CD47 Potentiates Inflammatory Response in Systemic Lupus Erythematosus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cell Isolation
2.3. Cell Stimulation
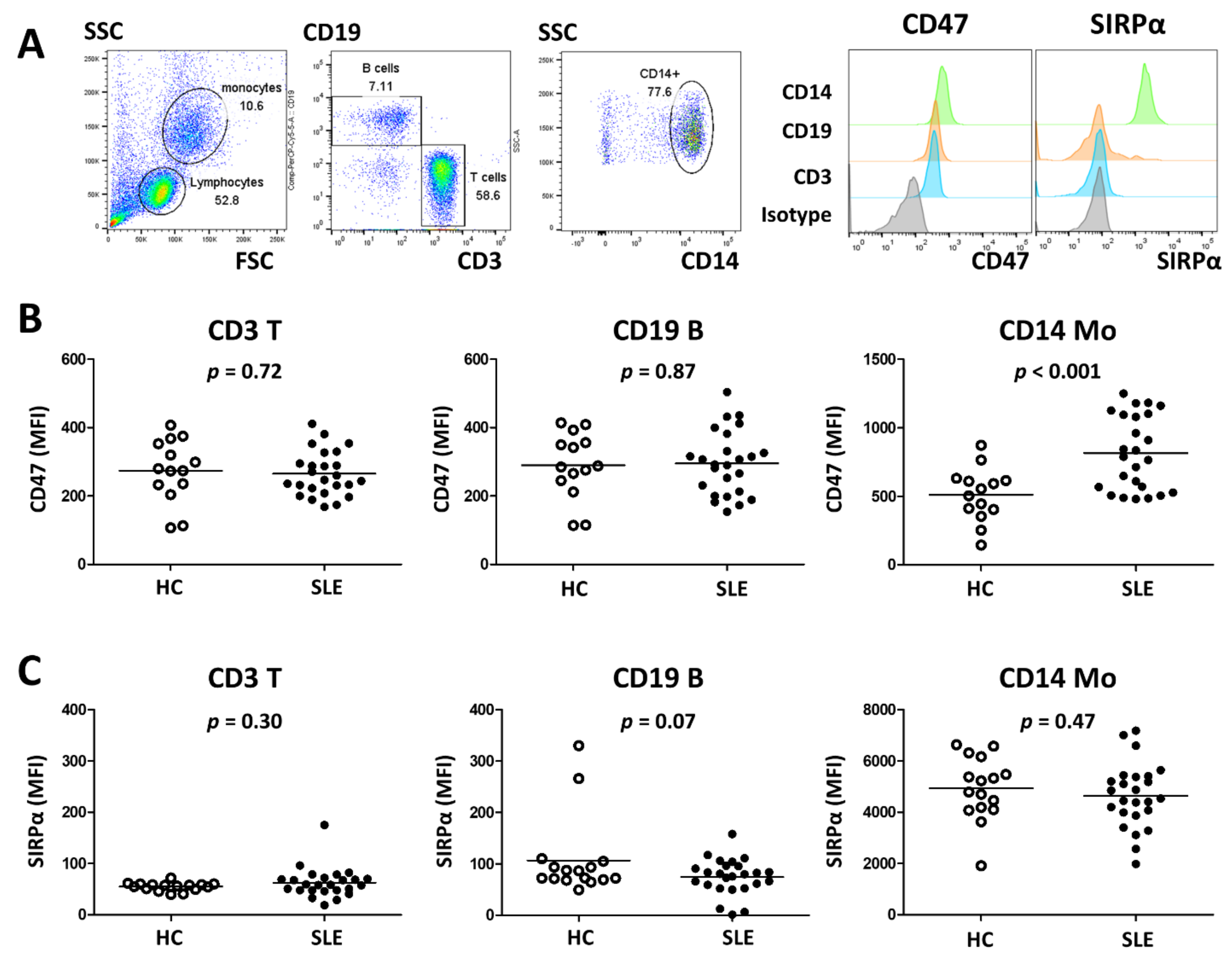
2.4. Flow Cytometry Analysis
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Hematoxylin and Eosin Staining
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. SLE Monocytes Show Increased Expression of CD47
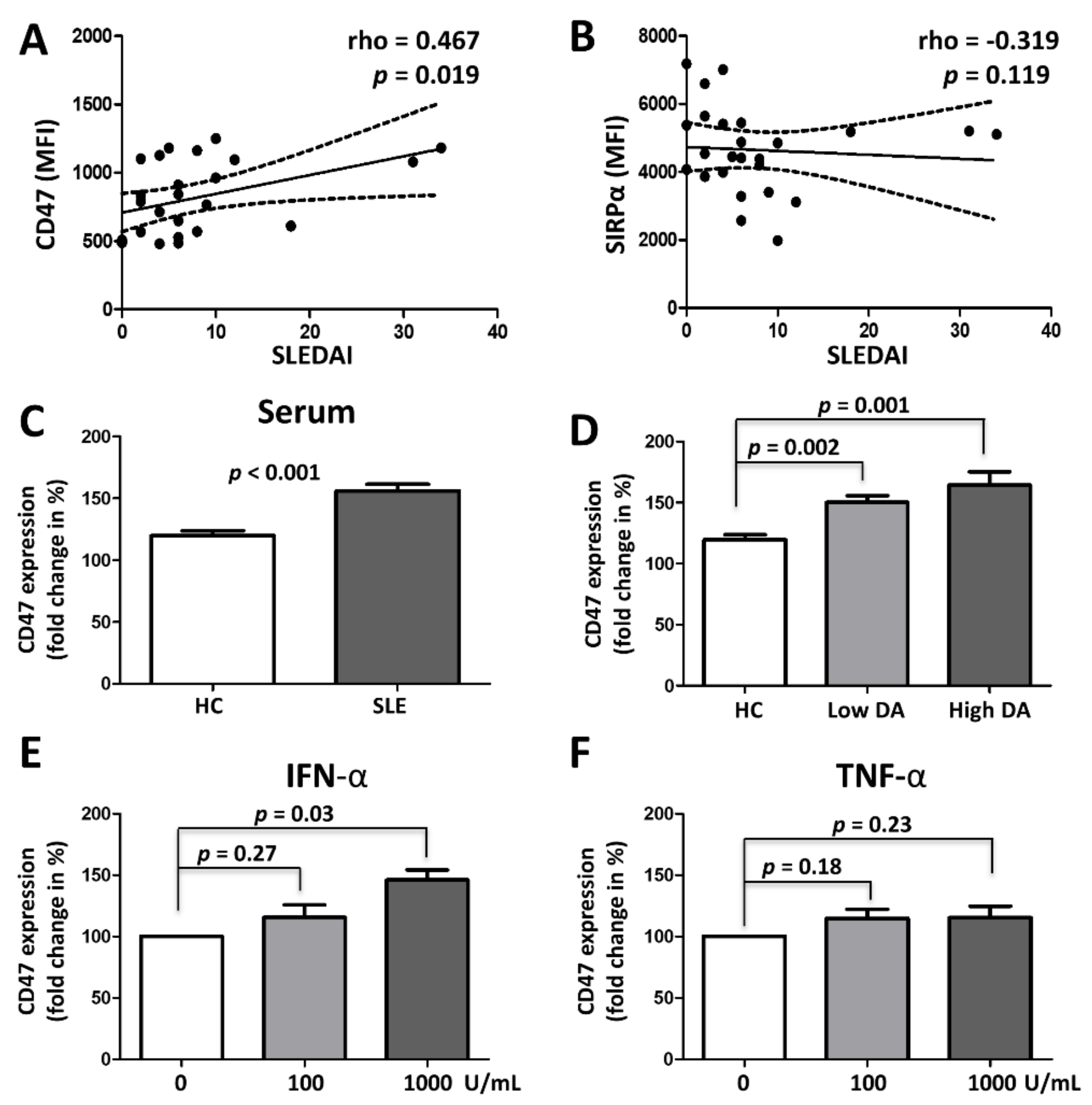
3.3. CD47 Expression Is Associated with SLE Disease Activity and Upregulated by Type 1 Interferon
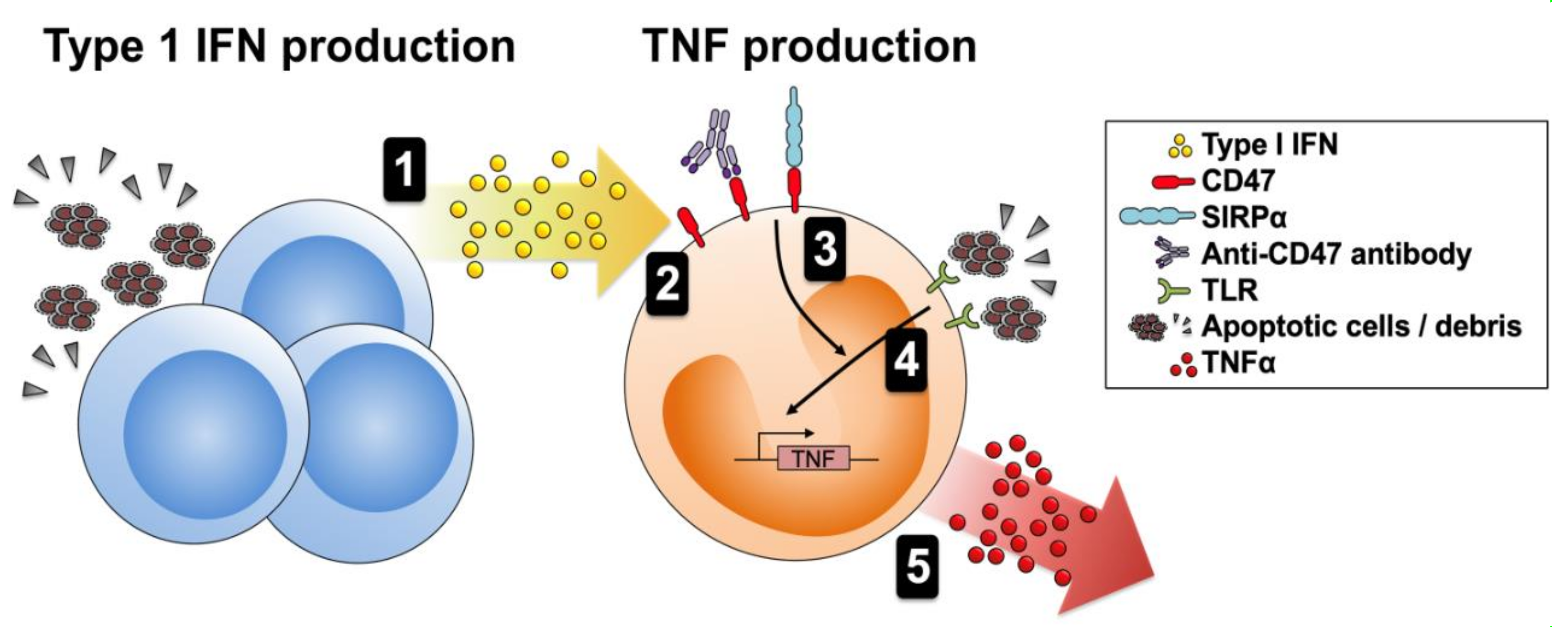
3.4. CD47 Activation Potentiates Proinflammatory Responses
3.5. Autoantibodies Directed Against CD47 Are Present in SLE
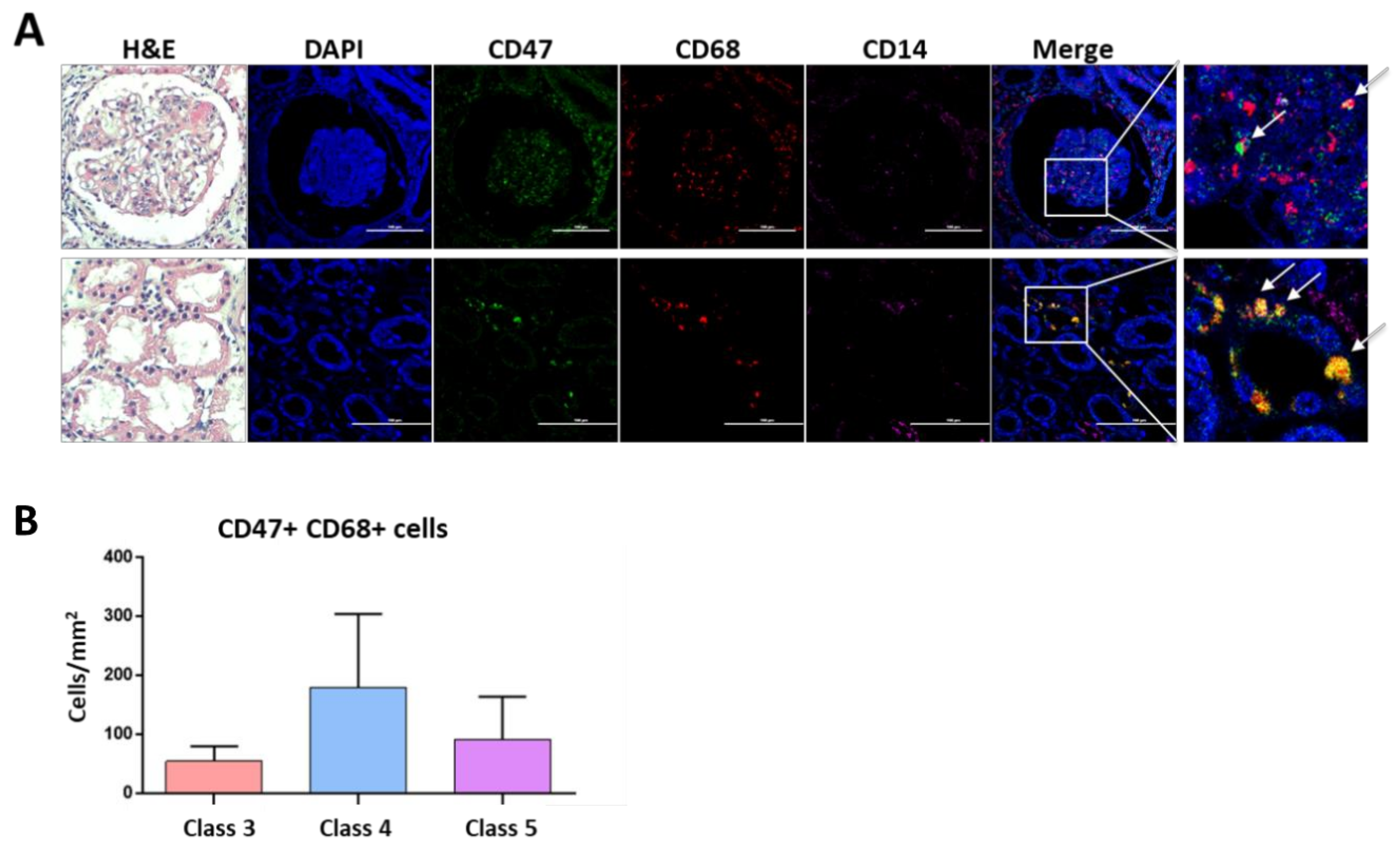
3.6. CD47 Expressing Macrophages Are Present in Lupus Nephritis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, A.; Isenberg, D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008, 358, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Davidson, A. Taming lupus—A new understanding of pathogenesis is leading to clinical advances. Nat. Med. 2012, 18, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, S.J.; Daridon, C.; Fleischer, V.; Lipsky, P.E.; Dorner, T. Enhanced Tyrosine Phosphatase Activity Underlies Dysregulated B Cell Receptor Signaling and Promotes Survival of Human Lupus B Cells. Arthritis Rheumatol. 2016, 68, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Dohring, C.; Samaridis, J.; Dessing, M.; Brockhaus, M.; Lanzavecchia, A.; Colonna, M. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J. Exp. Med. 1997, 185, 1743–1751. [Google Scholar] [CrossRef]
- Lu, H.K.; Rentero, C.; Raftery, M.J.; Borges, L.; Bryant, K.; Tedla, N. Leukocyte Ig-like receptor B4 (LILRB4) is a potent inhibitor of FcgammaRI-mediated monocyte activation via dephosphorylation of multiple kinases. J. Biol. Chem. 2009, 284, 34839–34848. [Google Scholar] [CrossRef]
- Navarro-Alvarez, N.; Yang, Y.G. CD47: A new player in phagocytosis and xenograft rejection. Cell. Mol. Immunol. 2011, 8, 285–288. [Google Scholar] [CrossRef]
- Barclay, A.N.; Van den Berg, T.K. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: Structure, function, and therapeutic target. Annu. Rev. Immunol. 2014, 32, 25–50. [Google Scholar] [CrossRef]
- Kong, F.; Gao, F.; Li, H.; Liu, H.; Zhang, Y.; Zheng, R.; Zhang, Y.; Chen, J.; Li, X.; Liu, G.; et al. CD47: A potential immunotherapy target for eliminating cancer cells. Clin. Transl. Oncol. 2016, 18, 1051–1055. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Kaur, S.; Roberts, D.D. CD47 signaling pathways controlling cellular differentiation and responses to stress. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 212–230. [Google Scholar] [CrossRef]
- Weiskopf, K.; Ring, A.M.; Ho, C.C.; Volkmer, J.P.; Levin, A.M.; Volkmer, A.K.; Ozkan, E.; Fernhoff, N.B.; van de Rijn, M.; Weissman, I.L.; et al. Engineered SIRPalpha variants as immunotherapeutic adjuvants to anticancer antibodies. Science 2013, 341, 88–91. [Google Scholar] [CrossRef]
- Kukreja, A.; Radfar, S.; Sun, B.H.; Insogna, K.; Dhodapkar, M.V. Dominant role of CD47-thrombospondin-1 interactions in myeloma-induced fusion of human dendritic cells: Implications for bone disease. Blood 2009, 114, 3413–3421. [Google Scholar] [CrossRef]
- Lundberg, P.; Koskinen, C.; Baldock, P.A.; Lothgren, H.; Stenberg, A.; Lerner, U.H.; Oldenborg, P.A. Osteoclast formation is strongly reduced both in vivo and in vitro in the absence of CD47/SIRPalpha-interaction. Biochem. Biophys. Res. Commun. 2007, 352, 444–448. [Google Scholar] [CrossRef]
- Londino, J.D.; Gulick, D.; Isenberg, J.S.; Mallampalli, R.K. Cleavage of Signal Regulatory Protein alpha (SIRPalpha) Enhances Inflammatory Signaling. J. Biol. Chem. 2015, 290, 31113–31125. [Google Scholar] [CrossRef]
- Stein, E.V.; Miller, T.W.; Ivins-O’Keefe, K.; Kaur, S.; Roberts, D.D. Secreted Thrombospondin-1 Regulates Macrophage Interleukin-1beta Production and Activation through CD47. Sci. Rep. 2016, 6, 19684. [Google Scholar] [CrossRef]
- Van, V.Q.; Baba, N.; Rubio, M.; Wakahara, K.; Panzini, B.; Richard, C.; Soucy, G.; Franchimont, D.; Fortin, G.; Torres, A.C.; et al. CD47(Low) Status on CD4 Effectors Is Necessary for the Contraction/Resolution of the Immune Response in Humans and Mice. PLoS ONE 2012, 7, e41972. [Google Scholar] [CrossRef]
- Shi, L.; Bian, Z.; Chen, C.X.; Guo, Y.N.; Lv, Z.; Zeng, C.; Liu, Z.; Zen, K.; Liu, Y. CD47 deficiency ameliorates autoimmune nephritis in Fas(lpr) mice by suppressing IgG autoantibody production. J. Pathol. 2015, 237, 285–295. [Google Scholar] [CrossRef]
- Sule, S.; Rosen, A.; Petri, M.; Akhter, E.; Andrade, F. Abnormal production of pro- and anti-inflammatory cytokines by lupus monocytes in response to apoptotic cells. PLoS ONE 2011, 6, e17495. [Google Scholar] [CrossRef]
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002, 2, 965–975. [Google Scholar] [CrossRef]
- Tas, S.W.; Quartier, P.; Botto, M.; Fossati-Jimack, L. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann. Rheum. Dis. 2006, 65, 216–221. [Google Scholar] [CrossRef]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Immunol. 2016, 12, 716–730. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Gladman, D.D.; Ibanez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar]
- Davidson, A. What is damaging the kidney in lupus nephritis? Nat. Rev. Immunol. 2016, 12, 143–153. [Google Scholar] [CrossRef]
- Ronnblom, L. The importance of the type I interferon system in autoimmunity. Clin. Exp. Rheumatol. 2016, 34, 21–24. [Google Scholar]
- Steffen, U.; Schett, G.; Bozec, A. How Autoantibodies Regulate Osteoclast Induced Bone Loss in Rheumatoid Arthritis. Front. Immunol. 2019, 10, 1483. [Google Scholar] [CrossRef]
- Meroni, P.L.; Raschi, E.; Grossi, C.; Pregnolato, F.; Trespidi, L.; Acaia, B.; Borghi, M.O. Obstetric and vascular APS: Same autoantibodies but different diseases? Lupus 2012, 21, 708–710. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, Y.; Han, C.; Hu, X.; Zhang, H.; Xu, X.; Tian, J.; Liu, Y.; Ding, Y.; Liu, J.; et al. Blockade of CD47 ameliorates autoimmune inflammation in CNS by suppressing IL-1-triggered infiltration of pathogenic Th17 cells. J. Autoimmun. 2016, 69, 74–85. [Google Scholar] [CrossRef]
- Han, M.H.; Lundgren, D.H.; Jaiswal, S.; Chao, M.; Graham, K.L.; Garris, C.S.; Axtell, R.C.; Ho, P.P.; Lock, C.B.; Woodard, J.I.; et al. Janus-like opposing roles of CD47 in autoimmune brain inflammation in humans and mice. J. Exp. Med. 2012, 209, 1325–1334. [Google Scholar] [CrossRef]
- Shah, K.; Cragg, M.; Leandro, M.; Reddy, V. Anti-CD20 monoclonal antibodies in Systemic Lupus Erythematosus. Biologicals 2021, 69, 1–14. [Google Scholar] [CrossRef]
- Gallagher, S.; Turman, S.; Lekstrom, K.; Wilson, S.; Herbst, R.; Wang, Y. CD47 limits antibody dependent phagocytosis against non-malignant B cells. Mol. Immunol. 2017, 85, 57–65. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, Y.J.; Bitoun, S.; Winthrop, K.L.; Choi, Y.; Lee, E.B.; Mariette, X. Interaction between B-cell activation factor and methotrexate impacts immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Ko, E.J.; Lee, Y.; Lee, Y.N.; Bian, Z.; Liu, Y.; Kang, S.M. CD47 Plays a Role as a Negative Regulator in Inducing Protective Immune Responses to Vaccination against Influenza Virus. J. Virol. 2016, 90, 6746–6758. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Park, J.K.; Lee, Y.J.; Yang, J.A.; Lee, E.Y.; Song, Y.W.; Lee, E.B. Comparison of cancer incidence among patients with rheumatic disease: A retrospective cohort study. Arthritis Res. Ther. 2014, 16, 428. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, E.F.; Michaud, K.; Katz, R.; Wolfe, F. Increased incidence of herpes zoster among patients with systemic lupus erythematosus. Lupus 2013, 22, 238–244. [Google Scholar] [CrossRef]

| SLE Patients (n = 25) | HCs (n = 16) | |
|---|---|---|
| Age, years | 38.7 ± 12.8 | 28.8 ± 4.2 |
| Female, n (%) | 23 (92.0) | 12 (75) |
| SLE duration, years | 4.2 (0.9–14.0) | |
| White blood cell, ×103/μL | 5.7 ± 2.9 | |
| Hematocrit, % | 38.6 (34.5–39.9) | |
| Platelet, ×103/μL | 190.7 ± 71.0 | |
| ESR, mm/hour | 19 (14.5–33.0) | |
| Anti-dsDNA, IU/mL (ref: 0–10 IU/mL) | 16.7 (7.7–40.5) | |
| C3, mg/dL (ref: 83–193 mg/dL) | 86.0 (57.5–98.7) | |
| C4, mg/dL (ref: 15–57 mg/dL) | 14.5 (7.0–16.7) | |
| SLEDAI-2K | 6 (2–9.5) | |
| Treatment | ||
| Corticosteroids | 20 (80.0) | |
| Prednisolone equivalent, mg/day | 5 (0–125) | |
| Hydroxychloroquine | 16 (64.0) | |
| Azathioprine | 2 (8.0) | |
| Mycophenolate mofetil | 1 (4.0) | |
| Sulfasalazine | 1 (4.0) | |
| NSAIDs | 6 (24.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.K.; Lee, Y.J.; Park, J.S.; Lee, E.B.; Song, Y.W. CD47 Potentiates Inflammatory Response in Systemic Lupus Erythematosus. Cells 2021, 10, 1151. https://doi.org/10.3390/cells10051151
Park JK, Lee YJ, Park JS, Lee EB, Song YW. CD47 Potentiates Inflammatory Response in Systemic Lupus Erythematosus. Cells. 2021; 10(5):1151. https://doi.org/10.3390/cells10051151
Chicago/Turabian StylePark, Jin Kyun, Ye Ji Lee, Ji Soo Park, Eun Bong Lee, and Yeong Wook Song. 2021. "CD47 Potentiates Inflammatory Response in Systemic Lupus Erythematosus" Cells 10, no. 5: 1151. https://doi.org/10.3390/cells10051151
APA StylePark, J. K., Lee, Y. J., Park, J. S., Lee, E. B., & Song, Y. W. (2021). CD47 Potentiates Inflammatory Response in Systemic Lupus Erythematosus. Cells, 10(5), 1151. https://doi.org/10.3390/cells10051151






