The Discovery of Naringenin as Endolysosomal Two-Pore Channel Inhibitor and Its Emerging Role in SARS-CoV-2 Infection
Abstract
1. Introduction
2. Plants and TPC Channels
3. The SV Channels Are Modulated by Redox Agents and Flavonoids
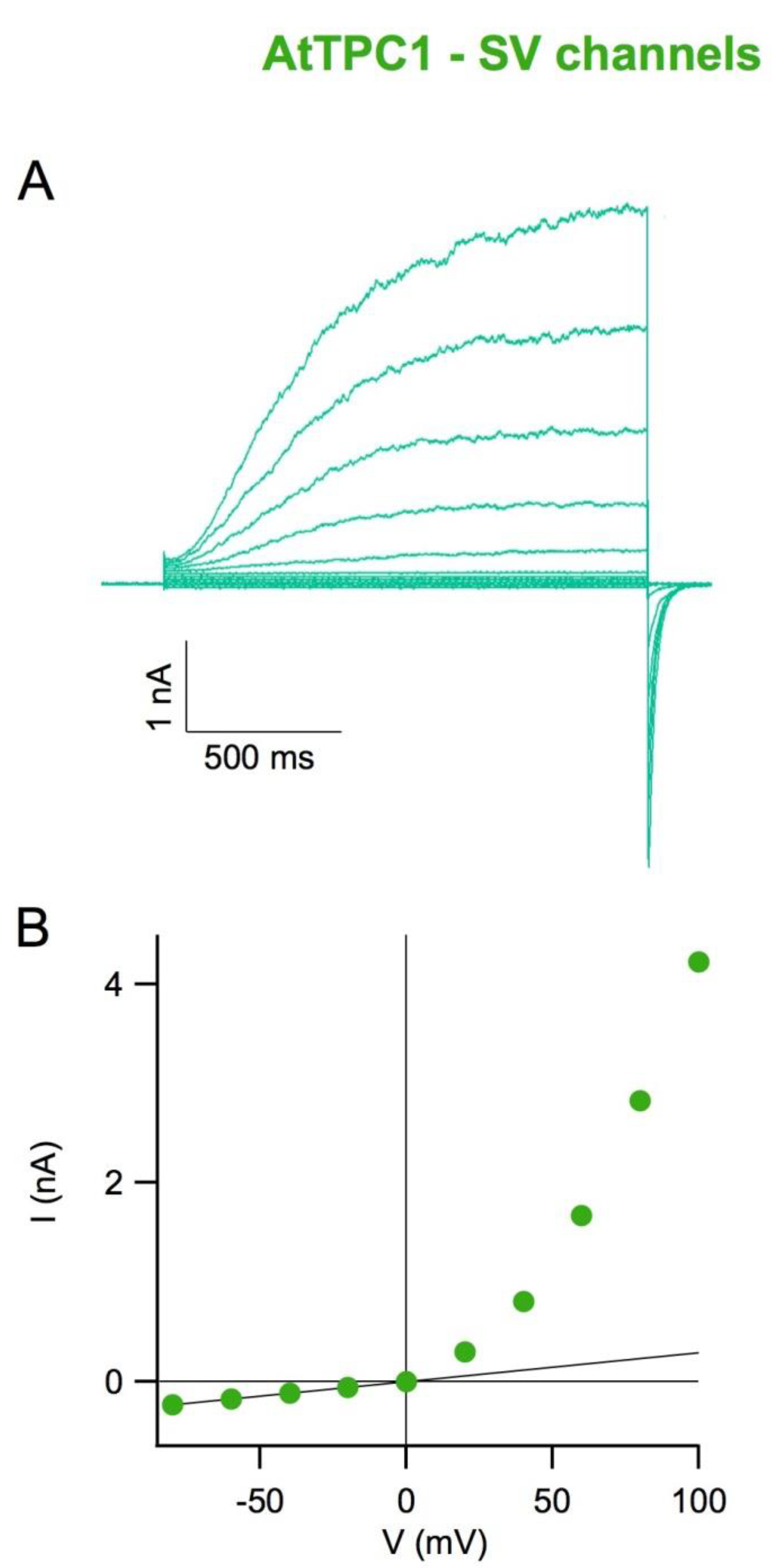
4. The SV Channel Protein in Arabidopsis thaliana Is Encoded by the TPC1 Gene
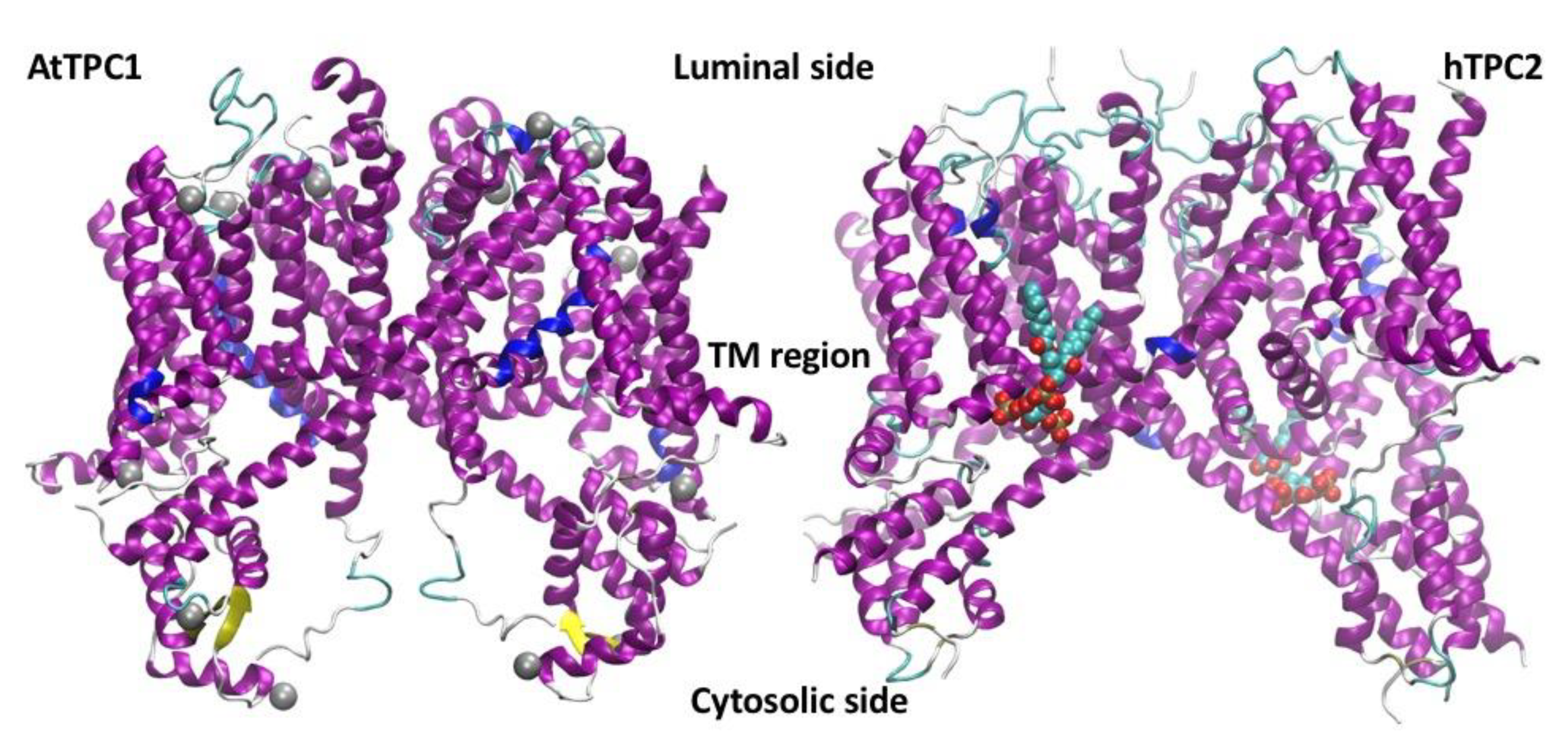
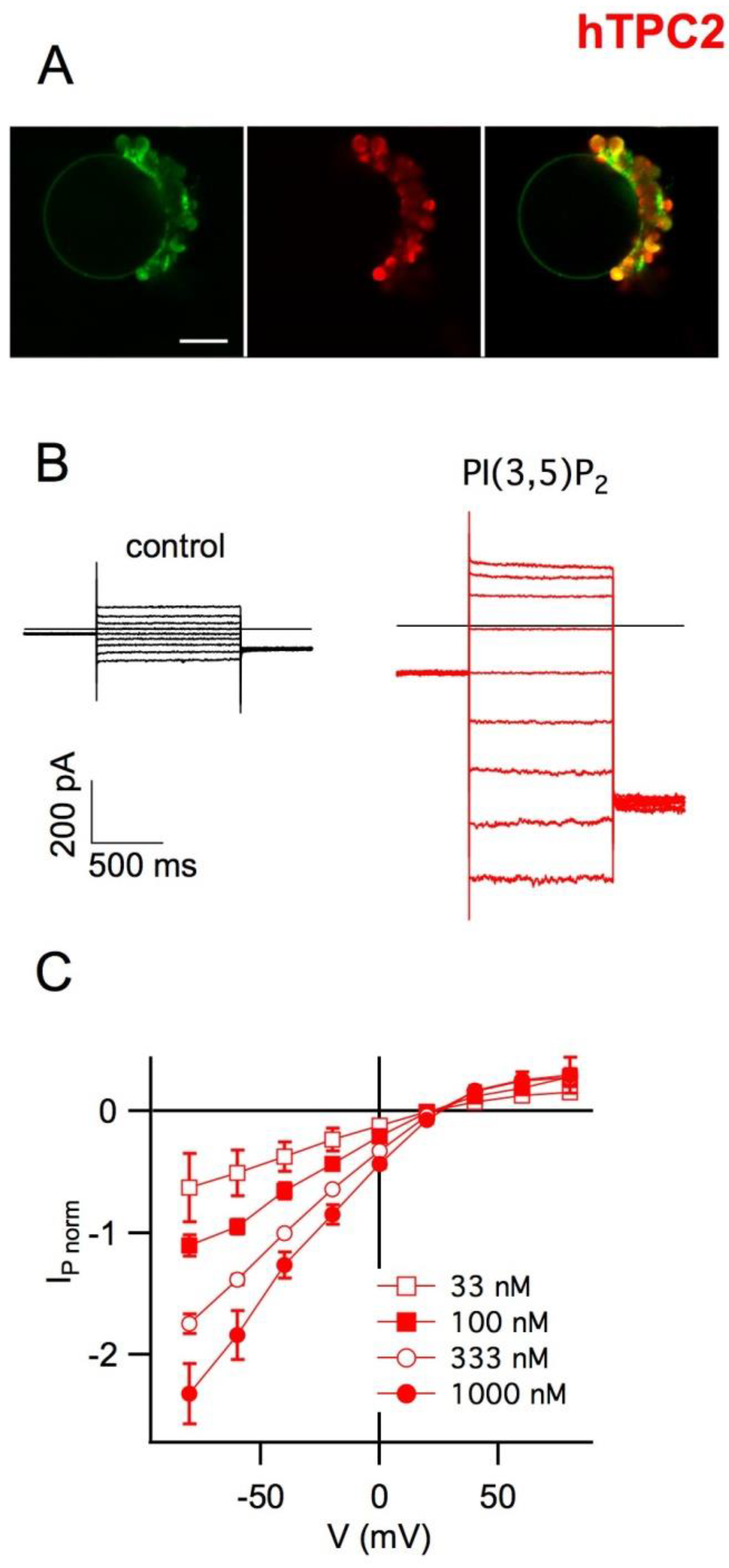
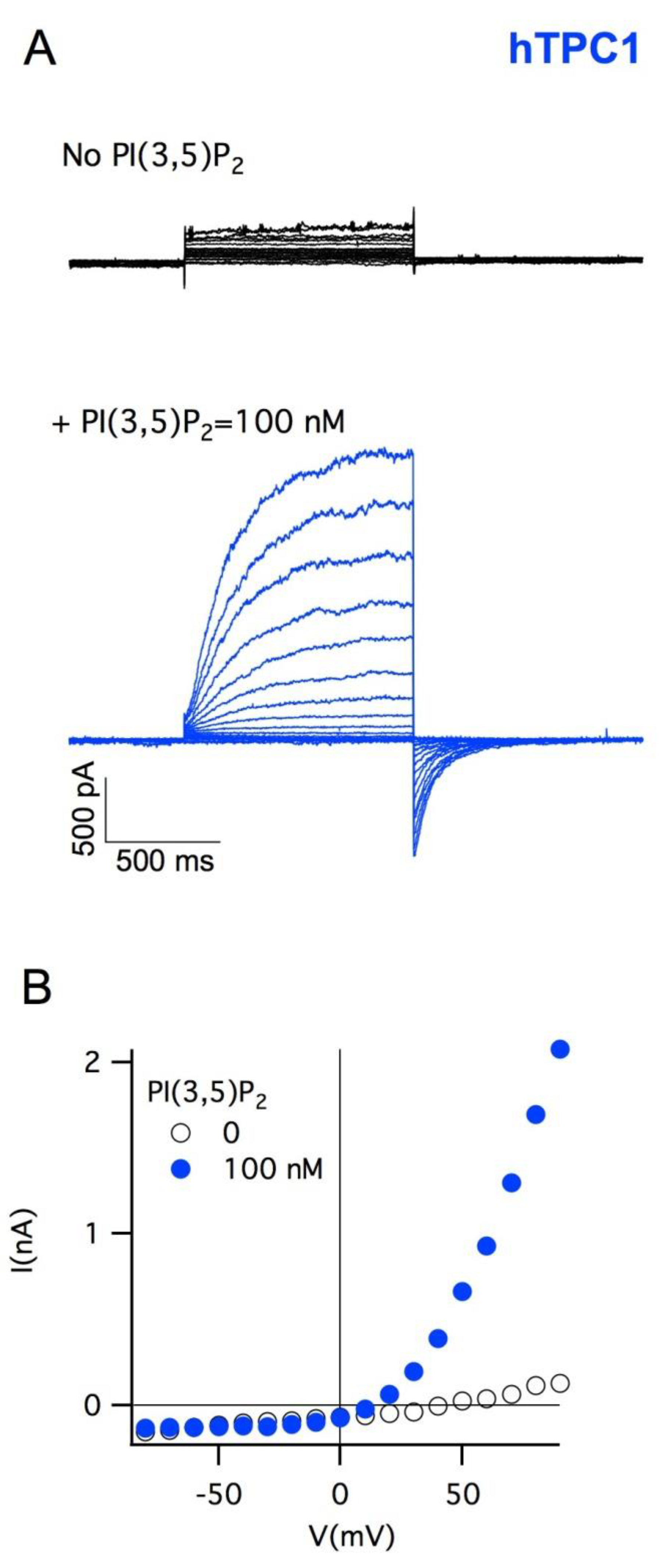
5. Plant Vacuoles as a Heterologous System of Expression and Characterization of Human TPCs
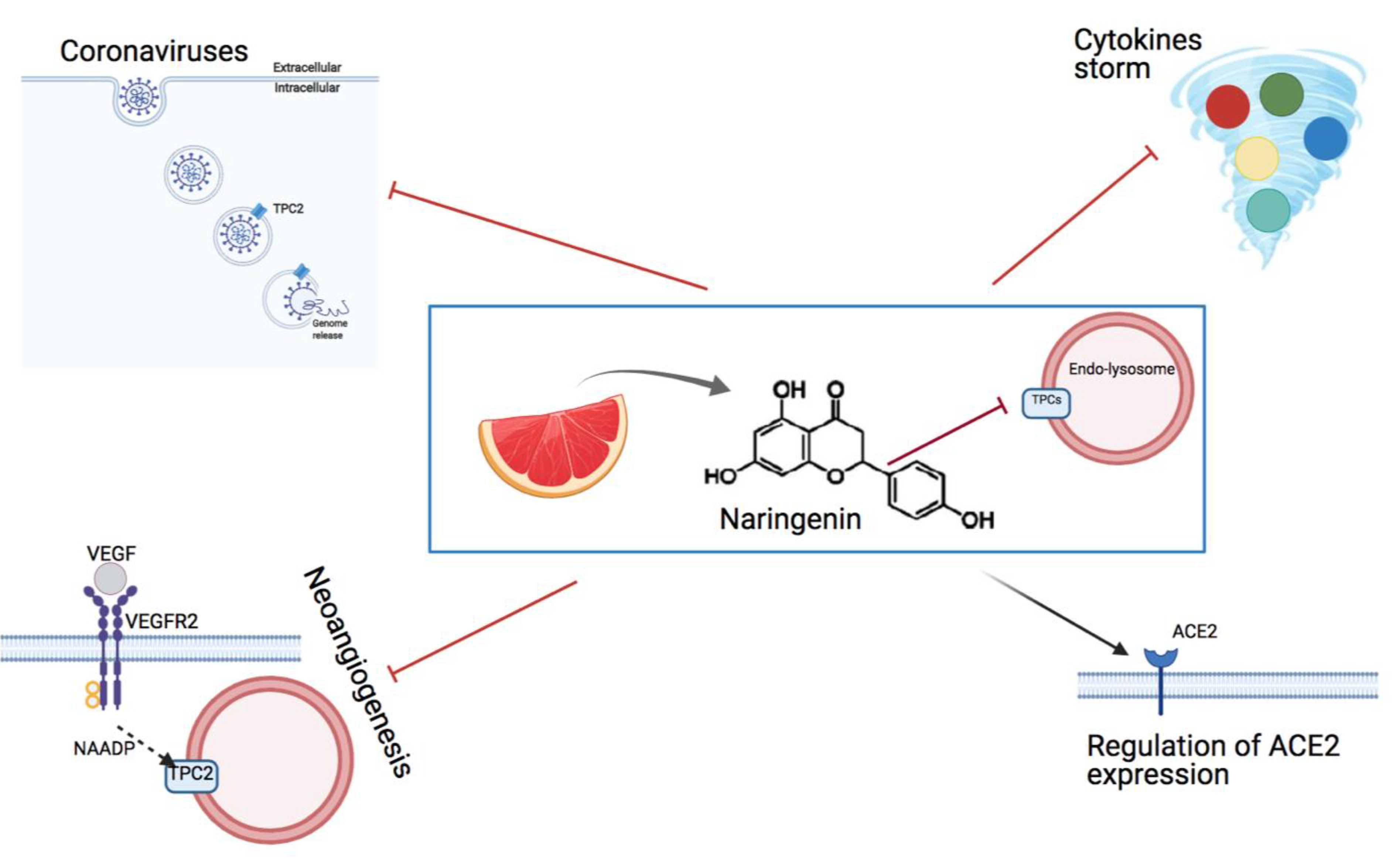
6. The Effect of the Flavonoid Naringenin on Neoangiogenesis
7. Inhibition of TPCs by Naringenin as an Option to Fight Viral Infections
8. Naringenin Is a Powerful Anti-Coronavirus Drug In Vitro
9. Perspectives and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hedrich, R.; Mueller, T.D.; Becker, D.; Marten, I. Structure and Function of TPC1 Vacuole SV Channel Gains Shape. Mol. Plant 2018, 11, 764–775. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, Y.; Alharbi, A.; Hanbashi, A.; Alhoshani, A.; Parrington, J. Targeting Two-Pore Channels: Current Progress and Future Challenges. Trends Pharmacol. Sci. 2020, 41, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.S.; Tang, R.; Grimm, C. Two-Pore and TRPML Cation Channels: Regulators of Phagocytosis, Autophagy and Lysosomal Exocytosis. Pharmacol. Ther. 2021, 220, 107713. [Google Scholar] [CrossRef]
- Vassileva, K.; Marsh, M.; Patel, S. Two-Pore Channels as Master Regulators of Membrane Trafficking and Endocytic Well-Being. Curr. Opin. Physiol. 2020, 17, 163–168. [Google Scholar] [CrossRef]
- Patel, S.; Marchant, J.S.; Brailoiu, E. Two-Pore Channels: Regulation by NAADP and Customized Roles in Triggering Calcium Signals. Cell Calcium 2010, 47, 480–490. [Google Scholar] [CrossRef]
- Brailoiu, E.; Churamani, D.; Cai, X.; Schrlau, M.G.; Brailoiu, G.C.; Gao, X.; Hooper, R.; Boulware, M.J.; Dun, N.J.; Marchant, J.S.; et al. Essential Requirement for Two-Pore Channel 1 in NAADP-Mediated Calcium Signaling. J. Cell Biol. 2009, 186, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Marten, I. TPC1-SV Channels Gain Shape. Mol. Plant 2011, 4, 428–441. [Google Scholar] [CrossRef]
- Martinoia, E. Vacuolar Transporters—Companions on a Longtime Journey. Plant Physiol. 2018, 176, 1384–1407. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.M.; Schroeder, J.I. Calcium-Activated K+ Channels and Calcium-Induced Calcium Release by Slow Vacuolar Ion Channels in Guard Cell Vacuoles Implicated in the Control of Stomatal Closure. Plant Cell 1994, 6, 669–683. [Google Scholar] [CrossRef]
- Gradogna, A.; Scholz-Starke, J.; Gutla, P.V.K.; Carpaneto, A. Fluorescence Combined with Excised Patch: Measuring Calcium Currents in Plant Cation Channels. Plant J. 2009, 58, 175–182. [Google Scholar] [CrossRef]
- Carpaneto, A.; Gradogna, A. Modulation of Calcium and Potassium Permeation in Plant TPC Channels. Biophys. Chem. 2018, 236, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zeng, W.; Jiang, Y. Tuning the Ion Selectivity of Two-Pore Channels. Proc. Natl. Acad. Sci. USA 2017, 114, 1009–1014. [Google Scholar] [CrossRef]
- Ivashikina, N.; Hedrich, R. K+ Currents through SV-Type Vacuolar Channels Are Sensitive to Elevated Luminal Sodium Levels. Plant J. Cell Mol. Biol. 2005, 41, 606–614. [Google Scholar] [CrossRef]
- Jaślan, D.; Dreyer, I.; Lu, J.; O’Malley, R.; Dindas, J.; Marten, I.; Hedrich, R. Voltage-Dependent Gating of SV Channel TPC1 Confers Vacuole Excitability. Nat. Commun. 2019, 10, 2659. [Google Scholar] [CrossRef] [PubMed]
- Cang, C.; Bekele, B.; Ren, D. The Voltage-Gated Sodium Channel TPC1 Confers Endolysosomal Excitability. Nat. Chem. Biol. 2014, 10, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-G.; Toyota, M.; Kim, S.-H.; Hilleary, R.; Gilroy, S. Salt Stress-Induced Ca2+ Waves Are Associated with Rapid, Long-Distance Root-to-Shoot Signaling in Plants. Proc. Natl. Acad. Sci. USA. 2014, 111, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Choi, W.-G.; Gilroy, S.; Morris, R.J. A ROS-Assisted Calcium Wave Dependent on the AtRBOHD NADPH Oxidase and TPC1 Cation Channel Propagates the Systemic Response to Salt Stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef]
- Choi, W.-G.; Miller, G.; Wallace, I.; Harper, J.; Mittler, R.; Gilroy, S. Orchestrating Rapid Long-Distance Signaling in Plants with Ca2+, ROS and Electrical Signals. Plant J. Cell Mol. Biol. 2017, 90, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Lenglet, A.; Jaślan, D.; Toyota, M.; Mueller, M.; Müller, T.; Schönknecht, G.; Marten, I.; Gilroy, S.; Hedrich, R.; Farmer, E.E. Control of Basal Jasmonate Signalling and Defence through Modulation of Intracellular Cation Flux Capacity. New Phytol. 2017, 216, 1161–1169. [Google Scholar] [CrossRef]
- Morgan, A.J.; Platt, F.M.; Lloyd-Evans, E.; Galione, A. Molecular Mechanisms of Endolysosomal Ca2+ Signalling in Health and Disease. Biochem. J. 2011, 439, 349–374. [Google Scholar] [CrossRef]
- Patel, S.; Kilpatrick, B.S. Two-Pore Channels and Disease. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Favia, A.; Desideri, M.; Gambara, G.; D’Alessio, A.; Ruas, M.; Esposito, B.; Del Bufalo, D.; Parrington, J.; Ziparo, E.; Palombi, F.; et al. VEGF-Induced Neoangiogenesis Is Mediated by NAADP and Two-Pore Channel-2-Dependent Ca2+ Signaling. Proc. Natl. Acad. Sci. USA. 2014, 111, E4706–E4715. [Google Scholar] [CrossRef]
- Pafumi, I.; Festa, M.; Papacci, F.; Lagostena, L.; Giunta, C.; Gutla, V.; Cornara, L.; Favia, A.; Palombi, F.; Gambale, F.; et al. Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis. Sci. Rep. 2017, 7, 5121. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Neher, E. Cytoplasmic Calcium Regulates Voltage-Dependent Ion Channels in Plant Vacuoles. Nature 1987, 329, 833. [Google Scholar] [CrossRef]
- Carpaneto, A.; Cantu’, A.M.; Busch, H.; Gambale, F. Ion Channels in the Vacuoles of the Seagrass Posidonia Oceanica. FEBS Lett. 1997, 412, 236–240. [Google Scholar] [CrossRef]
- Gambale, F.; Bregante, M.; Stragapede, F.; Cantu’, A.M. Ionic Channels of the Sugar Beet Tonoplast Are Regulated by a Multi-Ion Single-File Permeation Mechanism. J. Membr. Biol. 1996, 154, 69–79. [Google Scholar] [CrossRef]
- Carpaneto, A.; Cantù, A.M.; Gambale, F. Redox Agents Regulate Ion Channel Activity in Vacuoles from Higher Plant Cells. FEBS Lett. 1999, 442, 129–132. [Google Scholar] [CrossRef]
- Paganetto, A.; Carpaneto, A.; Gambale, F. Ion Transport and Metal Sensitivity of Vacuolar Channels from the Roots of the Aquatic Plant Eichhornia Crassipes. Plant Cell Environ. 2001, 24, 1329–1336. [Google Scholar] [CrossRef]
- Gambale, F.; Cantu, A.M.; Carpaneto, A.; Keller, B.U. Fast and Slow Activation of Voltage-Dependent Ion Channels in Radish Vacuoles. Biophys. J. 1993, 65, 1837–1843. [Google Scholar] [CrossRef][Green Version]
- Carpaneto, A.; Cantù, A.M.; Gambale, F. Effects of Cytoplasmic Mg2+ on Slowly Activating Channels in Isolated Vacuoles of Beta Vulgaris. Planta 2001, 213, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Carpaneto, A. Nickel Inhibits the Slowly Activating Channels of Radish Vacuoles. Eur. Biophys. J. EBJ 2003, 32, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Corem, S.; Carpaneto, A.; Soliani, P.; Cornara, L.; Gambale, F.; Scholz-Starke, J. Response to Cytosolic Nickel of Slow Vacuolar Channels in the Hyperaccumulator Plant Alyssum Bertolonii. Eur. Biophys. J. EBJ 2009, 38, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Starke, J.; Carpaneto, A.; Gambale, F. On the Interaction of Neomycin with the Slow Vacuolar Channel of Arabidopsis Thaliana. J. Gen. Physiol. 2006, 127, 329–340. [Google Scholar] [CrossRef]
- Dobrovinskaya, O.R.; Muñiz, J.; Pottosin, I.I. Inhibition of Vacuolar Ion Channels by Polyamines. J. Membr. Biol. 1999, 167, 127–140. [Google Scholar] [CrossRef]
- Dobrovinskaya, O.R.; Muñiz, J.; Pottosin, I.I. Asymmetric Block of the Plant Vacuolar Ca(2+)-Permeable Channel by Organic Cations. Eur. Biophys. J. EBJ 1999, 28, 552–563. [Google Scholar] [CrossRef]
- Pottosin, I.I.; Dobrovinskaya, O.R.; Muñiz, J. Cooperative Block of the Plant Endomembrane Ion Channel by Ruthenium Red. Biophys. J. 1999, 77, 1973–1979. [Google Scholar] [CrossRef]
- Gutla, P.V.K.; Boccaccio, A.; De Angeli, A.; Gambale, F.; Carpaneto, A. Modulation of Plant TPC Channels by Polyunsaturated Fatty Acids. J. Exp. Bot. 2012, 63, 6187–6197. [Google Scholar] [CrossRef] [PubMed]
- Pottosin, I.I.; Tikhonova, L.I.; Hedrich, R.; Schönknecht, G. Slowly Activating Vacuolar Channels Can Not Mediate Ca2+-Induced Ca2+ Release. Plant J. 1997, 12, 1387–1398. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; De Angeli, A.; Ferraretto, C.; Paluzzi, S.; Gambale, F.; Carpaneto, A. Redox-Dependent Modulation of the Carrot SV Channel by Cytosolic PH. FEBS Lett. 2004, 576, 449–454. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Gambale, F.; Carpaneto, A. Modulation of Plant Ion Channels by Oxidizing and Reducing Agents. Arch. Biochem. Biophys. 2005, 434, 43–50. [Google Scholar] [CrossRef]
- Scholz-Starke, J.; Naso, A.; Carpaneto, A. A Perspective on the Slow Vacuolar Channel in Vacuoles from Higher Plant Cells. J. Chem. Inf. Model. 2005, 45, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. Int. J. Exp. Plant Biol. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Benkerrou, D.; Minicozzi, V.; Gradogna, A.; Milenkovic, S.; Bodrenko, I.V.; Festa, M.; Lagostena, L.; Cornara, L.; D’Amore, A.; Ceccarelli, M.; et al. A Perspective on the Modulation of Plant and Animal Two Pore Channels (TPCs) by the Flavonoid Naringenin. Biophys. Chem. 2019, 254, 106246. [Google Scholar] [CrossRef]
- Peiter, E.; Maathuis, F.J.M.; Mills, L.N.; Knight, H.; Pelloux, J.; Hetherington, A.M.; Sanders, D. The Vacuolar Ca2+-Activated Channel TPC1 Regulates Germination and Stomatal Movement. Nature 2005, 434, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Sticht, H.; Meyerhoff, P.; Dietrich, P. Differential Contribution of EF-Hands to the Ca2+-Dependent Activation in the Plant Two-Pore Channel TPC1. Plant J. Cell Mol. Biol. 2011, 68, 424–432. [Google Scholar] [CrossRef]
- Kintzer, A.F.; Stroud, R.M. Structure, Inhibition and Regulation of Two-Pore Channel TPC1 from Arabidopsis Thaliana. Nature 2016, 531, 258–262. [Google Scholar] [CrossRef]
- Guo, J.; Zeng, W.; Chen, Q.; Lee, C.; Chen, L.; Yang, Y.; Cang, C.; Ren, D.; Jiang, Y. Structure of the Voltage-Gated Two-Pore Channel TPC1 from Arabidopsis Thaliana. Nature 2016, 531, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Kintzer, A.F.; Stroud, R.M. On the Structure and Mechanism of Two-Pore Channels. FEBS J. 2018, 285, 233–243. [Google Scholar] [CrossRef]
- Kintzer, A.F.; Green, E.M.; Dominik, P.K.; Bridges, M.; Armache, J.-P.; Deneka, D.; Kim, S.S.; Hubbell, W.; Kossiakoff, A.A.; Cheng, Y.; et al. Structural Basis for Activation of Voltage Sensor Domains in an Ion Channel TPC1. Proc. Natl. Acad. Sci. USA 2018, 115, E9095–E9104. [Google Scholar] [CrossRef]
- She, J.; Zeng, W.; Guo, J.; Chen, Q.; Bai, X.-C.; Jiang, Y. Structural Mechanisms of Phospholipid Activation of the Human TPC2 Channel. eLife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, C.; Francisco, R.; Martinoia, E. Plant Vacuoles. Curr. Biol. CB 2015, 25, R136–R137. [Google Scholar] [CrossRef][Green Version]
- Carpaneto, A.; Dalla Serra, M.; Menestrina, G.; Fogliano, V.; Gambale, F. The Phytotoxic Lipodepsipeptide Syringopeptin 25A from Pseudomonas Syringae Pv Syringae Forms Ion Channels in Sugar Beet Vacuoles. J. Membr. Biol. 2002, 188, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Dalla Serra, M.; Menestrina, G.; Carpaneto, A.; Gambale, F.; Fogliano, V.; Ballio, A. Molecular Mechanism of Action of Syringopeptins, Antifungal Peptides from Pseudomonas Syringae Pv. Syringae. Pore-Form. Pept. Proteins Toxins 2003, 272–295. [Google Scholar]
- Menestrina, G.; Coraiola, M.; Fogliano, V.; Fiore, A.; Grgurina, I.; Carpaneto, A.; Gambale, F.; Dalla Serra, M. Antimicrobial lipodepsipeptides from Pseudomonas spp: A comparison of their activity on model membranes. In Pseudomonas Syringae and Related Pathogens; Springer: Berlin/Heidelberg, Germany, 2003; pp. 185–198. [Google Scholar]
- Festa, M.; Lagostena, L.; Carpaneto, A. Using the Plant Vacuole as a Biological System to Investigate the Functional Properties of Exogenous Channels and Transporters. Biochim. Biophys. Acta 2016, 1858, 607–612. [Google Scholar] [CrossRef]
- Costa, A.; Gutla, P.V.K.; Boccaccio, A.; Scholz-Starke, J.; Festa, M.; Basso, B.; Zanardi, I.; Pusch, M.; Schiavo, F.L.; Gambale, F.; et al. The Arabidopsis Central Vacuole as an Expression System for Intracellular Transporters: Functional Characterization of the Cl-/H+ Exchanger CLC-7. J. Physiol. 2012, 590, 3421–3430. [Google Scholar] [CrossRef]
- Yoo, S.-D.; Cho, Y.-H.; Sheen, J. Arabidopsis Mesophyll Protoplasts: A Versatile Cell System for Transient Gene Expression Analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Tzfira, T.; Tian, G.-W.; Lacroix, B.; Vyas, S.; Li, J.; Leitner-Dagan, Y.; Krichevsky, A.; Taylor, T.; Vainstein, A.; Citovsky, V. PSAT Vectors: A Modular Series of Plasmids for Autofluorescent Protein Tagging and Expression of Multiple Genes in Plants. Plant Mol. Biol. 2005, 57, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Boccaccio, A.; Scholz-Starke, J.; Hamamoto, S.; Larisch, N.; Festa, M.; Gutla, P.V.K.; Costa, A.; Dietrich, P.; Uozumi, N.; Carpaneto, A. The Phosphoinositide PI(3,5)P₂ Mediates Activation of Mammalian but Not Plant TPC Proteins: Functional Expression of Endolysosomal Channels in Yeast and Plant Cells. Cell. Mol. Life Sci. CMLS 2014, 71, 4275–4283. [Google Scholar] [CrossRef] [PubMed]
- Lagostena, L.; Festa, M.; Pusch, M.; Carpaneto, A. The Human Two-Pore Channel 1 Is Modulated by Cytosolic and Luminal Calcium. Sci. Rep. 2017, 7, 43900. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, S.A.; Kugemann, A.; Carpaneto, A.; Böckmann, R.A.; Dietrich, P. Phosphatidylinositol-3,5-Bisphosphate Lipid-Binding-Induced Activation of the Human Two-Pore Channel 2. Cell. Mol. Life Sci. CMLS 2018, 75, 3803–3815. [Google Scholar] [CrossRef]
- Carpaneto, A.; Boccaccio, A.; Lagostena, L.; Di Zanni, E.; Scholz-Starke, J. The Signaling Lipid Phosphatidylinositol-3,5-Bisphosphate Targets Plant CLC-a Anion/H+ Exchange Activity. EMBO Rep. 2017, 18, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Gradogna, A.; Scholz-Starke, J.; Pardo, J.M.; Carpaneto, A. Beyond the Patch-Clamp Resolution: Functional Activity of Nonelectrogenic Vacuolar NHX Proton/Potassium Antiporters and Inhibition by Phosphoinositides. New Phytol. 2021, 229, 3026–3036. [Google Scholar] [CrossRef]
- Milenkovic, S.; Bodrenko, I.V.; Lagostena, L.; Gradogna, A.; Serra, G.; Bosin, A.; Carpaneto, A.; Ceccarelli, M. The Mechanism and Energetics of a Ligand-Controlled Hydrophobic Gate in a Mammalian Two Pore Channel. Phys. Chem. Chem. Phys. PCCP 2020, 22, 15664–15674. [Google Scholar] [CrossRef]
- Porée, F.; Wulfetange, K.; Naso, A.; Carpaneto, A.; Roller, A.; Natura, G.; Bertl, A.; Sentenac, H.; Thibaud, J.-B.; Dreyer, I. Plant K(in) and K(out) Channels: Approaching the Trait of Opposite Rectification by Analyzing More than 250 KAT1-SKOR Chimeras. Biochem. Biophys. Res. Commun. 2005, 332, 465–473. [Google Scholar] [CrossRef]
- Carpaneto, A.; Koepsell, H.; Bamberg, E.; Hedrich, R.; Geiger, D. Sucrose-and H+-Dependent Charge Movements Associated with the Gating of Sucrose Transporter ZmSUT1. PLoS ONE 2010, 5, e12605. [Google Scholar] [CrossRef] [PubMed]
- Picco, C.; Scholz-Starke, J.; Naso, A.; Preger, V.; Sparla, F.; Trost, P.; Carpaneto, A. How Are Cytochrome B561 Electron Currents Controlled by Membrane Voltage and Substrate Availability? Antioxid. Redox Signal. 2014, 21, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Picco, C.; Scholz-Starke, J.; Festa, M.; Costa, A.; Sparla, F.; Trost, P.; Carpaneto, A. Direct Recording of Trans-Plasma Membrane Electron Currents Mediated by a Member of the Cytochrome B561 Family of Soybean. Plant Physiol. 2015, 169, 986–995. [Google Scholar] [CrossRef]
- Carpaneto, A.; Accardi, A.; Pisciotta, M.; Gambale, F. Chloride Channels Activated by Hypotonicity in N2A Neuroblastoma Cell Line. Exp. Brain Res. 1999, 124, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, B.; Tang, B.; Gong, G.; Kam, H.; Gao, C.; Chen, Y.; Wang, R.; Lee, S.M.Y. Differential Angiogenic Activities of Naringin and Naringenin in Zebrafish in Vivo and Human Umbilical Vein Endothelial Cells in Vitro. J. Funct. Foods 2018, 49, 369–377. [Google Scholar] [CrossRef]
- Maniotis, A.J.; Folberg, R.; Hess, A.; Seftor, E.A.; Gardner, L.M.; Pe’er, J.; Trent, J.M.; Meltzer, P.S.; Hendrix, M.J. Vascular Channel Formation by Human Melanoma Cells in Vivo and in Vitro: Vasculogenic Mimicry. Am. J. Pathol. 1999, 155, 739–752. [Google Scholar] [CrossRef]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A.; Duarte Dos Santos, C.N.; Bordignon, J. The Citrus Flavanone Naringenin Impairs Dengue Virus Replication in Human Cells. Sci. Rep. 2017, 7, 41864. [Google Scholar] [CrossRef]
- Cataneo, A.H.D.; Kuczera, D.; Koishi, A.C.; Zanluca, C.; Silveira, G.F.; de Arruda, T.B.; Suzukawa, A.A.; Bortot, L.O.; Dias-Baruffi, M.; Verri, W.A.; et al. The Citrus Flavonoid Naringenin Impairs the in Vitro Infection of Human Cells by Zika Virus. Sci. Rep. 2019, 9, 16348. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, J.; Cohen, P.Y.; Lin, W.; Kitsberg, D.; Balaguer, P.; Polyak, S.J.; Chung, R.T.; Yarmush, M.L.; Nahmias, Y. Naringenin Inhibits the Assembly and Long-Term Production of Infectious Hepatitis C Virus Particles through a PPAR-Mediated Mechanism. J. Hepatol. 2011, 55, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Pohjala, L.; Utt, A.; Varjak, M.; Lulla, A.; Merits, A.; Ahola, T.; Tammela, P. Inhibitors of Alphavirus Entry and Replication Identified with a Stable Chikungunya Replicon Cell Line and Virus-Based Assays. PloS ONE 2011, 6, e28923. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Hassandarvish, P.; Lani, R.; Yadollahi, P.; Jokar, A.; Bakar, S.A.; Zandi, K. Inhibition of Chikungunya Virus Replication by Hesperetin and Naringenin. RSC Adv. 2016, 6, 69421–69430. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kolokoltsov, A.A.; Chen, C.-C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Ebola Virus. Two-Pore Channels Control Ebola Virus Host Cell Entry and Are Drug Targets for Disease Treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharm. Basel Switz. 2019, 12, 11. [Google Scholar] [CrossRef]
- Bai, Y.; Peng, W.; Yang, C.; Zou, W.; Liu, M.; Wu, H.; Fan, L.; Li, P.; Zeng, X.; Su, W. Pharmacokinetics and Metabolism of Naringin and Active Metabolite Naringenin in Rats, Dogs, Humans, and the Differences Between Species. Front. Pharmacol. 2020, 11, 364. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and Pharmacokinetics of Naringenin: A Randomized, Controlled, Single-Ascending-Dose Clinical Trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef]
- Weiss, S.R.; Navas-Martin, S. Coronavirus Pathogenesis and the Emerging Pathogen Severe Acute Respiratory Syndrome Coronavirus. Microbiol. Mol. Biol. Rev. MMBR 2005, 69, 635–664. [Google Scholar] [CrossRef]
- Filippini, A.; D’Amore, A.; Palombi, F.; Carpaneto, A. Could the Inhibition of Endo-Lysosomal Two-Pore Channels (TPCs) by the Natural Flavonoid Naringenin Represent an Option to Fight SARS-CoV-2 Infection? Front. Microbiol. 2020, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Tang, R. Could an Endo-Lysosomal Ion Channel Be the Achilles Heel of SARS-CoV2? Cell Calcium 2020, 88, 102212. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O.H.; Gerasimenko, O.V.; Gerasimenko, J.V. Endocytic Uptake of SARS-CoV-2: The Critical Roles of PH, Ca2+, and NAADP. Function 2020, 1, zqaa003. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and Its Immune Cross-Reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Clementi, N.; Scagnolari, C.; D’Amore, A.; Palombi, F.; Criscuolo, E.; Frasca, F.; Pierangeli, A.; Mancini, N.; Antonelli, G.; Clementi, M.; et al. Naringenin Is a Powerful Inhibitor of SARS-CoV-2 Infection in Vitro. Pharmacol. Res. 2020, 105255. [Google Scholar] [CrossRef]
- Netcharoensirisuk, P.; Abrahamian, C.; Tang, R.; Chen, C.-C.; Rosato, A.S.; Beyers, W.; Chao, Y.-K.; Filippini, A.; Di Pietro, S.; Bartel, K.; et al. Flavonoids Increase Melanin Production and Reduce Proliferation, Migration and Invasion of Melanoma Cells by Blocking Endolysosomal/Melanosomal TPC2. Sci. Rep. 2021, 11, 8515. [Google Scholar] [CrossRef]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a Potential Immunomodulator in Therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, S.; Zhao, J.; Yu, C.; Hu, Y.; Tu, Y.; Yang, Z.; Zheng, J.; Wang, Y.; Gao, Y. Naringenin Ameliorates Renovascular Hypertensive Renal Damage by Normalizing the Balance of Renin-Angiotensin System Components in Rats. Int. J. Med. Sci. 2019, 16, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zeng, W.; Zhang, F.; Zhang, C.; Liang, W. Naringenin Ameliorates Acute Inflammation by Regulating Intracellular Cytokine Degradation. J. Immunol. Baltim. Md 1950 2017, 199, 3466–3477. [Google Scholar] [CrossRef]
- Wang, J.; Niu, X.; Wu, C.; Wu, D. Naringenin Modifies the Development of Lineage-Specific Effector CD4+ T Cells. Front. Immunol. 2018, 9, 2267. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. Covid-19 and Autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting Potential Drivers of COVID-19: Neutrophil Extracellular Traps. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, C.; Shen, F.; Wang, M.; Jia, N.; Wang, C. Naringenin Ameliorates LPS-Induced Acute Lung Injury through Its Anti-Oxidative and Anti-Inflammatory Activity and by Inhibition of the PI3K/AKT Pathway. Exp. Ther. Med. 2017, 14, 2228–2234. [Google Scholar] [CrossRef]
- Chtourou, Y.; Fetoui, H.; Jemai, R.; Ben Slima, A.; Makni, M.; Gdoura, R. Naringenin Reduces Cholesterol-Induced Hepatic Inflammation in Rats by Modulating Matrix Metalloproteinases-2, 9 via Inhibition of Nuclear Factor ΚB Pathway. Eur. J. Pharmacol. 2015, 746, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.F.; Gaspar, V.M.; Conde, T.A.; Mano, J.F.; Duarte, I.F. Flavonoid-Mediated Immunomodulation of Human Macrophages Involves Key Metabolites and Metabolic Pathways. Sci. Rep. 2019, 9, 14906. [Google Scholar] [CrossRef]
- Yoshida, H.; Watanabe, H.; Ishida, A.; Watanabe, W.; Narumi, K.; Atsumi, T.; Sugita, C.; Kurokawa, M. Naringenin Suppresses Macrophage Infiltration into Adipose Tissue in an Early Phase of High-Fat Diet-Induced Obesity. Biochem. Biophys. Res. Commun. 2014, 454, 95–101. [Google Scholar] [CrossRef]
- Bodet, C.; La, V.D.; Epifano, F.; Grenier, D. Naringenin Has Anti-Inflammatory Properties in Macrophage and Ex Vivo Human Whole-Blood Models. J. Periodontal Res. 2008, 43, 400–407. [Google Scholar] [CrossRef] [PubMed]
| Naringenin (Concentration/Dose) and Model | Immune Regulation Effects | References |
|---|---|---|
| 50 mg/kg Hepatocytes/Hypercholesterolemia | Naringenin reduces TNF- α, IL-6, and IL-1β by suppressing NF-kB. | [95] |
| 100 µM Pre-polarized M1 macrophages | Naringenin reduces TNF- α production. | [96] |
| 50–100 µM T cells 100 µM Macrophages | Naringenin reduces TNF- α, IL-6 secretion regulating cytokines degradation through lysosome-TFEB dependent mechanisms. | [90] |
| 100 mg/Kg Mouse model of ARDS | Naringenin reduces neutrophil infiltration reducing airway inflammation and lung injury. | [94] |
| 100 mg/Kg in vivo and in vitro studies | Naringenin reduces Monocyte chemoattractant protein (MCP)1 secretion suppressing macrophages infiltration in adipose tissue. | [97] |
| 25–50 µg/mL Macrophages and ex vivo whole blood | Naringenin reduces proinflammatory cytokines (IL-8, IL-6, IL-1β, TNF-α) in macrophages and ex vivo whole blood samples. | [98] |
| 80 µM CD4+T cells | Naringenin inhibits Th1 and Th17 differentiation. | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amore, A.; Gradogna, A.; Palombi, F.; Minicozzi, V.; Ceccarelli, M.; Carpaneto, A.; Filippini, A. The Discovery of Naringenin as Endolysosomal Two-Pore Channel Inhibitor and Its Emerging Role in SARS-CoV-2 Infection. Cells 2021, 10, 1130. https://doi.org/10.3390/cells10051130
D’Amore A, Gradogna A, Palombi F, Minicozzi V, Ceccarelli M, Carpaneto A, Filippini A. The Discovery of Naringenin as Endolysosomal Two-Pore Channel Inhibitor and Its Emerging Role in SARS-CoV-2 Infection. Cells. 2021; 10(5):1130. https://doi.org/10.3390/cells10051130
Chicago/Turabian StyleD’Amore, Antonella, Antonella Gradogna, Fioretta Palombi, Velia Minicozzi, Matteo Ceccarelli, Armando Carpaneto, and Antonio Filippini. 2021. "The Discovery of Naringenin as Endolysosomal Two-Pore Channel Inhibitor and Its Emerging Role in SARS-CoV-2 Infection" Cells 10, no. 5: 1130. https://doi.org/10.3390/cells10051130
APA StyleD’Amore, A., Gradogna, A., Palombi, F., Minicozzi, V., Ceccarelli, M., Carpaneto, A., & Filippini, A. (2021). The Discovery of Naringenin as Endolysosomal Two-Pore Channel Inhibitor and Its Emerging Role in SARS-CoV-2 Infection. Cells, 10(5), 1130. https://doi.org/10.3390/cells10051130






