; Kurihara, I.; Kobayashi, S.; Yokota, K.; Murai-Takeda, A.; Hayashi, T.; Jo, R.; Nakamura, T.; Morisaki, M.; et al. Epidermal growth factor receptor/extracellular signal-regulated kinase pathway enhances mineralocorticoid receptor transcriptional activity through protein stabilization. Mol. Cell. Endocrinol. 2018, 473, 89–99. [Google Scholar] [CrossRef]
- Ong, G.S.; Young, M.J. Mineralocorticoid regulation of cell function: The role of rapid signalling and gene transcription path-ways. J. Mol. Endocrinol. 2017, 58, R33–R57. [Google Scholar] [CrossRef]
- Shrestha, A.; Che, R.-C.; Zhang, A.-H. Role of Aldosterone in Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 325–346. [Google Scholar] [CrossRef]
- Sheng, L.; Yang, M.; Ding, W.; Zhang, M.; Niu, J.; Qiao, Z.; Gu, Y. Epidermal growth factor receptor signaling mediates aldosterone-induced profibrotic responses in kidney. Exp. Cell Res. 2016, 346, 99–110. [Google Scholar] [CrossRef]
- McEneaney, V.; Harvey, B.J.; Thomas, W. Aldosterone rapidly activates protein kinase D via a mineralocorticoid receptor/EGFR trans-activation pathway in the M1 kidney CCD cell line. J. Steroid Biochem. Mol. Biol. 2007, 107, 180–190. [Google Scholar] [CrossRef]
- Sanomachi, T.; Suzuki, S.; Togashi, K.; Sugai, A.; Seino, S.; Okada, M.; Yoshioka, T.; Kitanaka, C.; Yamamoto, M. Spironolactone, a Classic Potassium-Sparing Diuretic, Reduces Survivin Expression and Chemosensitizes Cancer Cells to Non-DNA-Damaging Anticancer Drugs. Cancers 2019, 11, 1550. [Google Scholar] [CrossRef]
- Elinoff, J.M.; Chen, L.-Y.; Dougherty, E.J.; Awad, K.S.; Wang, S.; Biancotto, A.; Siddiqui, A.H.; Weir, N.A.; Cai, R.; Sun, J.; et al. Spironolactone-induced degradation of the TFIIH core complex XPB subunit suppresses NF-κB and AP-1 signalling. Cardiovasc. Res. 2018, 114, 65–76. [Google Scholar] [CrossRef]
- Kemp, M.G.; Krishnamurthy, S.; Kent, M.N.; Schumacher, D.L.; Sharma, P.; Excoffon, K.J.; Travers, J.B. Spironolactone Depletes the XPB Protein and Inhibits DNA Damage Responses in UVB-Irradiated Human Skin. J. Investig. Dermatol. 2019, 139, 448–454. [Google Scholar] [CrossRef]
- Compe, E.; Egly, J.-M. Nucleotide Excision Repair and Transcriptional Regulation: TFIIH and Beyond. Annu. Rev. Biochem. 2016, 85, 265–290. [Google Scholar] [CrossRef]
- Varbanov, H.P.; Kuttler, F.; Banfi, D.; Turcatti, G.; Dyson, P.J. Screening-based approach to discover effective platinum-based chemotherapies for cancers with poor prognosis. PLoS ONE 2019, 14, e0211268. [Google Scholar] [CrossRef]
- Shahar, O.D.; Kalousi, A.; Eini, L.; Fisher, B.; Weiss, A.; Darr, J.; Mazina, O.; Bramson, S.; Kupiec, M.; Eden, A.; et al. A high-throughput chemical screen with FDA approved drugs reveals that the antihypertensive drug Spironolactone impairs cancer cell survival by inhibiting homology directed repair. Nucleic Acids Res. 2014, 42, 5689–5701. [Google Scholar] [CrossRef]
- Rickard, A.J.; Fuller, P.J. Mineralocorticoid and Epidermal Growth Factor Receptors: Partners in Vivo. Hypertension 2011, 57, 144–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Drug | T 1/2 | Plasma Level | OPALS Function |
|---|---|---|---|
| Cyproheptadine | 16 h | 33 microg/L av 669 microg/L max | anticholinergic, Bcl-2 inhibition, antihistamine, |
| Pyrimethamine | 4 d | 500 microg/L | DHFR inhibition, |
| Azithromycin | 2–3 d | 31 mg/L | MMP-9 reduction, autophagy inhibition |
| Loratadine | 8 h | 30 μg/L | lysosomal leakage |
| Spironolactone canrenone | 2 h 17 h | 140 microg/L | EGFR transactivation DNA repair inhibition |
| Receptor | Ki nM |
|---|---|
| H1 | 0.06 |
| M1 | 12 |
| M2 | 7 |
| M3 | 12 |
| M4 | 8 |
| M5 | 12 |
| 5HT1a | 59 |
| 5HT2a | 1.7 |
| 5HT2b | 1.5 |
| 5HT2c | 2.2 |
| D3 | 8 |
| Post-Dose | Brain Microg/g | CSF Microg/mL | Serum Microg/mL |
|---|---|---|---|
| 24 h | 2.63 +/− 2.58 | <0.015 | 0.031 +/− 0.044 |
| 48 h | 3.64 +/− 3.81 | <0.015 | 0.016 +/− 0.011 |
| 72 h | 0.74 +/− 0.37 | <0.015 | 0.012 +/− 0.005 |
| 96 h | 0.41 | <0.015 | 0.008 |
| Changes in Pyrimethamine Resistant Cancer Cells Compared to Sensitive Counterpart: |
|---|
| lower external folate requirement for growth |
| 3 x increased intracellular polyglutamated folate content |
| increased lysosome number |
| increased folylpolyglutamate synthetase |
| increased P-gp export activity |
| DHFR gene amplification |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kast, R.E.; Halatsch, M.-E.; Rosell, R. OPALS: A New Osimertinib Adjunctive Treatment of Lung Adenocarcinoma or Glioblastoma Using Five Repurposed Drugs. Cells 2021, 10, 1148. https://doi.org/10.3390/cells10051148
Kast RE, Halatsch M-E, Rosell R. OPALS: A New Osimertinib Adjunctive Treatment of Lung Adenocarcinoma or Glioblastoma Using Five Repurposed Drugs. Cells. 2021; 10(5):1148. https://doi.org/10.3390/cells10051148
Chicago/Turabian StyleKast, Richard E., Marc-Eric Halatsch, and Rafael Rosell. 2021. "OPALS: A New Osimertinib Adjunctive Treatment of Lung Adenocarcinoma or Glioblastoma Using Five Repurposed Drugs" Cells 10, no. 5: 1148. https://doi.org/10.3390/cells10051148
APA StyleKast, R. E., Halatsch, M.-E., & Rosell, R. (2021). OPALS: A New Osimertinib Adjunctive Treatment of Lung Adenocarcinoma or Glioblastoma Using Five Repurposed Drugs. Cells, 10(5), 1148. https://doi.org/10.3390/cells10051148







