Impact of the Uncoupling Protein 1 on Cardiovascular Risk in Patients with Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Cardiovascular Risk Assessment
2.2.1. Pocock’s Risk Score
2.2.2. Framingham Risk Score
2.3. Prospective Cardiovascular Follow-Up
2.4. Collection and Preparation of Blood and Fat Tissue Samples
2.5. Preparation and Stimulation of Primary Adipocyte Cultures
2.6. Preparation of mRNA
2.7. Gene Expression Analysis
2.8. Serological Parameters
2.9. Statistical Analysis
3. Results
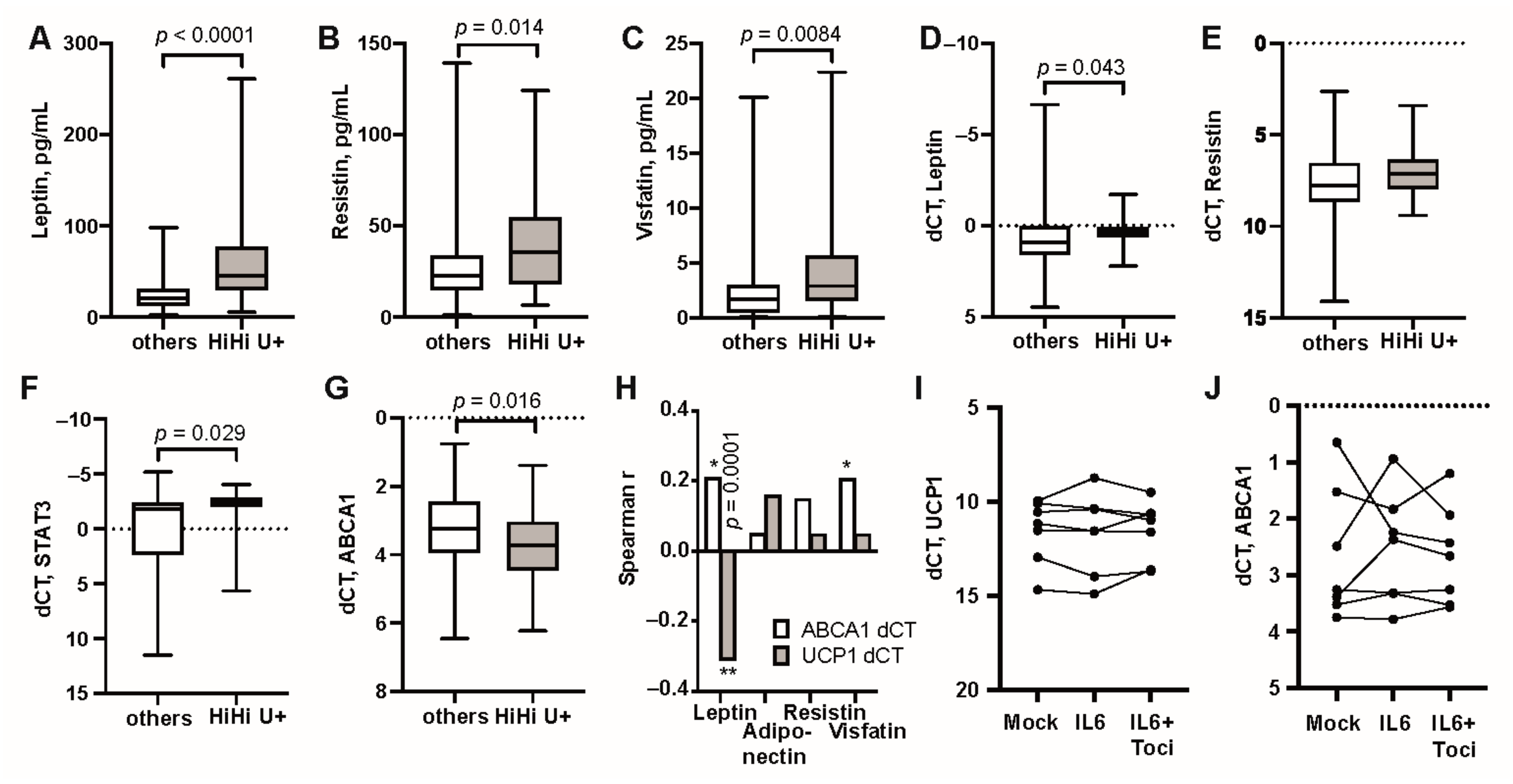
3.1. UCP1 Transcription in Adipose Tissue Associates with High Serum Levels of IL6 and Overweight
3.2. Modulation of IL-6 Receptor Signaling Changes UCP1 Transcription
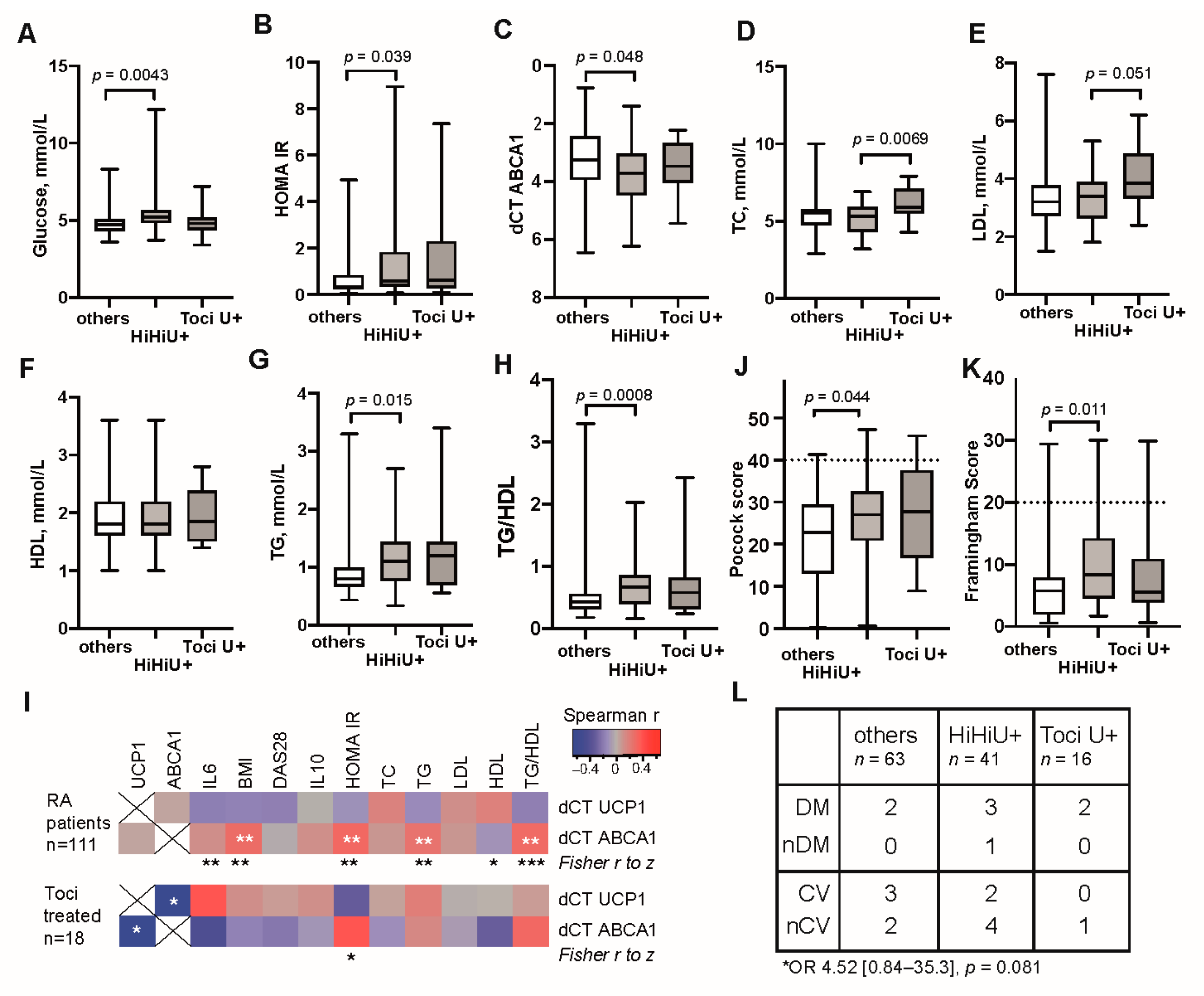
3.3. Unfavourable Metabolic Profile in RA Patients with High Transcription of UCP1
3.4. High UCP1 Transcription Provided No Protection against Cardiovascular Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudina, A.; Cooney, M.T.; De Bacquer, D.; De Backer, G.; Ducimetière, P.; Jousilahti, P.; Keil, U.; Menotti, A.; Njølstad, I.; Oganov, R.; et al. Relationships between body mass index, cardiovascular mortality, and risk factors: A report from the SCORE investigators. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 731–742. [Google Scholar] [CrossRef]
- Matsushita, M.; Yoneshiro, T.; Aita, S.; Kameya, T.; Sugie, H.; Saito, M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int. J. Obes. 2014, 38, 812–817. [Google Scholar] [CrossRef]
- Matthias, A.; Ohlson, K.B.E.; Fredriksson, J.M.; Jacobsson, A.; Nedergaard, J.; Cannon, B. Thermogenic Responses in Brown Fat Cells Are Fully UCP1-dependent. J. Biol. Chem. 2000, 275, 25073–25081. [Google Scholar] [CrossRef]
- Berbée, J.F.; Boon, M.R.; Khedoe, P.P.S.J.; Bartelt, A.; Schlein, C.; Worthmann, A.; Kooijman, S.; Hoeke, G.; Mol, I.M.; John, C.; et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015, 6, 6356. [Google Scholar] [CrossRef]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Tews, D.; Pula, T.; Funcke, J.; Jastroch, M.; Keuper, M.; Debatin, K.; Wabitsch, M.; Fischer-Posovszky, P. Elevated UCP1 levels are sufficient to improve glucose uptake in human white adipocytes. Redox Biol. 2019, 26, 101286. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Tsoli, M.; Moore, M.; Burg, D.; Painter, A.; Taylor, R.; Lockie, S.H.; Turner, N.; Warren, A.; Cooney, G.; Oldfield, B.; et al. Activation of Thermogenesis in Brown Adipose Tissue and Dysregulated Lipid Metabolism Associated with Cancer Cachexia in Mice. Cancer Res. 2012, 72, 4372–4382. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Auger, C.; Stanojcic, M.; Patsouris, D.; Parousis, A.; Epelman, S.; Jeschke, M.G. Alternatively Activated Macrophages Drive Browning of White Adipose Tissue in Burns. Ann. Surg. 2019, 269, 554–563. [Google Scholar] [CrossRef]
- Boström, E.A.; Svensson, M.; Andersson, S.; Jonsson, I.-M.; Ekwall, A.-K.H.; Eisler, T.; Dahlberg, L.E.; Smith, U.; Bokarewa, M.I. Resistin and insulin/insulin-like growth factor signaling in rheumatoid arthritis. Arthritis Rheum. 2011, 63, 2894–2904. [Google Scholar] [CrossRef]
- Neumann, E.; Frommer, K.W.; Vasile, M.; Müller-Ladner, U. Adipocytokines as driving forces in rheumatoid arthritis and related inflammatory diseases? Arthritis Rheum. 2011, 63, 1159–1169. [Google Scholar] [CrossRef]
- Erlandsson, M.C.; Medina, R.D.; Silfverswärd, S.T.; Bokarewa, M.I. Smoking Functions as a Negative Regulator of IGF1 and Impairs Adipokine Network in Patients with Rheumatoid Arthritis. Mediat. Inflamm. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Crowson, C.S.; Matteson, E.L.; Davis, J.M.; Gabriel, S.E. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Rheum. 2013, 65, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Hiraki, L.T.; Sparks, J.A.; Malspeis, S.; Chen, C.-Y.; Awosogba, J.A.; Arkema, E.V.; Costenbader, K.H.; Karlson, E.W. Being overweight or obese and risk of developing rheumatoid arthritis among women: A prospective cohort study. Ann. Rheum. Dis. 2014, 73, 1914–1922. [Google Scholar] [CrossRef]
- Kaipiainen-Seppanen, O.; Kautiainen, H. Declining trend in the incidence of rheumatoid factor-positive rheumatoid arthritis in Finland 1980–2000. J. Rheumatol. 2006, 33, 2132–2138. [Google Scholar]
- Bartfai, T.; Waalen, J.; Buxbaum, J.N. Adipose tissue as a modulator of clinical inflammation: Does obesity reduce the prevalence of rheumatoid arthritis? J. Rheumatol. 2007, 34, 488–492. [Google Scholar] [PubMed]
- Ajeganova, S.; Andersson, M.L.; Hafström, I. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: A long-term followup from disease onset. Arthritis Rheum. 2012, 65, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.E.C.; Bengtsson, C.; Källberg, H.; Wesley, A.; Klareskog, L.; Alfredsson, L.; Saevarsdottir, S. Overweight decreases the chance of achieving good response and low disease activity in early rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 2029–2033. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.-Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef]
- Westhoff, G.; Rau, R.; Zink, A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007, 56, 3575–3582. [Google Scholar] [CrossRef]
- Kaufmann, J.; Kielstein, V.; Kilian, S.; Stein, G.; Hein, G. Relation between body mass index and radiological progression in patients with rheumatoid arthritis. J. Rheumatol. 2003, 30, 2350–2355. [Google Scholar]
- Escalante, A.; Haas, R.W.; Del Rincón, I. Paradoxical Effect of Body Mass Index on Survival in Rheumatoid Arthritis: Role of comorbidity and systemic inflammation. Arch. Intern. Med. 2005, 165, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Engvall, I.; Elkan, A.; Tengstrand, B.; Cederholm, T.; Brismar, K.; Hafström, I. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand. J. Rheumatol. 2008, 37, 321–328. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Pocock, S.J.; McCormack, V.; Gueyffier, F.; Boutitie, F.; Fagard, R.H.; Boissel, J.-P. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trials. BMJ 2001, 323, 75–81. [Google Scholar] [CrossRef]
- Sohn, C.; Kim, J.; Bae, W. The framingham risk score, diet, and inflammatory markers in Korean men with metabolic syndrome. Nutr. Res. Pr. 2012, 6, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, M.C.; Lyngfelt, L.; Åberg, N.D.; Wasén, C.; Espino, R.A.; Silfverswärd, S.T.; Nadali, M.; Jood, K.; Andersson, K.M.; Pullerits, R.; et al. Low serum IGF1 is associated with hypertension and predicts early cardiovascular events in women with rheumatoid arthritis. BMC Med. 2019, 17, 141. [Google Scholar] [CrossRef] [PubMed]
- Nadali, M.; Pullerits, R.; Andersson, K.M.E.; Silfverswärd, S.T.; Erlandsson, M.C.; Bokarewa, M.I. High Expression of STAT3 in Subcutaneous Adipose Tissue Associates with Cardiovascular Risk in Women with Rheumatoid Arthritis. Int. J. Mol. Sci. 2017, 18, 2410. [Google Scholar] [CrossRef]
- Gustafson, B.; Smith, U. The WNT Inhibitor Dickkopf 1 and Bone Morphogenetic Protein 4 Rescue Adipogenesis in Hypertrophic Obesity in Humans. Diabetes 2012, 61, 1217–1224. [Google Scholar] [CrossRef]
- Jia, L.; Long, S.; Fu, M.; Yan, B.; Tian, Y.; Xu, Y.; Gou, L. Relationship between total cholesterol/high-density lipoprotein cholesterol ratio, triglyceride/high-density lipoprotein cholesterol ratio, and high-density lipoprotein subclasses. Metabolism 2006, 55, 1141–1148. [Google Scholar] [CrossRef]
- D’Agostino, S.R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General Cardiovascular Risk Profile for Use in Primary Care. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Colaco, K.; Ocampo, V.; Ayala, A.P.; Harvey, P.; Gladman, D.D.; Piguet, V.; Eder, L. Predictive Utility of Cardiovascular Risk Prediction Algorithms in Inflammatory Rheumatic Diseases: A Systematic Review. J. Rheumatol. 2019, 47, 928–938. [Google Scholar] [CrossRef]
- Alemao, E.; Cawston, H.; Bourhis, F.; Al, M.; Mölken, M.P.M.H.R.-V.; Liao, K.P.; Solomon, D.H. Cardiovascular risk factor management in patients with RA compared to matched non-RA patients. Rheumatology 2016, 55, 809–816. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Z.; Feng, F.; Wu, T.; Luo, D.; Hu, C.; Sun, C. Foxc2 coordinates inflammation and browning of white adipose by leptin-STAT3-PRDM16 signal in mice. Int. J. Obes. 2018, 42, 252–259. [Google Scholar] [CrossRef]
- Derecka, M.; Gornicka, A.; Koralov, S.B.; Szczepanek, K.; Morgan, M.; Raje, V.; Sisler, J.; Zhang, Q.; Otero, D.; Cichy, J.; et al. Tyk2 and Stat3 Regulate Brown Adipose Tissue Differentiation and Obesity. Cell Metab. 2012, 16, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.G.; Murholm, M.; Carey, A.L.; Biensø, R.S.; Basse, A.L.; Allen, T.L.; Hidalgo, J.; Kingwell, B.A.; Febbraio, M.A.; Hansen, J.B.; et al. Role of IL-6 in Exercise Training- and Cold-Induced UCP1 Expression in Subcutaneous White Adipose Tissue. PLoS ONE 2014, 9, e84910. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.; Chen, P.; Stanojcic, M.; Sadri, A.-R.; Coburn, N.; Jeschke, M.G. IL-6 Signal From the Bone Marrow is Required for the Browning of White Adipose Tissue Post Burn Injury. Shock 2017, 47, 33–39. [Google Scholar] [CrossRef]
- Han, J.; Meng, Q.; Shen, L.; Wu, G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018, 17, 1–8. [Google Scholar] [CrossRef]
- Bettini, S.; Favaretto, F.; Compagnin, C.; Belligoli, A.; Sanna, M.; Fabris, R.; Serra, R.; Prà, C.D.; Prevedello, L.; Foletto, M.; et al. Resting Energy Expenditure, Insulin Resistance and UCP1 Expression in Human Subcutaneous and Visceral Adipose Tissue of Patients With Obesity. Front. Endocrinol. 2019, 10, 548. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Xu, M.; Gu, W.; Xi, Y.; Qi, L.; Li, B.; Wang, W. Brown Adipose Tissue Activation Is Inversely Related to Central Obesity and Metabolic Parameters in Adult Human. PLoS ONE 2015, 10, e0123795. [Google Scholar] [CrossRef] [PubMed]
- Brondani, L.D.A.; Assmann, T.S.; Duarte, G.C.K.; Gross, J.L.; Canani, L.H.; Crispim, D. The role of the uncoupling protein 1 (UCP1) on the development of obesity and type 2 diabetes mellitus. Arq. Bras. Endocrinol. Metabol. 2012, 56, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.S.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nat. Cell Biol. 2011, 480, 104–108. [Google Scholar] [CrossRef]
- Fischer, K.; Ruiz, H.H.; Jhun, K.; Finan, B.; Oberlin, D.J.; Van Der Heide, V.; Kalinovich, A.V.; Petrovic, N.; Wolf, Y.; Clemmensen, C.; et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 2017, 23, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Chapman, M.J.; Piraino, P.; Lamerz, J.; Schindler, T.; Cutler, P.; Dernick, G. Remodeling of plasma lipoproteins in patients with rheumatoid arthritis: Interleukin-6 receptor-alpha inhibition with tocilizumab. Proteom. Clin. Appl. 2015, 10, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Sattar, N.; Gabriel, S.; Ridker, P.M.; Gay, S.; Warne, C.; Musselman, D.; Brockwell, L.; Shittu, E.; Klearman, M.; et al. Cardiovascular Safety of Tocilizumab Versus Etanercept in Rheumatoid Arthritis: A Randomized Controlled Trial. Arthritis Rheumatol. 2019, 72, 31–40. [Google Scholar] [CrossRef]
- Gallagher, L.; Cregan, S.; Biniecka, M.; Cunningham, C.; Veale, D.J.; Kane, D.J.; Fearon, U.; Mullan, R.H. Insulin-Resistant Pathways Are Associated With Disease Activity in Rheumatoid Arthritis and Are Subject to Disease Modification Through Metabolic Reprogramming: A Potential Novel Therapeutic Approach. Arthritis Rheumatol. 2019, 72, 896–902. [Google Scholar] [CrossRef]
- Nicolau, J.; Lequerré, T.; Bacquet, H.; Vittecoq, O. Rheumatoid arthritis, insulin resistance, and diabetes. Jt. Bone Spine 2017, 84, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Peyrou, M.; Giralt, M. Transcriptional regulation of the uncoupling protein-1 gene. Biochimie 2017, 134, 86–92. [Google Scholar] [CrossRef]
- Metsios, G.S.; Stavropoulos-Kalinoglou, A.; Van Zanten, J.J.C.S.V.; Treharne, G.J.; Panoulas, V.F.; Douglas, K.M.J.; Koutedakis, Y.; Kitas, G.D. Rheumatoid arthritis, cardiovascular disease and physical exercise: A systematic review. Rheumatology 2007, 47, 239–248. [Google Scholar] [CrossRef]
- Panoulas, V.F.; Metsios, G.S.; Pace, A.V.; John, H.; Treharne, G.J.; Banks, M.J.; Kitas, G.D. Hypertension in rheumatoid arthritis. Rheumatology 2008, 47, 1286–1298. [Google Scholar] [CrossRef] [PubMed]


| UCP1 Positive n = 89 | UCP1 Negative n = 22 | Tocilizumab n = 18 | |
|---|---|---|---|
| Age, years | 56 (48–63) | 61 (46–63) | 65 (56–69) |
| Disease duration, years | 7 (4–13) | 8 (6–12) | 16 (6–27) |
| Autoantibodies | |||
| RF positive, n (%) | 70 (80.4%) | 15 (68.2%) | 11 (69%) |
| ACPA positive, n (%) | 56 (64.4%) | 13 (59.1%) | 9 (75%) |
| Tender joints, n | 3 (0–10) | 1 (0–5) | 3 (1.5–8) |
| Swollen joints, n | 2 (1–6) | 1 (0–5.8) | 2 (0–4.5) |
| VAS pain, mm | 25 (12–61) | 19 (9.5–33) | 27 (21–41) |
| Methotrexate monotherapy, n (%) | 33 (37%) | 12 (54%) | |
| + TNFa inhibitors, n (%) | 23 (25%) | 6 (27%) | |
| + Other biologics, n (%) | 12 (13%) | 2 (9%) | |
| + Conventional DMARDS, n (%) | 16 (18%) | 1 (5%) | |
| No DMARDS, n (%) | 5 (6%) | 1 (5%) | |
| Oral corticosteroids, n (%) | 13 (15%) | 1 (5%) | 0 |
| Levothyroxine, n (%) | 10 (11%) | 2 (9%) | 1 (6%) |
| Estradiol, n (%) | 3 (3%) | 0 | 1 (6%) |
| Statins, n (%) | 2 (2%) | 0 | 3 (17%) |
| Diabetes type II, n (%) | 4 (4%) | 0 | 2 (11%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyngfelt, L.I.; Erlandsson, M.C.; Nadali, M.; Hedjazifar, S.; Pullerits, R.; Andersson, K.M.; Brembeck, P.; Silfverswärd, S.T.; Smith, U.; Bokarewa, M.I. Impact of the Uncoupling Protein 1 on Cardiovascular Risk in Patients with Rheumatoid Arthritis. Cells 2021, 10, 1131. https://doi.org/10.3390/cells10051131
Lyngfelt LI, Erlandsson MC, Nadali M, Hedjazifar S, Pullerits R, Andersson KM, Brembeck P, Silfverswärd ST, Smith U, Bokarewa MI. Impact of the Uncoupling Protein 1 on Cardiovascular Risk in Patients with Rheumatoid Arthritis. Cells. 2021; 10(5):1131. https://doi.org/10.3390/cells10051131
Chicago/Turabian StyleLyngfelt, Lovisa I., Malin C. Erlandsson, Mitra Nadali, Shahram Hedjazifar, Rille Pullerits, Karin M. Andersson, Petra Brembeck, Sofia Töyrä Silfverswärd, Ulf Smith, and Maria I. Bokarewa. 2021. "Impact of the Uncoupling Protein 1 on Cardiovascular Risk in Patients with Rheumatoid Arthritis" Cells 10, no. 5: 1131. https://doi.org/10.3390/cells10051131
APA StyleLyngfelt, L. I., Erlandsson, M. C., Nadali, M., Hedjazifar, S., Pullerits, R., Andersson, K. M., Brembeck, P., Silfverswärd, S. T., Smith, U., & Bokarewa, M. I. (2021). Impact of the Uncoupling Protein 1 on Cardiovascular Risk in Patients with Rheumatoid Arthritis. Cells, 10(5), 1131. https://doi.org/10.3390/cells10051131