Calcium Signaling in Plant Programmed Cell Death
Abstract
1. Introduction
2. The role of Ca2+ in PCD
2.1. Biotic Stresses
2.2. Abiotic Stress
2.2.1. Salt Stress
2.2.2. Temperature Stress
2.2.3. Anoxic Stress
2.2.4. Heavy Metal Stress
2.2.5. Mechanical Damage
2.2.6. Comparison of Ca2+ Signaling Components under Biotic and Abiotic Stresses
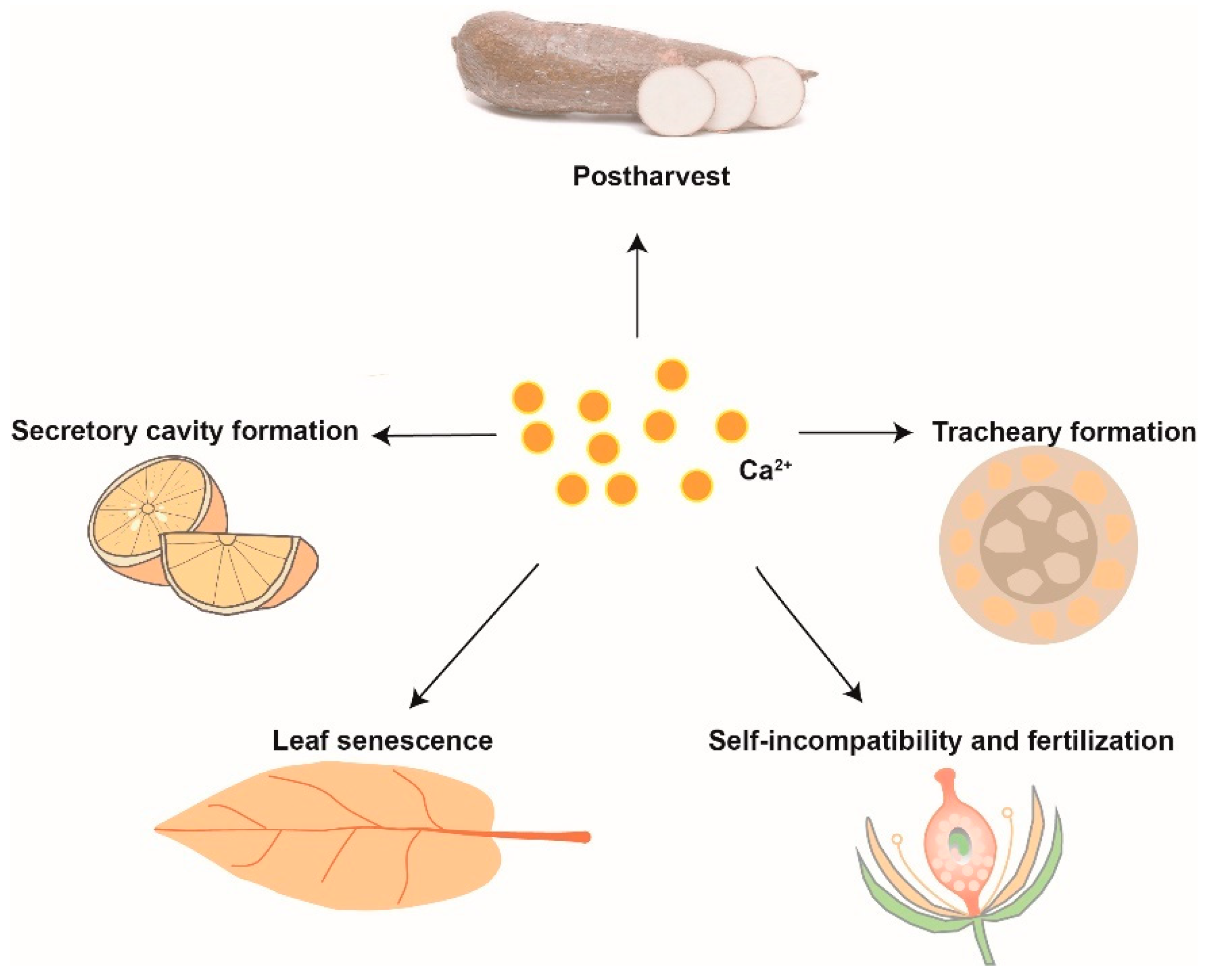
2.3. Plant Development and Postharvest Storage
2.4. Small Chemical Molecule
2.5. Metacaspases
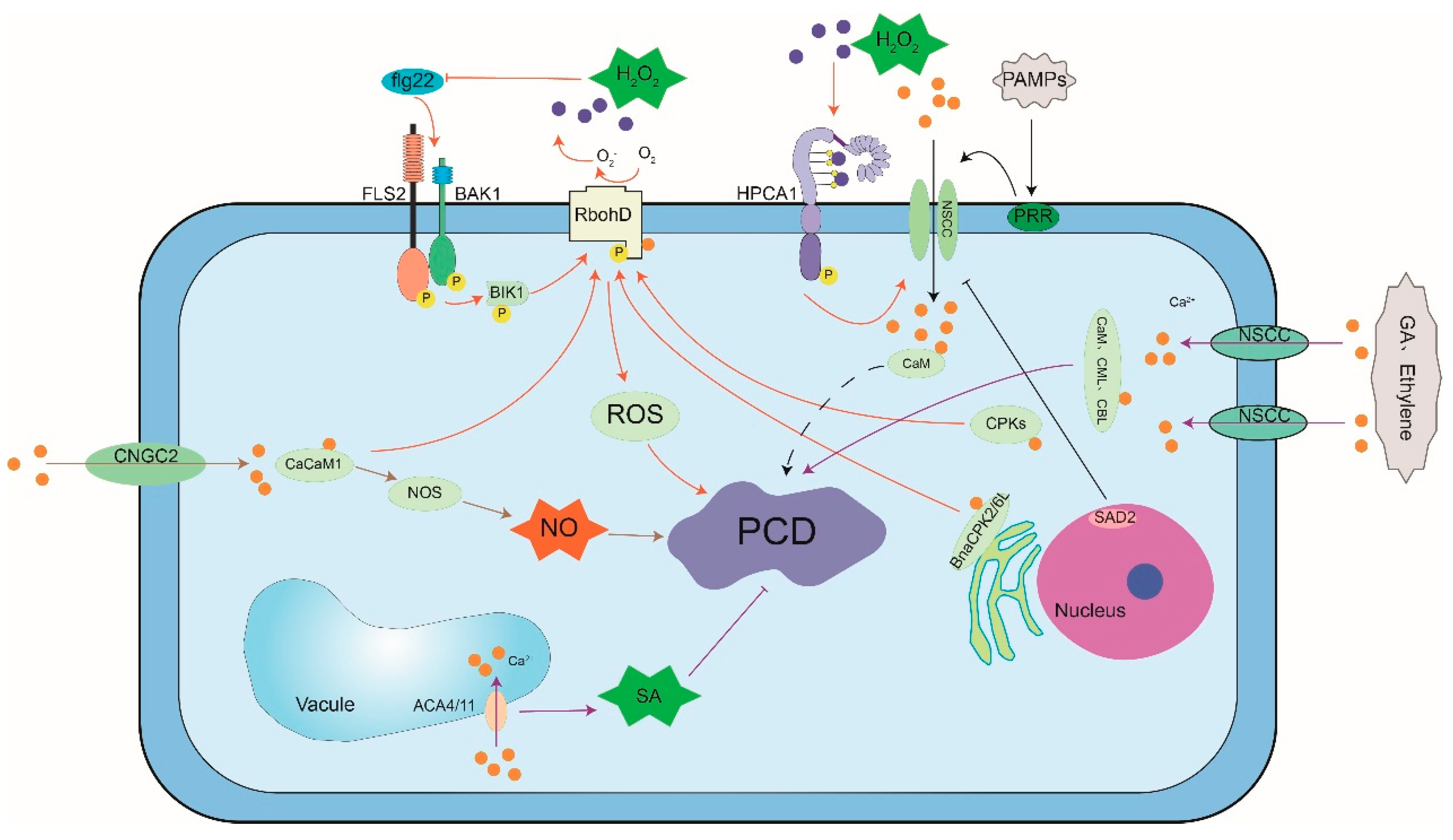
2.6. Crosstalk between Ca2+ and Other Signaling Molecules in PCD
3. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCD | Programmed Cell Death |
| dPCD | Developmental Programmed Cell Death |
| ePCD | Environmental Programmed Cell Death |
| CNGC | Cyclic Nucleotide-Gated Channel |
| CaM | Calmodulin |
| PPD | Postharvest Physiological Deterioration |
| DHS | D-Erythro-Sphinganine |
| MCPs | Metacaspases |
| PG | Polygalacturonase |
| MPTP | Mitochondrial Permeability Transition Pore |
| CBL | Calcineurin B-Like Protein |
| CIPK | CBL-Interacting Protein Kinase |
| CPK | Ca2+-Dependent Protein Kinase |
| PTI | Pattern-Triggered Immunity |
| ETI | Effector-Triggered Immunity |
| PAMP | Pathogen-Associated Molecular Pattern |
| HR | Hypersensitive Response |
| EGTA | Ethylenebis (Oxyethylenenitrilo) Tetraacetic Acid |
| TPCs | Two-Pore Channels |
| CAXs | Ca2+/H+ exchangers |
| cAMP | 3′-5′-Cyclic Adenosine Monophosphate |
| cGMP | Cyclic Guanosine Monophosphate |
| PEPRs | Pep Receptors |
| DAMPs | Damage-Associated Molecular Patterns |
| ETH | Ecdysis Triggering Hormone |
| CML | CaM-Like Protein |
| EFR | Elongation Factor Tu Receptor |
| AC | Adenylate Cyclase |
| PDE | Phosphodiesterase |
| PM | Plasma Membrane |
| TvX | Tichoderma Viride Xylanase |
| MAPK | Mitogen-Activated Protein Kinase |
| BAP | Biofilm Associated Protein |
| SA | Salicylic Acid |
| RBOHB | Respiratory Burst Oxidase Homolog B |
| ROS | Reactive Oxygen Species |
| ETH | Ecdysis Triggering Hormone |
| GIPCs | Glycosyl Inositol Phosphorylceramides |
| NOS | Nitric Oxide Synthase |
| KEAs | Plastid K+ Exchange Antiporters |
| VPE | Vacuolar Processing Enzyme |
| PTP | Permeability Transition Pore |
| BAPTA-AM | Bis-(O-Aminophenoxy)-N,N,N,N’-Tetraacetic Acid Acetoxymethyl Ester |
| PGIP | Polygalacturonase-Inhibiting Protein |
| PG | Pyoderma Gangrenosum |
| HPCA1 | Hydrogen Peroxide Sensor |
| GLR | Glutamate Receptors |
| PEPs | Plant Elicitor Peptides |
| PEPRs | Extracellular Pep Receptors |
| ER stress | Endoplasmic Reticulum Stress |
| SERCA | Er-Type Iia Ca2+ Pumps |
| PHS | Phytosphingosine |
References
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Mccabe, P.F.; Leaver, C.J. Programmed cell death in cell cultures. Plant Mol. Biol. 2000, 44, 359–368. [Google Scholar] [CrossRef]
- Sychta, K.; Tbomka, A.; Kuta, E. Insights into Plant Programmed Cell Death Induced by Heavy Metals-Discovering a Terra Incognita. Cells 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.F.; Li, J.; Bi, F.C.; Liu, Z.; Yao, N. Ceramide-Induced Cell Death Depends on Calcium and Caspase-Like Activity in Rice. Front. Plant Sci. 2020, 11, 145. [Google Scholar] [CrossRef]
- Tian, D.; Wang, J.; Zeng, X.; Gu, K.; Qiu, C.; Yang, X.; Zhou, Z.; Goh, M.; Luo, Y.; Murata-Hori, M.; et al. The Rice TAL Effecto’ Dependent Resistance Protein XA10 Triggers Cell Death and Calcium Depletion in the Endoplasmic Reticulum. Plant Cell 2014, 26, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhan, Q.D.; Shi, T.T.; Liu, J.; Zhao, K.J.; Gao, Y. The nuclear transporter SAD2 plays a role in calcium- and H2O2-mediated cell death in Arabidopsis. Plant J. 2020, 101, 324–333. [Google Scholar]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81. [Google Scholar] [CrossRef]
- Jones, J.; Dangl, J. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; He, S.Y. Innate Immunity in Plants: An Arms Race Between Pattern Recognition Receptors in Plants and Effectors in Microbial Pathogens. Science 2009, 324, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Zhang, H.; Chen, B.; Gao, S.; Yang, B.; Jiang, Y. Rapeseed calcium-dependent protein kinase CPK6L modulates reactive oxygen species and cell death through interacting and phosphorylating RBOHD. Biochem. Biophys. Res. Commun. 2019, 518, 719–725. [Google Scholar] [CrossRef]
- Johansson, O.N.; Nilsson, A.K.; Gustavsson, M.B.; Backhaus, T.; Andersson, M.; Ellerström, M. A quick and robust method for quantification of the hypersensitive response in plants. Peer J. 2015, 3, e1469. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, H.; Wei, X.; Yang, L.; Yang, B.; Zhang, L.; Li, J.; Jiang, Y. Functional characterization of calcium-dependent protein kinase (CPK) 2 gene from oilseed rape (Brassica napus L.) in regulating reactive oxygen species signaling and cell death control. Gene 2018, 651, 49–56. [Google Scholar] [CrossRef]
- Gao, X.; Cox, K.L.; He, P. Functions of Calcium-Dependent Protein Kinases in Plant Innate Immunity. Plants 2014, 3, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Brown, I.; Adams, S.; Knight, M.; Ainslie, A.; Mansfield, J. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 2000, 23, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.H. Current Status of the Gene-For-Gene Concept. Annu. Rev. Phytopathol. 1971, 9, 275–296. [Google Scholar] [CrossRef]
- Yang, Y.; Shah, J.; Klessig, D.F. Signal perception and transduction in plant defense responses. Genes Dev. 1997, 11, 1621–1639. [Google Scholar] [CrossRef]
- Rudd, J.J.; Franklin-Tong, V.E. Calcium signaling in plants. Cell Mol. Life Sci. 1999, 55, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Kudla, J.; Sanders, D. The Language of Calcium Signalling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, K.A.; Matthus, E.; Swarbreck, S.M.; Davies, J.M. Calcium-Mediated Abiotic Stress Signaling in Roots. Front. Plant Sci. 2016, 7, 1296. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Smigel, A.; Tsai, Y.C.; Braam, J.; Berkowitz, G.A. Innate immunity signaling: Cytosolic Ca2+ elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 2008, 148, 818–828. [Google Scholar] [CrossRef]
- Qi, Z.; Verma, R.; Gehring, C.; Yamaguchi, Y.; Zhao, Y.; Ryan, C.A.; Berkowita, G.A. Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc. Natl. Acad. Sci. USA 2010, 107, 21193–21198. [Google Scholar] [CrossRef]
- Chin, K.; Defalco, T.A.; Moeder, W.; Yoshioka, K. The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol. 2013, 163, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Jurkowski, G.I.; Smith, R.K., Jr.; Yu, I.C.; Ham, J.H.; Sharma, S.B.; Klessig, D.F.; Fengler, K.A.; Bent, A. Arabidopsis DND2, a Second Cyclic Nucleotide-Gated Ion Channel Gene for Which Mutation Causes the “Defense, No Death” Phenotype. Mol. Plant Microbe. Interact. 2004, 17, 511–520. [Google Scholar] [CrossRef]
- Clough, S.J.; Fengler, K.A.; Yu, I.C.; Lippok, B.; Smith Jr, R.K.; Bent, A.F. The Arabidopsis dnd1 "defense, no death" gene encodes a mutated cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. USA 2000, 97, 9323–9328. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Hou, C.; Ren, Z.; Wang, C.; Zhao, F.; Ahlbeck, D.D.; Hu, S.; Zhang, L.; Niu, Q.; Li, L.; et al. A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 2019, 572, 131–135. [Google Scholar] [CrossRef]
- Thuleau, P.; Aldon, D.; Cotelle, V.; Brière, C.; Ranty, B.; Galaud, J.; Mazars, C. Relationships between calcium and sphingolipid-dependent signalling pathways during the early steps of plant-pathogen interactions. Biochim. Biophys. Acta 2013, 1833, 1590–1594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdel-Hamid, H.; Chin, K.; Moeder, W.; Yoshioka, K. High throughput chemical screening supports the involvement of Ca2+ in cyclic nucleotide-gated ion channel-mediated programmed cell death in Arabidopsis. Plant Signal Behav. 2011, 6, 1817–1819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hou, C.; Tian, W.; Li, L.; Zhu, H.E. Electrophysiological Studies Revealed CaM1-Mediated Regulation of the Arabidopsis Calcium Channel CNGC12. Front. Plant Sci. 2019, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Moeder, W.; Urquhart, W.; Ung, H.; Yoshioka, K. The Role of Cyclic Nucleotide-Gated Ion Channels in Plant Immunity. Mol. Plant 2011, 4, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Moeder, W.; Kang, H.; Kachroo, P.; Masmoudi, K.; Berkowitz, G.; Klessig, D. The Chimeric Cyclic Nucleotide-Gated Ion Channel AtCNGC11/12 Activates Multiple Pathogen Resistance Responses. Plant Cell 2006, 18, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Baxter, J.; Moeder, W.; Urquhart, W.; Shahinas, D.; Chin, K.; Christendat, D.; Kang, H.G.; Angelova, M.; Kato, N.; Yoshioka, K. Identification of a functionally essential amino acid for Arabidopsis cyclic nucleotide gated ion channels using the chimeric AtCNGC11/12 gene. Plant J. 2008, 56, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Urquhart, W.; Gunawardena, A.; Moeder, W.; Ali, R.; Berkowitz, G.; Yoshioka, K. The chimeric cyclic nucleotide-gated ion channel ATCNGC11/12 constitutively induces programmed cell death in a Ca2+ dependent manner. Plant Mol. Biol. 2007, 65, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, G.; Li, B.; Vespoli, L.; Liu, H.; Moeder, W.; Chen, S.; Oliveira, M.; Souza, S.; Shao, W.; et al. The Receptor Kinases BAK1/SERK4 Regulate Ca2+ Channel-Mediated Cellular Homeostasis for Cell Death Containment. Curr. Biol. 2019, 29, 3778–3790. [Google Scholar] [CrossRef]
- Hilleary, R.; Paez-Valencia, J.; Vens, C.; Toyota, M.; Palmgren, M.; Gilroy, S. Tonoplast-localized Ca2+ pumps regulate Ca2+ signals during pattern-triggered immunity in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2020, 117, 18849–18857. [Google Scholar] [CrossRef]
- Boursiac, Y.; Lee, S.M.; Romanowsky, S.M.; Blank, R.; Sladek, C.; Chung, W.; Harper, J. Disruption of the Vacuolar Calcium-ATPases in Arabidopsis Results in the Activation of a Salicylic Acid-Dependent Programmed Cell Death Pathway. Plant Physiol. 2010, 154, 1158–1171. [Google Scholar] [CrossRef]
- Kurusu, T.; Yagala, T.; Miyao, A.; Hirochika, H.; Kuchitsu, K. Identification of a putative voltage-gated Ca2+ channel as a key regulator of elicitor-induced hypersensitive cell death and mitogen-activated protein kinase activation in rice. Plant J. 2005, 42, 798–809. [Google Scholar] [CrossRef]
- Bjornson, M.; Pimprikar, P.; Nürnberger, T.; Zipfel, C. The transcriptional landscape of Arabidopsis thaliana pattern-triggered immunity. Nat. Plants 2021. [Google Scholar] [CrossRef] [PubMed]
- Zuppini, A.; Navazio, L.; Mariani, P. Endoplasmic reticulum stress-induced programmed cell death in soybean cells. J. Cell Sci. 2004, 117, 2591–2598. [Google Scholar] [CrossRef]
- Zhu, X.; Caplan, J.; Mamillapalli, P.; Czymmek, K.; Dinesh-Kumar, S. Function of endoplasmic reticulum calcium ATPase in innate immunity-mediated programmed cell death. EMBO J. 2010, 29, 1007–1018. [Google Scholar] [CrossRef]
- Ihara-Ohori, Y.; Nagano, M.; Muto, S.; Uchimiya, H.; Kawai-Yamada, M. Cell Death Suppressor Arabidopsis Bax Inhibitor-1 Is Associated with Calmodulin Binding and Ion Homeostasis. Plant Physiol. 2007, 143, 650–660. [Google Scholar] [CrossRef]
- Lemtiri-Chlieh, F.; Berkowitz, G.A. Cyclic Adenosine Monophosphate Regulates Calcium Channels in the Plasma Membrane of Arabidopsis Leaf Guard and Mesophyll Cells. J. Biol. Chem. 2004, 279, 35306–35312. [Google Scholar] [CrossRef] [PubMed]
- Volotovski, I.D.; Sokolovsky, S.G.; Molchan, O.V.; Knight, M.R. Second Messengers Mediate Increases in Cytosolic Calcium in Tobacco Protoplasts. Plant Physiol. 1998, 117, 1023–1030. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.C.; Walker, R.K.; Berkowitz, G.A. Molecular Steps in the Immune Signaling Pathway Evoked by Plant Elicitor Peptides: Ca2+-Dependent Protein Kinases, Nitric Oxide, and Reactive Oxygen Species Are Downstream from the Early Ca2+ Signal. Plant Physiol. 2013, 163, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Hander, T.; Fernández-Fernández, Á.D.; Kumpf, R.P.; Willems, P.; Schatowitz, H.; Rombaut, D.; Staes, A.; Nolf, J.; Pottie, R.; Yao, P.; et al. Damage on plants activates Ca2+-dependent metacaspases for release of immunomodulatory peptides. Science 2019, 363, eaar7486. [Google Scholar] [CrossRef]
- Zhu, P.; Yu, X.; Wang, C.; Zhang, Q.; Liu, W.; Mcsweeney, S.; Shanklin, J.; Lam, E.; Liu, Q. Structural Basis for Ca2+-Dependent Activation of a Plant Metacaspase. Nat. Commun. 2020, 11, 2249. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Kunze, G.; Chinchilla, D.; Caniard, A.; Felix, G. Perception of the Bacterial PAMP EF-Tu by the Receptor EFR Restricts Agrobacterium-Mediated Transformation. Cell 2006, 125, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef]
- Gómez-Gómez, L.; Boller, T. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell 2000, 5, 1003–1011. [Google Scholar] [CrossRef]
- Lin, W.; Lu, D.; Gao, X.; Jiang, S.; Ma, X.; Wang, Z.; Mengiste, T.; He, P.; Shan, L. Inverse modulation of plant immune and brassinosteroid signaling pathways by the receptor-like cytoplasmic kinase BIK1. Proc. Natl. Acad. Sci. USA 2013, 110, 12114–12119. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, P.; Claus, L.a.N.; Savatin, D.V.; Xu, G.; Wu, S.; Meng, X.; Russinova, E.; He, P.; Shan, L. Proteolytic Processing of SERK3/BAK1 Regulates Plant Immunity, Development, and Cell Death. Plant Physiol. 2019, 180, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, X.; Li, M.; He, P.; Zhang, Y. Loss-of-function of Arabidopsis receptor-like kinase BIR1 activates cell death and defense responses mediated by BAK1 and SOBIR1. New Phytol. 2016, 212, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Jung, W.Y.; Kang, Y.H.; Kim, J.Y.; Kim, D.G.; Jeong, J.C.; Baek, D.W.; Jin, J.B.; Lee, J.Y.; Kim, M.O.; et al. AtBAG6, a novel calmodulin-binding protein, induces programmed cell death in yeast and plants. Cell Death Differ. 2006, 13, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Bouché, N.; Yellin, A.; Snedden, W.A.; Fromm, H. Plant-Specific Calmodulin-Binding Protens. Annu. Rev. Plant Biol. 2005, 56, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, D.; Ekengren, S.K.; Martin, G.B.; Dobney, S.L.; Snedden, W.A. Calmodulin-like Proteins from Arabidopsis and Tomato are Involved in Host Defense Against Pseudomonas syringae pv. tomato. Plant Mol. Biol. 2005, 58, 887–897. [Google Scholar] [CrossRef]
- Mei, J.; Ding, Y.; Li, Y.; Tong, C.; Du, H.; Yu, Y.; Wan, H.; Xiong, Q.; Yu, J.; Liu, S.; et al. Transcriptomic comparison between Brassica oleracea and rice (Oryza sativa) reveals diverse modulations on cell death in response to Sclerotinia sclerotiorum. Sci. Rep. 2016, 6, 33706. [Google Scholar] [CrossRef]
- Lachaud, C.; Prigent, E.; Thuleau, P.; Grat, S.; Silva, D.D.; Brière, C.; Mazars, C.; Cotelle, V. 14-3-3-Regulated Ca2+-dependent protein kinase CPK3 is required for sphingolipid-induced cell death in Arabidopsis. Cell Death Differ. 2013, 20, 209–217. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, N.; Zhang, L.; He, Y.X.; Chao, C.; Zhou, J.G.; Li, J.; Meng, X. Perception of the pathogen-induced peptide RGF7 by the receptor-like kinases RGI4 and RGI5 triggers innate immunity in Arabidopsis thaliana. New Phytol. 2021, 230, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Torre, F.D.L.; Gutiérrez-Beltrán, E.; Pareja-Jaime, Y.; Chakravarthy, S.; Martin, G.B.; Pozo, O.D. The tomato calcium sensor Cbl10 and its interacting protein kinase Cipk6 define a signaling Pathway in plant immunity. Plant Cell 2013, 25, 2748–2764. [Google Scholar] [CrossRef]
- Yang, H.J.; Yang, S.H.; Li, Y.Q.; Hua, J. The Arabidopsis BAP1 and BAP2 genes are general inhibitors of programmed cell death. Plant Physiol. 2007, 145, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.H.; Jiang, Z.Y.; Bi, G.; Nomura, K.; Liu, K.; He, M.; Zhou, J.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar]
- Ahmed, I.; Michael, R.; Nick, P. The jasmonate pathway mediates salt tolerance in grapevines. J. Exp. Bot. 2012, 63, 2127–2139. [Google Scholar]
- Emanuela, M.; Takashi, K.; Daniel, T.; Elisa, A.; Delphine, A.B.; Bernadette, B.; Joël, B.; Tomonori, K.; Stefano, M.; Franois, B. Deciphering early events involved in hyperosmotic stress-induced programmed cell death in tobacco BY-2 cells. J. Exp. Bot. 2014, 65, 1361–1375. [Google Scholar]
- Katsuhara, M.; Kawasaki, T. Salt stress induced nuclear and DNA degradation in meristematic cells of Barley Roots. Plant Cell Physiol. 1996, 37, 169–173. [Google Scholar] [CrossRef]
- Ismail, A.; El-Sharkawy, I.; Sherif, S. Salt Stress Signals on Demand: Cellular Events in the Right Context. Int. J. Mol. Sci. 2020, 21, 3918. [Google Scholar] [CrossRef]
- Lin, J.S.; Wang, Y.; Wang, G.X. Salt stress-induced programmed cell death via Ca2+-mediated mitochondrial permeability transition in tobacco protoplasts. Plant Growth Regul. 2005, 45, 243–250. [Google Scholar] [CrossRef]
- Li, J.Y.; Jiang, A.L.; Chen, H.Y.; Wang, Y.; Zhang, W. Lanthanum Prevents Salt Stress-induced Programmed Cell Death in Rice Root Tip Cells by Controlling Early Induction Events. J. Integr. Plant Biol. 2007, 49, 1024–1031. [Google Scholar] [CrossRef]
- Kim, Y.; Wang, M.Q.; Bai, Y.; Zeng, Z.H.; Guo, F.; Han, N.; Bian, H.W.; Wang, J.H.; Pan, J.W.; Zhu, M.Y. Bcl-2 suppresses activation of VPEs by inhibiting cytosolic Ca2+ level with elevated K+ efflux in NaCl-induced PCD in rice. Plant Physiol. Biochem. 2014, 80, 168–175. [Google Scholar] [CrossRef]
- Chaloupka, J.; Vinter, V. Programmed cell death in bacteria. Folia Microbiol. 1996, 41, 451–464. [Google Scholar] [CrossRef]
- Lee, D.Y.; Rhee, G.Y. Kinetics of cell death in the cyanobacterium anabaena flos-aquae and the production of dissolved organic carbon. J. Appl. Psychol. 2008, 33, 991–998. [Google Scholar]
- Ning, S.B.; Guo, H.L.; Wang, L.; Song, Y.C. Salt stress induces programmed cell death in prokaryotic organism Anabaena. J. Appl. Microbiol. 2002, 93, 15–28. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Zhou, X.P.; Tao, M.; Yuan, F.; Liu, l.l.; Wu, F.H.; Wu, X.M.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Richards, S.l.; Shabala, l.; Chen, C.; Renato, D.D.R.C.; Swarbreck, S.M.; Shaw, E.; Dark, A.; Shabala, S.; Shang, Z. Salinity-Induced Calcium Signaling and Root Adaptation in Arabidopsis Require the Calcium Regulatory Protein Annexin1. Plant Physiol. 2013, 163, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Theerawitaya, C.; Cha-Um, S.; Kirdmanee, C.; Takabe, T. Expression and functional analysis of putative vacuolar Ca2+-transporters (CAXs and ACAs) in roots of salt tolerant and sensitive rice cultivars. Protoplasma 2014, 251, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Zuppini, A.; Bugno, V.; Baldan, B. Monitoring programmed cell death triggered by mild heat shock in soybean-cultured cells. Funct. Plant Biol. 2006, 33, 617–627. [Google Scholar] [CrossRef]
- Kratsch, H.; Wise, R.R. The ultrastructure of chilling stress. Plant Cell Environ. 2000, 23, 337–350. [Google Scholar] [CrossRef]
- Snedden, W.A.; Fromm, H. Calmodulin, calmodulin-related proteins and plant responses to the environment. Trends Plant Sci. 1998, 3, 299–304. [Google Scholar] [CrossRef]
- Liu, Q.B.; Ding, Y.L.; Shi, Y.T.; Ma, L.; Wang, Y.; Song, C.P.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 2021, 40, 104559. [Google Scholar] [CrossRef] [PubMed]
- Nicotera, P.; Orrenius, S. The role of calcium in apoptosis. Cell Calcium 1998, 23, 173–180. [Google Scholar] [CrossRef]
- Chen, J.X.; Zhao, Y.Y.; Chen, X.H.; Peng, Y.; Hurr, B.M.; Mao, L.C. The Role of Ethylene and Calcium in Programmed Cell Death of Cold-Stored Cucumber Fruit. J. Food Biochem. 2013, 38, 337–344. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef]
- Laohavisit, A.; Shang, Z.; Rubio, L.; Cuin, T.A.; Véry, A.A.; Wang, A.; Mortimer, J.C.; Macpherson, N.; Coxon, K.M.; Battey, N.H.; et al. Arabidopsis Annexin1 Mediates the Radical-Activated Plasma Membrane Ca2+ and K+ Permeable Conductance in Root Cells. Plant Cell 2012, 24, 1522–1533. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.Y.; Xu, Y.Y.; Luo, W.; Zheng, X.M.; Zeng, D.; Pan, Y.J.; Lin, X.L.; Liu, H.H.; Zhang, D.J.; et al. COLD1 Confers Chilling Tolerance in Rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Liu, D.F.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, X.L.; Wang, H.; Li, B.J.; Clark, G.; Guo, Y.; Roux, S.; Sun, D.; Tang, W.Q. Proteomic Study of Microsomal Proteins Reveals a Key Role for Arabidopsis Annexin 1 in Mediating Heat Stress-Induced Increase in Intracellular Calcium Levels. Mol. Cell Proteom. 2015, 14, 686–694. [Google Scholar] [CrossRef]
- Liao, C.; Zheng, Y.; Guo, Y. MYB30 transcription factor regulates oxidative and heat stress responses through ANNEXIN-mediated cytosolic calcium signaling in Arabidopsis. New Phytol. 2017, 216, 163–177. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Finka, A.; Cuendet, A.; Maathuis, F.; Saidi, Y.; Goloubinoff, P. Plasma Membrane Cyclic Nucleotide Gated Calcium Channels Control Land Plant Thermal Sensing and Acquired Thermotolerance. Plant Cell 2012, 24, 3333–3348. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Han, X.; Wu, J.; Zheng, S.; Shang, Z.; Sun, D.; Zhou, R.; Li, B. A heat-activated calcium-permeable channel-Arabidopsis cyclic nucleotide-gated ion channel 6-is involved in heat shock responses. Plant J. 2012, 70, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, X.; Lv, X.; Ahammed, G.J.; Guo, Z.; Qi, Z.; Yu, J.; Zhou, Y. Tomato GLR3.3 and GLR3.5 mediate cold acclimation-induced chilling tolerance by regulating apoplastic H2O2 production and redox homeostasis. Plant Cell Environ. 2019, 42, 3326–3339. [Google Scholar] [CrossRef]
- Lenzoni, G.; Knight, M.R. Increases in Absolute Temperature Stimulate Free Calcium Concentration Elevations in the Chloroplast. Plant Cell Physiol. 2019, 60, 538–548. [Google Scholar] [CrossRef]
- D’angeli, S.; Altamura, M.M. Osmotin induces cold protection in olive trees by affecting programmed cell death and cytoskeleton organization. Planta 2007, 225, 1147–1163. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Saidi, Y.; Peter, M.; Finka, A.; Cicekli, C.; Vigh, L.; Goloubinoff, P. Membrane lipid composition affects plant heat sensing and modulates Ca2+-dependent heat shock response. Plant Signal Behav. 2010, 5, 1530–1533. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yue, H.; Xing, D. MAP Kinase 6-mediated activation of vacuolar processing enzyme modulates heat shock-induced programmed cell death in Arabidopsis. New Phytol. 2012, 195, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.C.; He, C.J.; Morgan, P.W. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000, 5, 123–127. [Google Scholar] [CrossRef]
- He, C.J.; Morgan, P.W.; Drew, M.C. Transduction of an Ethylene Signal Is Required for Cell Death and Lysis in the Root Cortex of Maize during Aerenchyma Formation Induced by Hypoxia. Plant Physiol. 1996, 112, 463–472. [Google Scholar] [CrossRef]
- Virolainen, E.; Blokhina, O.; Fagerstedt, K. Ca2+-induced high amplitude swelling and cytochrome c release from wheat (Triticum aestivum L.) mitochondria under anoxic stress. Ann. Bot. 2002, 90, 509–516. [Google Scholar]
- Huang, D.; Gong, X.; Liu, Y.; Zeng, G.; Lai, C.; Bashir, H.; Zhou, L.; Wang, D.; Xu, P.; Cheng, M. Effects of calcium at toxic concentrations of cadmium in plants. Planta 2017, 245, 863. [Google Scholar] [CrossRef]
- Dayod, M.; Tyerman, S.D.; Leigh, R.A.; Gilliham, M. Calcium storage in plants and the implications for calcium biofortification. Protoplasma 2010, 247, 215–231. [Google Scholar] [CrossRef]
- Huda, K.M.; Banu, M.S.; Tuteja, R.; Tuteja, N. Global calcium transducer P-type Ca2+-ATPases open new avenues for agriculture by regulating stress signalling. J. Exp. Bot. 2013, 64, 3099–3109. [Google Scholar] [CrossRef]
- Qiao, Z.; Tao, J.; Jin, Z.; Liang, Y.; Zhang, L.; Liu, Z. CDPKs enhance Cd tolerance through intensifying H2S signal in Arabidopsis thaliana. Plant Soil 2016, 398, 99–110. [Google Scholar] [CrossRef]
- Jiang, J.H.; Ge, G.; Gao, K.; Pang, Y.; Chai, R.C.; Jia, X.H.; Kong, J.G.; Yu, A.C.H. Calcium Signaling Involvement in Cadmium-Induced Astrocyte Cytotoxicity and Cell Death Through Activation of MAPK and PI3K/Akt Signaling Pathways. Neurochem. Res. 2015, 40, 1929–1944. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Trebotich, J.; Vergara, E.; Medina, C.; Morales, B.; Moenne, A. Copper-induced calcium release from ER involves the activation of ryanodine-sensitive and IP3-sensitive channels in Ulva Compressa. Plant Signal. Behav. 2010, 5, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Vera, J.; Castro, J.; Dennett, G.; Mellado, M.; Morales, B.; Correa, J.A.; Moenne, A. Co-occurring increases of calcium and organellar reactive oxygen species determine differential activation of antioxidant and defense enzymes in Ulva compressa (Chlorophyta) exposed to copper excess. Plant Cell Environ. 2010, 33, 1627–1640. [Google Scholar] [CrossRef]
- González, A.; Cabrera, M.L.; Henríquez, M.; Contreras, R.A.; Morales, B.; Moenne, A. Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in ulva compressa exposed to copper excess. Plant Physiol. 2012, 158, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Huang, W.; Oo, T.L.; Gu, M.; He, L.F. Nitric oxide inhibits aluminum-induced programmed cell death in peanut (Arachis hypoganea L.) root tips. J. Hazard. Mater. 2017, 333, 285–292. [Google Scholar] [CrossRef]
- Huang, T.L.; Huang, H.J. ROS and CDPK-like kinase-mediated activation of MAP kinase in rice roots exposed to lead. Chemosphere 2008, 71, 1377–1385. [Google Scholar] [CrossRef]
- Ahmad, A.; Hadi, F.; Ali, N. Effective Phytoextraction of Cadmium (Cd) with Increasing Concentration of Total Phenolics and Free Proline in Cannabis sativa (L) Plant Under Various Treatments of Fertilizers, Plant Growth Regulators and Sodium Salt. Int. J. Phytoremediation 2015, 17, 56–65. [Google Scholar]
- Fang, H.; Tao, J.; Liu, Z.; Zhang, L.; Pei, Y. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 2014, 56, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Huang, L.Y.; Fu, S.F.; Trinh, N.N.; Huang, H.J. Genomic profiling of rice roots with short- and long-term chromium stress. Plant Mol. Biol. 2014, 86, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Norambuena, L.; Maturana, D.; Toro, M.; Vergara, C.; Orellana, A.; Zurita-Silva, A.; Ordenes, V.R. AtHMA1 is a thapsigargin-sensitive Ca2+/heavy metal pump. J. Biol. Chem. 2008, 283, 9633–9641. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Chauvin, A.; Pascaud, F.; Kellenberger, S.; Farmer, E.E. Glutamate Receptor-Like genes mediate leaf-to-leaf wound signalling. Nature 2013, 500, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Fan, M.; Yang, M.; Zhao, J.; Zhang, W.; Su, Y.; Xiao, L.; Deng, H.; Xie, D. Injury Activates Ca2+/Calmodulin-Dependent Phosphorylation of JAV1-JAZ8-WRKY51 Complex for Jasmonate Biosynthesis. Mol. Cell 2018, 70, 136–149. [Google Scholar] [CrossRef]
- Steinhorst, L.; Kudla, J. How plants perceive salt. Nature 2019, 572, 318–320. [Google Scholar] [CrossRef]
- Wang, C.; Teng, Y.; Zhu, S.; Zhang, L.; Liu, X. NaCl- and cold-induced stress activate different Ca2+-permeable channels in Arabidopsis thaliana. Plant Growth Regul. 2019, 87, 217–225. [Google Scholar] [CrossRef]
- Cao, X.Q.; Jiang, Z.H.; Yi, Y.Y.; Yang, Y.; Ke, L.P.; Pei, Z.M.; Shan, Z. Biotic and Abiotic Stresses Activate Different Ca2+ Permeable Channels in Arabidopsis. Front. Plant Sci. 2017, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, S.; Ye, R.; Xue, Y.; Chen, A.; An, L.; Pei, Z.M. Relationship between NaCl- and H2O2-Induced Cytosolic Ca2+ Increases in Response to Stress in Arabidopsis. PLoS ONE 2013, 8, e76130. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Franklin-Tong, V.E. Self-incompatibility in Papaver: Signalling to trigger PCD in incompatible pollen. J. Exp. Bot. 2008, 146, 481–490. [Google Scholar] [CrossRef]
- Groover, A.; Jones, A.M. Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol. 1999, 119, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, D.Y.; Li, Q.; Xu, Y.J.; Cui, K.M.; Zhu, Y.X. PPF1 inhibits programmed cell death in apical meristems of both G2 pea and transgenic Arabidopsis plants possibly by delaying cytosolic Ca2+ elevation. Cell Calcium 2004, 35, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Bai, M.; Chen, Y.; Liu, P.W.; Gao, L.; Liang, S.J.; Wu, H. Programmed cell death of secretory cavity cells of citrus fruits is associated with Ca2+ accumulation in the nucleus. Trees 2014, 28, 1137–1144. [Google Scholar] [CrossRef]
- Bai, M.; Liang, M.; Huai, B.; Gao, H.; Tong, P.; Shen, R.; He, H.; Wu, H. Ca2+-dependent nuclease is involved in DNA degradation during the programmed cell death of secretory cavity formation in fruit of Citrus grandis ‘Tomentosa’. J. Exp. Bot. 2020, 71, 4812–4827. [Google Scholar] [CrossRef] [PubMed]
- Durian, G.; Sedaghatmehr, M.; Matallana-Ramirez, L.P.; Schilling, S.M.; Schaepe, S.; Guerra, T.; Herde, M.T.; Witte, C.P.; Schulze, W.X.; Mueller-Roeber, B.; et al. Calcium-Dependent Protein Kinase CPK1 Controls Cell Death by In Vivo Phosphorylation of Senescence Master Regulator ORE1. Plant Cell 2020, 32, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhao, P.; Liang, W.; Cheng, Q.; Mu, B.; Niu, F.; Yan, J.; Liu, C.; Xie, H.; Kav, N.N.V.; et al. A rapeseed WRKY transcription factor phosphorylated by CPK modulates cell death and leaf senescence by regulating the expression of ROS and SA-synthesis-related genes. J. Agric. Food Chem. 2020, 68, 7348–7359. [Google Scholar] [CrossRef] [PubMed]
- Ngo, Q.; Vogler, H.; Lituiev, D.; Nestorova, A.; Grossniklaus, U. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev. Cell 2014, 29, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.G.; Franklin-Tong, V.E. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 2004, 429, 305–309. [Google Scholar] [CrossRef]
- Franklin-Tong, V.E.; Ride, J.P.; Read, N.D.; Trewavas, A.J.; Franklin, F.C.H. The self-incompatibility response in Papaver rhoeas is mediated 818by cytosolic free calcium. Plant J. 1993, 4, 163–177. [Google Scholar] [CrossRef]
- Jordan, N.D.; Franklin, F.C.H.; Franklin-Tong, V.E. Evidence for DNA fragmentation triggered in the selfincompatibility response in pollen of Papaver Rhoeas. Plant J. 2000, 23, 471–479. [Google Scholar] [CrossRef]
- Wang, X.P.; Li, X.S.U.; Centre, J.W. Distribution changes of calcium and programmed cell death in the pistil of litchi (Litchi chinensis Sonn.) flower during its development. J. Physiol. Mol. Biol. 2006, 32, 607. [Google Scholar]
- Djabou, A.S.M.; Carvalho, L.J.C.B.; Li, Q.X.; Niemenak, N.; Chen, S. Cassava postharvest physiological deterioration: A complex phenomenon involving calcium signaling, reactive oxygen species and programmed cell death. Acta Physiol. Plant 2017, 39, 91. [Google Scholar] [CrossRef] [PubMed]
- Owiti, J.; Grossmann, J.; Gehrig, P.; Dessimoz, C.; Laloi, C.; Hansen, M.B.; Gruissem, W.; Vanderschuren, H. iTRAQ-based analysis of changes in the cassava root proteome reveals pathways associated with post-harvest physiological deterioration. Plant J. 2011, 67, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, N.; Inoue, M.; Ogihara, Y. Reactive oxygen species and intracellular Ca2+, common signals for apoptosis induced by gallic acid. Biochem. Pharmacol. 1998, 55, 1973–1981. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, C.L.; Deng, S.R.; Lu, C.F.; Shen, X.; Zhou, X.Y.; Zheng, X.J.; Hu, Z.M.; Chen, S.L. An ATP signalling pathway in plant cells: Extracellular ATP triggers programmed cell death in Populus euphratica. Plant Cell Environ. 2012, 35, 893–916. [Google Scholar] [CrossRef]
- Zuppini, A.; Baldan, B.; Millioni, R.; Favaron, F.; Navazio, L.; Mariani, P. Chitosan induces Ca2+-mediated programmed cell death in soybean cells. New Phytol. 2004, 161, 557–568. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Yin, H.; Zhao, X.; Du, Y. Oligochitosan induces programmed cell death in tobacco suspension cells. Carbohydr. Polym. 2012, 87, 2270–2278. [Google Scholar] [CrossRef]
- Zuppini, A.; Navazio, L.; Sella, L.; Castiglioni, C.; Favaron, F.; Mariani, P. An endopolygalacturonase from Sclerotinia sclerotiorum induces calcium-mediated signaling and programmed cell death in soybean cells. Mol. Plant Microbe Interact. 2005, 18, 849–855. [Google Scholar] [CrossRef]
- Hao, L.; Goodwin, P.H.; Hsiang, T. Expression of a metacaspase gene of Nicotiana benthamiana after inoculation with Colletotrichum destructivum or Pseudomonas syringae pv. tomato, and the effect of silencing the gene on the host response. Plant Cell Rep. 2007, 26, 1879–1888. [Google Scholar] [CrossRef]
- Suarez, M.F.; Filonova, L.H.; Smertenko, A.; Savenkov, E.I.; Clapham, D.H.; Arnold, S.; Zhivotovsky, B.; Bozhkov, P.V. Metacaspase-dependent programmed cell death is essential for plant embryogenesis. Curr. Biol. 2004, 14, 339–340. [Google Scholar] [CrossRef]
- Tsiatsiani, L.; Breusegem, F.V.; Gallois, P.; Zavialov, A.; Bozhkov, P.V. Metacaspases. Cell Death Differ. 2011, 18, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lam, E. Sheathing the swords of death: Post-translational modulation of plant metacaspases. Plant Signal. Behav. 2011, 6, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Riemann, M.; Dong, D.; Stoeffler, N.; Gross, B.; Markel, A.; Nick, P. Two grapevine metacaspase genes mediate ETI-like cell death in grapevine defence against infection of Plasmopara Viticola. Protoplasma 2019, 256, 951–969. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Drury, G.E.; Rotari, V.I.; Gordon, A.; Willer, M.; Farzaneh, T.; Woltering, E.J.; Gallois, P. Metacaspase-8 modulates programmed cell death induced by ultraviolet light and H2O2 in Arabidopsis. J. Biol. Chem. 2008, 283, 774–783. [Google Scholar] [CrossRef]
- Locato, V.; De Gara, L. Programmed Cell Death in Plants: An Overview. Methods Mol. Biol. 2018, 1743, 1–8. [Google Scholar]
- Heath, M.C. Hypersensitive response-related death. Plant Mol. Biol. 2000, 44, 321–334. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yoshioka, M.; Asai, S.; Nomura, H.; Kuchimura, K.; Mori, H.; Doke, N.; Yoshioka, H. StCDPK5 confers resistance to late blight pathogen but increases susceptibility to early blight pathogen in potato via reactive oxygen species burst. New Phytol. 2012, 196, 223–237. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L.; Jones, J. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef]
- Asai, S.; Yoshioka, H. Nitric oxide as a partner of reactive oxygen species participates in disease resistance to necrotrophic pathogen botrytis cinerea in Nicotiana Benthamiana. Mol. Plant Microbe Interact. 2009, 22, 619–629. [Google Scholar] [CrossRef]
- Kwak, J.M.; Jones, J.; Pei, Z.M.; Torres, M.A.; Dangl, J.L.; Mori, I.C.; Leonhardt, N.; Bloom, R.E.; Bodde, S.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Levine, A.; Pennell, R.; Alvarez, M.E.; Palmer, R.; Lamb, C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 1996, 6, 427–437. [Google Scholar]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, J.S.; Wang, G.X. Calcium-Mediated Mitochondrial Permeability Transition Involved in Hydrogen Peroxide-Induced Apoptosis in Tobacco Protoplasts. J. Integr. Plant Biol. 2006, 48, 433–439. [Google Scholar] [CrossRef]
- Takahashi, F.; Mizoguchi, T.; Yoshida, R.; Ichimura, K.; Shinozaki, K. Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol. Cell 2011, 41, 649–660. [Google Scholar] [CrossRef]
- Lamotte, O.; Gould, K.; Lecourieux, D.; Sequeira-Legrand, A.; Lebrun-Garcia, A.; Durner, J.; Pugin, A.; Wendehenne, D. Analysis of nitric oxide signaling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiol. 2004, 135, 516–529. [Google Scholar] [CrossRef]
- Choi, H.W.; Lee, D.H.; Hwang, B.K. The pepper calmodulin gene CaCaM1 is involved in reactive oxygen species and nitric oxide generation required for cell death and the defense response. Mol. Plant Microbe Interact. 2009, 22, 1389–1400. [Google Scholar] [CrossRef]
- Delledonne, M. NO news is good news for plants. Curr. Opin. Plant Biol. 2005, 8, 390–396. [Google Scholar] [CrossRef]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence-signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, D.; Zahringer, U.; Gerber, I.; Dubery, I.; Hartung, T.; Bors, W.; Hutzler, P.; Durner, J. Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc. Natl. Acad. Sci. USA 2004, 101, 15811–15816. [Google Scholar] [CrossRef]
- Moeder, W.; Yoshioka, K. Lesion mimic mutants: A classical, yet still fundamental approach to study programmed cell death. Plant Signal. Behav. 2008, 3, 764–767. [Google Scholar] [CrossRef]
- Kuo, A.; Cappelluti, S.; Cervantes-Cervantes, M.; Bush, R. Okadaic acid, a protein phosphatase inhibitor, blocks calcium changes, gene expression, and cell death induced by gibberellin in wheat aleurone cells. Plant Cell 1996, 8, 259–269. [Google Scholar] [PubMed]
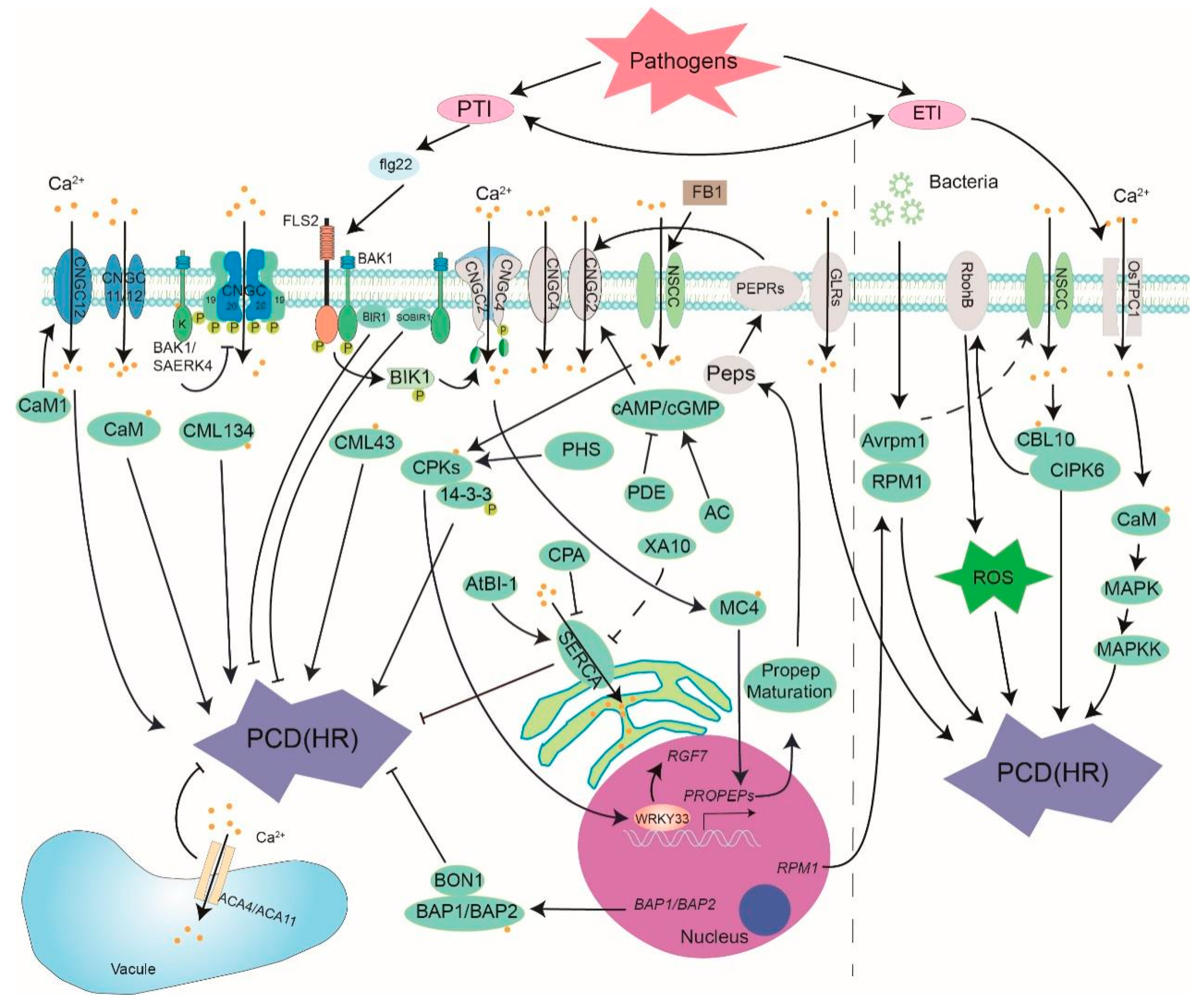
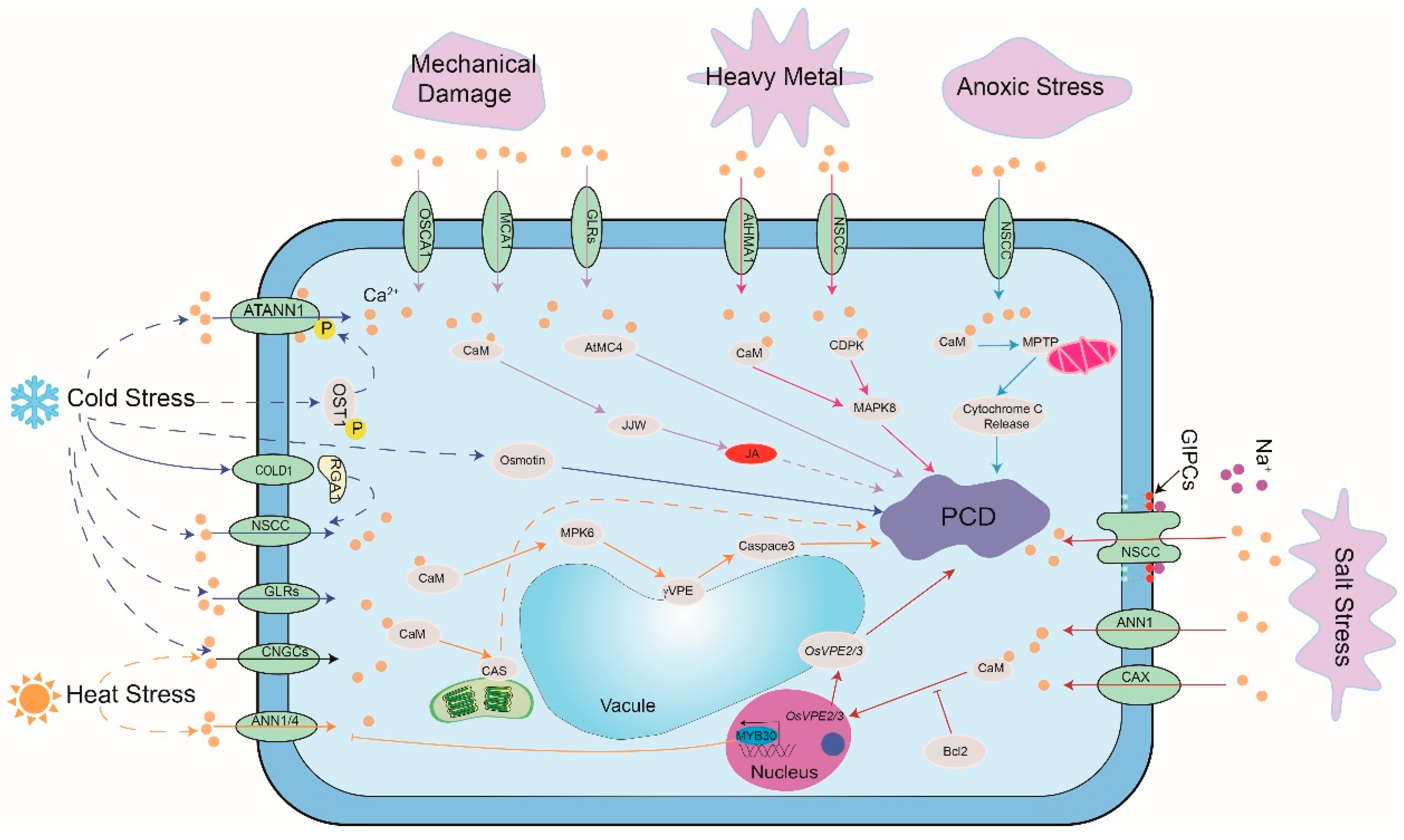
| PCD | Receptor | Calcium Channel | Regulation Factor of Ca2+ Channel | Calcium Sensor | Substrate | |
|---|---|---|---|---|---|---|
| Biotic stresses | PTI | FLS2/BAK1 | CNGC2/4/11/12/19/20 GLR2.7/2.8/2.9 ACA4/11 SERCA | cAMP/cGMP BAK1/BIK1 PEPR | CaM/CML CPK3/5/6 | RboHB 14-3-3 WRKY33 MC4 |
| ETI | ∕ | OsTPC1 | ∕ | CaM SlCBL10 | SlCIPK6 MPK | |
| Abiotic stresses | Salt | GIPC | ANN1 CAX1 | ∕ | CaM | OsVPE2/3 |
| Cold | COLD1 | ANN1 SlGLR3.3/3.5 CNGC2/4 OsCNGC14/16 | COLD1 OST1 | CaM | Osmotin | |
| Heat | ∕ | ANN1/4 OsCNGC14/16 CAS | MYB30 | CaM | MPK6 γVPE | |
| Anoxic | ∕ | ∕ | ∕ | CaM | MPTP Cytochrome C | |
| Heavy metal | ∕ | HMA1 | ∕ | CaM CDPKs | MAPK8 | |
| Damage | ∕ | GLR3.3/3.6 MCA1 OSCA1.2 | ∕ | CaM | JJW MC4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Zhao, X.; Li, W.; Hussain, J.; Qi, G.; Liu, S. Calcium Signaling in Plant Programmed Cell Death. Cells 2021, 10, 1089. https://doi.org/10.3390/cells10051089
Ren H, Zhao X, Li W, Hussain J, Qi G, Liu S. Calcium Signaling in Plant Programmed Cell Death. Cells. 2021; 10(5):1089. https://doi.org/10.3390/cells10051089
Chicago/Turabian StyleRen, Huimin, Xiaohong Zhao, Wenjie Li, Jamshaid Hussain, Guoning Qi, and Shenkui Liu. 2021. "Calcium Signaling in Plant Programmed Cell Death" Cells 10, no. 5: 1089. https://doi.org/10.3390/cells10051089
APA StyleRen, H., Zhao, X., Li, W., Hussain, J., Qi, G., & Liu, S. (2021). Calcium Signaling in Plant Programmed Cell Death. Cells, 10(5), 1089. https://doi.org/10.3390/cells10051089





