Abstract
Salicylic acid (SA) is well known hormonal molecule involved in cell death regulation. In response to a broad range of environmental factors (e.g., high light, UV, pathogens attack), plants accumulate SA, which participates in cell death induction and spread in some foliar cells. LESION SIMULATING DISEASE 1 (LSD1) is one of the best-known cell death regulators in Arabidopsis thaliana. The lsd1 mutant, lacking functional LSD1 protein, accumulates SA and is conditionally susceptible to many biotic and abiotic stresses. In order to get more insight into the role of LSD1-dependent regulation of SA accumulation during cell death, we crossed the lsd1 with the sid2 mutant, caring mutation in ISOCHORISMATE SYNTHASE 1 (ICS1) gene and having deregulated SA synthesis, and with plants expressing the bacterial nahG gene and thus decomposing SA to catechol. In response to UV A+B irradiation, the lsd1 mutant exhibited clear cell death phenotype, which was reversed in lsd1/sid2 and lsd1/NahG plants. The expression of PR-genes and the H2O2 content in UV-treated lsd1 were significantly higher when compared with the wild type. In contrast, lsd1/sid2 and lsd1/NahG plants demonstrated comparability with the wild-type level of PR-genes expression and H2O2. Our results demonstrate that SA accumulation is crucial for triggering cell death in lsd1, while the reduction of excessive SA accumulation may lead to a greater tolerance toward abiotic stress.
1. Introduction
In their natural environment plants are constantly and simultaneously exposed to many biotic and abiotic environmental factors, such as various pathogens, excess/deficiency of light, UV irradiation, drought, chilling, heat, and salinity. More and more data clearly indicate that plants have developed molecular and genetic systems to simultaneously respond to a mixture of biotic and abiotic stress factors [1,2,3,4,5]. One of the mechanisms important in plants’ response to stress is programmed cell death (PCD). PCD is a very sophisticated and selective molecular and physiological process leading to the death of some cells [1,6,7,8,9], which triggers a beneficial immune defense and acclimatory response in others [10,11,12,13,14].
Many PCD regulatory proteins in Arabidopsis thaliana have been described, but one of the best known is LESION SIMULATING DISEASE 1 (LSD1). LSD1 encodes a small C2C2 zinc finger protein that is a negative regulator of PCD [15], playing a molecular function of transcriptional regulator and scaffold protein [16]. It was shown that lsd1 mutant plants depleted in LSD1 functional protein are very susceptible to biotic stresses [17] and to abiotic stresses such as high light [18], chilling [19], and UV irradiation [1,5]. Importantly, LSD1 acts as a negative switch for two positive cell death regulators, ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1) and PHYTOALEXIN DEFICIENT 4 (PAD4) [16,20]. LSD1, EDS1, and PAD4 proteins form a specific hub that is responsible for triggering salicylic acid (SA)-, ethylene (ET)-, and reactive oxygen species (ROS)-dependent cell death and acclimatory responses to unfavorable conditions [1,20,21,22].
SA is one of the most important phytohormones in plant defense signaling [23]. It was shown that both biotic [17,24] and abiotic stresses [1,5] induce SA biosynthesis. Plants possess two different pathways to synthesize SA, both starting from chorismate. One of them is a pathway engaging isochorismate synthase (ICS), and the other is a pathway involving phenylalanine ammonia-lyase (PAL). The contribution of these two pathways is species-dependent. In Oryza sativa the PAL pathway is the most important in SA accumulation, while in Arabidopsis the ICS pathway prevails [25,26]. The ICS pathway starts from the conversion of chorismate into isochorismate (IC) by the ICS enzyme [27,28,29] and was first found in bacteria [30]. However, it was found that SA synthesis via the ICS pathway in Arabidopsis thaliana differs significantly from that in bacteria. The ICS pathway relies on amino acid conjugation of L-glutamate to IC, which is then spontaneously decomposed or enzymatically conversed, resulting in the formation of SA. The gene encoding enzyme responsible for this reaction, AVRPPHB SUSCEPTIBLE 3 (PBS3, AT5G13320), has been characterized in Arabidopsis but not in any other plant species so far [31,32]. The ICS pathway is very important in pathogen-induced SA accumulation [33] and in response to abiotic stress such as UV irradiation [34]. In Arabidopsis, two ICS homologs were found: ICS1 and ICS2. The mutation in ICS1 significantly lowers SA levels in response to UV stress, while the mutation in ICS2 does not [35]. This suggests that ICS1 is the main contributor to basal- and UV-induced SA accumulation in Arabidopsis [26], and the mutant in the ICS1 gene is called salicylic acid induction deficient 2 (sid2) [27].
It was shown that mutants with lower SA levels demonstrate better fitness, produce more seeds, and accumulate increased biomass [5,36], while mutants with higher SA content exhibit a dwarf phenotype [37,38]. Interestingly, some mutants with decreased SA biosynthesis are more susceptible to biotrophic pathogen infection (i.e., enhanced disease susceptibility 5 (eds5) and sid2) [39,40], while others are more resistant to abiotic stresses (i.e., eds1 or pad4) [5]. Findings indicate that the role of SA in response to biotic and abiotic stresses is different, and it was postulated that SA acts as double-edged sword for PCD in plants [41].
In response to stress, the lsd1 mutant was proved to accumulate SA and to strongly exhibit the cell death phenotype in laboratory conditions but not in the field [5,42,43]. SA accumulation in lsd1 is reverted in double eds1/lsd1 and pad4/lsd1 mutants, accumulating significantly less SA and ROS than the single lsd1 mutant [5]. However, the role of SA in LSD1-, EDS1-, and PAD4-dependent PCD regulation has not been broadly studied in response to abiotic stresses. Therefore, in this study we aimed to check the relationship between the SA content and the LSD1/EDS1/PAD4-dependent PCD in response to UV A+B stress. We crossed the lsd1 mutant with the sid2 mutant or with a transgenic line expressing nahG (NahG) [27,36,44], which allowed us to conclude a role of SA in the regulation of PCD that is dependent on LSD1/EDS1/PAD4 during abiotic stress response.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
Arabidopsis thaliana plants (Col-0, lsd1, sid2, NahG, sid2/lsd1 and NahG/lsd1) were grown in a walk in-type growing chamber (Siemens, München, Germany) under the following conditions: 8/16 h photoperiod, photosynthetic photon flux density of 80 μmol photons m−2·s−1, air humidity of 50%, and day/night temperature of 20/18 °C. The sid2/lsd1 and NahG/lsd1 plants were obtained by crossing. F3 generation was checked using PCR and qPCR (Figure 1A–C). Genotyping of sid2 mutant was described previously [38]. Genotyping of NahG transgenic plants was performed using the following PCR condition: initialization 3 min 95 °C, 30-times repeated denaturation 30 s 95 °C, annealing 30 s 56 °C, elongation 40 s 72 °C, and final elongation 1 min 72 °C. Each PCR mixture included 13.9 µL water, 2 µL 10× buffer (Sigma-Aldrich, St. Louis, MO, USA), 2 µL deoxyribonucleotide triphosphate (2.5 mmol), 1 µL mix of forward and reverse primers (10 mmol), 0.1 µL DreamTaq DNA Polymerase (5 U/µL) (Sigma-Aldrich, St. Louis, MO, USA), and 1 µL of total genomic DNA. All primers used in this study are listed in Table 1. A band of approximately 500 bp was obtained for the nahG gene [38]. All experiments were performed on 4-week-old plants.
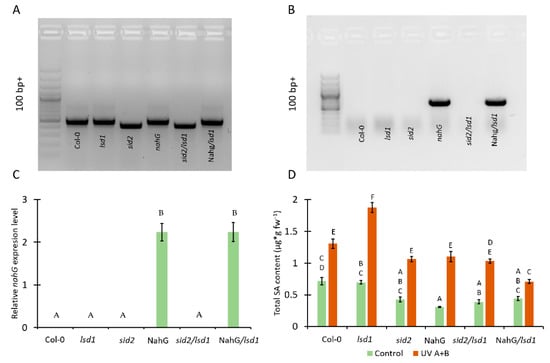
Figure 1.
Genotyping of plant material used in this study and the effect of genetic background on the level of total salicylic acid (SA). (A) Detection of mutation in the ICS1 gene using PCR, (B) detection of the presence of the nahG gene using PCR, (C) relative nahG expression level, (D) total SA content in tested plants before UV treatment (green bars) and after UV irradiation (2000 mJ·cm−2) (orange bars). Within a subgraph, values sharing common labels (letters) are not significantly different from each other (p > 0.001) (n = 9).

Table 1.
Primers used for PCR and qPCR.
2.2. Ultraviolet Irradiation Application
For UV A+B stress application, the UV 500 Crosslinker (Hoefer Pharmacia Biotech, San Francisco, CA, USA) was used. It was equipped with three UV-B lamps (type G8T5E, Sankyo Denki, peak wavelength 306 nm) and two UV-A lamps (type TL8WBLB, Philips, peak wavelength 365 nm). Arabidopsis mutants were exposed to a single irradiation dose 2000 mJ·cm−2. All analyses described in this study were performed 24 h after stress application.
2.3. Relative Electrolyte Leakage Measurement
The Arabidopsis rosettes were cut and placed in 50 mL falcon tubes filled with 35 mL of Milli-Q water (Merc Millipore, Darmstadt, Germany). The relative electrolyte leakage was measured with a conductance meter pHenomenal® CO 3100 L (VWR, Gdańsk, Poland) and calculated as a ratio between the value obtained after 1 h incubation and the total leakage evaluated after freezing the samples in −80 °C overnight followed by defrosting.
2.4. Trypan Blue Staining
Trypan blue (TB) stock (30 µmol trypan blue; Sigma-Aldrich, St. Louis, MO, USA) in a mixture of lactic acid, glycerol, and water (10 mL:10 mL:20 mL) was diluted with 96% ethanol (1:2) to obtain TB working solution. Fully developed leaves from non-treated plants and plants treated with UV A+B were collected and dipped immediately in TB working solution in 50 mL falcon tubes. Leaves were incubated in TB working solution for 30 min at room temperature and gently shaken several times. Subsequently, the TB working solution was removed and replaced which methanol. The leaves were incubated in methanol for 24 h, and methanol was changed several times for fresh methanol. Leaves deprived of chlorophyll were visualized using a Nikon SMZ18 stereomicroscope (Nikon Inc., Melville, NY, USA) with an adapted camera Nikon d5100 (Nikon Inc., Melville, NY, USA). Pictures of individual leaves were analyzed using ImageJ software version 1.8.0 (http://rsb.info.nih.gov/ij, accessed on 20 February 2021), and blue dots (micro-lesions) were counted per mm2 of leaf area.
2.5. RNA Isolation, cDNA Synthesis and qPCR Analysis
Arabidopsis rosettes were collected and immediately frozen in liquid nitrogen in three independent biological replicates, each containing 15–20 individual plants. Total RNA extraction was performed using a GeneMATRIX Universal RNA Purification Kit (EURX, Gdańsk, Poland) with an additional step of on-column DNaseI digestion. RNA concentration and purity were checked using an Eppendorf BioSpectrometer (Eppendorf, Hamburg, Germany). The RNA quality was controlled by electrophoretic separation in 1% agarose gel. cDNA synthesis was performed for equimolar RNA amounts of each sample using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). qPCRs were performed in three technical repetitions for each of the three biological replicates using the Power SYBR Green PCR Master Mix and the ABI 7500 Fast Real-Time PCR System (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Two reference genes were used: 5-FORMYLTETRAHYDROFOLATE CYCLOLIGASE (5-FCL, AT5G13050) and PROTEIN PHOSPHATASE 2A SUBUNIT A2 (PP2AA2, AT3G25800). Primers are listed in Table 1.
2.6. Measurement of SA and Hydrogen Peroxide (H2O2) Content and Ascorbate Peroxidase (APX) Activity
The methodology for measuring the content of SA, H2O2, and APX activity has been precisely described previously [42,45,46,47].
3. Results
3.1. Mutation in ICS1 and Expression of Bacterial Nahg Results in Lower Accumulation of SA in Lsd1 Mutant Background
In order to study the relationship between the SA content and LSD1/EDS1/PAD4-dependent response to UV A+B stress, the double mutant in ICS1 and LSD1 genes (sid2/lsd1) were obtained (Figure 1A). Moreover, the lsd1 mutant expressing bacterial nahG gene (NahG/lsd1) was generated by crossing (Figure 1A,B). ICS1 protein is known as a crucial component in SA synthesis [24], while bacterial nahG protein decomposes SA to catechol [36].
Before UV treatment, we found no significant differences in SA content between Col-0, lsd1, sid2, and NahG/lsd1, while sid2/lsd1 and NahG plants exhibited significantly lower levels of SA in their tissues. After UV irradiation, all tested genotypes accumulated more SA than before stress. The sid2, NahG, and sid2/lsd1 did not differ in terms of SA level when compared with the Col-0, while NahG/lsd1 accumulated significantly less SA than Col-0. However, the lsd1 mutant showed the highest content of SA, much higher than Col-0 or other genotype. Importantly, after UV stress the sid2/lsd1 and NahG/lsd1 plants exhibited significantly lower SA content in comparison with the lsd1 mutant (Figure 1D), which indicates that the mutations in ICS1 or nahG expression are able to revert the lsd1-specific SA accumulation.
3.2. Lower Foliar SA Level Mitigates the Lsd1-Specific Cell Death Phenotype
SA is an important molecule in PCD regulation [48,49], and it is accumulated in the lsd1 mutant [4,5]. In this work we wanted to know if deregulation in SA synthesis or metabolism can influence PCD in the lsd1 background. We found no differences in the phenotype among tested genotypes grown under non-stress conditions (Figure 2A). However, 24 h after UV irradiation the PCD symptoms started to be visible (Figure 2A). The wild-type plants showed only subtle changes, such as twisted leaves. In the lsd1 mutant, leaf curling was more visible. Other genotypes—sid2, NahG, sid2/lsd1 and NahG/lsd1—did not exhibit apparent changes after UV irradiation.
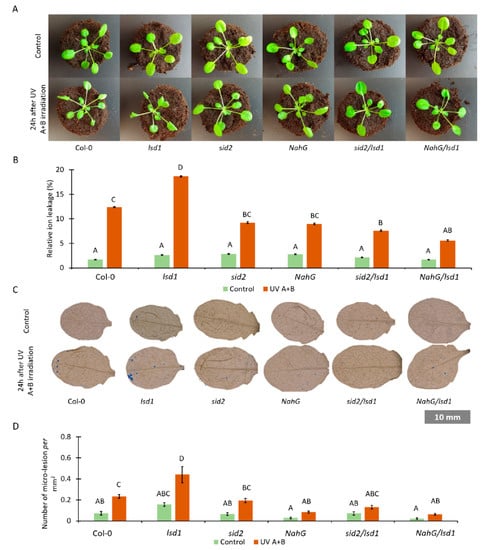
Figure 2.
The effect of SA synthesis or metabolism deregulation on LSD1-regulated cell death. (A) Pictures of plants cultivated under laboratory non-stress conditions (top row) and 24 h after episode of UV irradiation (2000 mJ·cm−2) (bottom row), (B) relative ion leakage in plants before stress (green bars) and 24 h after UV irradiation (2000 mJ·cm−2) (orange bars), (C) trypan blue staining of dead cells in plants before stress (top row) and after UV irradiation (bottom row), and (D) cell death quantified as micro-lesion number per mm2 in plants before stress (green bars) and after UV irradiation (2000 mJ·cm−2) (orange bars). Within a subgraph, values sharing common labels (letters) are not significantly different from each other (p > 0.001) (n = 10–15).
In order to assess the level of cell death in tested genotypes, relative electrolyte leakage was measured. Before UV treatment we did not observe any differences among tested genotypes (Figure 2B). Twenty-four hours after UV irradiation, all tested genotypes exhibited higher ion leakage when compared with non-treated plants. Ion leakage was significantly higher in UV-treated lsd1 when compared with the wild type (Figure 2B). However, sid2/lsd1 and NahG/lsd1 demonstrated significantly lower electrolyte leakage when compared with the lsd1 background and with Col-0 plants.
Moreover, micro-lesion formation using TB staining was checked. Micro-lesions constitute small lesion areas within the leaf tissue, comprising one or a couple of dead cells [50,51]. Before stress, we found no differences in the micro-lesion number among tested genotypes, while after UV irradiation in both the wild-type and lsd1 mutant we found more micro-lesions than in plants with deregulated SA synthesis or metabolism (Figure 3C,D). All these results demonstrate that lower foliar concentration of SA mitigates the lsd1-specific cell death phenotype after stress.
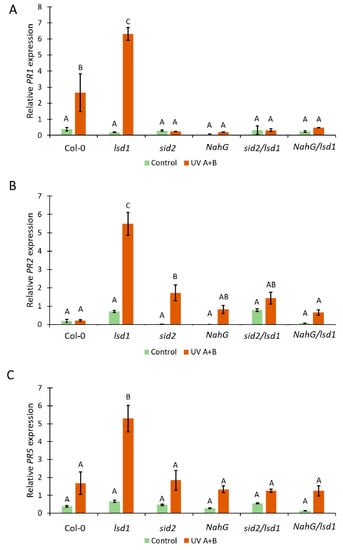
Figure 3.
The effect of deregulation in SA synthesis or metabolism on PR-genes expression. Relative expression of (A) PR1, (B) PR2, and (C) PR5 in tested genotypes before stress (green bars) and after UV irradiation (2000 mJ·cm−2) (orange bars). Within a subgraph, values sharing common labels (letters) are not significantly different from each other (p > 0.001) (n = 10–15). Results were normalized on two independent reference genes.
3.3. Deregulated SA Synthesis or SA Decomposition Leads to Alterations in PR-Genes Expression
SA is involved in the induction of PR-genes expression [52]. Therefore, we decided to check the relative expression level of PR1, PR2, and PR5 genes in genotypes tested within this study. Before stress, we found marginal but not statistically significant differences in the expression level of all tested PR-genes (Figure 3A–C). However, 24 h after the episode of UV irradiation in the wild type, the PR1 expression was higher when compared with the plants before stress. This effect was significantly stronger in the lsd1 mutant. The other tested Arabidopsis genotypes did not exhibit differences in PR1 expression between control and stress conditions (Figure 3A). Importantly, sid2/lsd1 and NahG/lsd1 plants demonstrated significantly lower expression of PR1 when compared with the single lsd1 mutant or even with Col-0. The expression of PR2 before stress did not differ between any tested genotypes. However, after UV irradiation, the expression of PR2 in lsd1 was almost 6 times higher than before stress. In the wild-type NahG, sid2/lsd1, and NahG/lsd1, there were no statistically significant increases in PR2 expression after UV irradiation. Interestingly, we found slightly but significantly higher expression of PR2 in sid2 in comparison with wild-type plants (Figure 3B). The expression of PR5 before stress and after UV irradiation followed a similar pattern as PR2 expression. After UV treatment, only lsd1 exhibited higher expression of PR5, while sid2/lsd1 and NahG/lsd1 had similar PR5 expression as Col-0 (Figure 2C). This part of our research shows that lower SA content reverts the lsd1-specific high expression of PR-genes after stress.
3.4. Deregulation in the SA Synthesis or Metabolism Results in Changes in the Antioxidant System
There is a strong relationship between SA and ROS content in plant tissues [5]. Moreover, SA can act as an inhibitor of some antioxidant enzymes, such as ascorbate peroxidase (APX) [53]. Therefore, we analyzed the level of H2O2 and APX activity in tested genotypes. Before stress, we found no statistical differences in the H2O2 content. Twenty-four hours after UV irradiation, the content of H2O2 increased in all tested genotypes in comparison with non-treated counterparts (Figure 4A). The UV-treated lsd1 mutant showed significantly higher H2O2 level when compared with UV irradiated Col-0. Importantly, the mutation in ICS1 or expression of nahG reversed this lsd1-specific accumulation of H2O2. The activity of APX before stress was higher in NahG plants and the sid2/lsd1 mutant in comparison with the wild type (Figure 4B). Interestingly, after UV irradiation, the APX activity dropped significantly in lsd1, NahG, and sid2/lsd1 in relation to non-treated counterparts. The lsd1 mutant demonstrated the lowest APX activity, while sid2/lsd1 and NahG/lsd1 had comparable APX activity to Col-0 (Figure 4B). These results indicate that stress-induced redox changes in the lsd1 mutant are modulated by SA content.
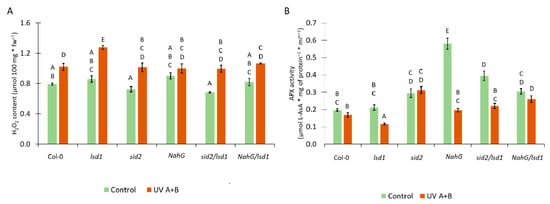
Figure 4.
The influence of SA synthesis or metabolism deregulation on foliar redox status. (A) Foliar H2O2 content before stress (green bars) and 24 h after UV irradiation (2000 mJ·cm−2) (orange bars), (B) ascorbate peroxidase (APX) activity before stress (green bars) and 24 h after UV irradiation (2000 mJ·cm−2) (orange bars). Within a subgraph, values sharing common labels (letters) are not significantly different from each other (p > 0.001) (n = 9).
4. Discussion
PCD is an ultimate end of the cell cycle, which occurs in all living multicellular organisms. It is essential for the appropriate response of plants to biotic and abiotic stresses, but is also important in the regulation of growth and development [6,7,8]. One of the most important signaling molecules being engaged in this process is SA [49,54]. It was demonstrated that plants with higher SA accumulation exhibit greater potential for PCD [4,41]. Contrariwise, Arabidopsis genotypes with ameliorated SA metabolism or deregulation in SA synthesis exhibit better growth and fitness and higher seed yield [5,27]. However, SA does not act alone during PCD since other molecules such as ethylene and ROS are also involved [43,54,55,56].
Some of the best described Arabidopsis proteins in the context of PCD regulation are LSD1, EDS1, and PAD4 [5,15,22,42,57,58]. LSD1 is a negative regulator of PCD, suppressing EDS1 and PAD4 activities since the double mutants eds1/lsd1 and pad4/lsd1 demonstrate a reverted lsd1-specific phenotype in terms of SA, ethylene, and ROS accumulation and cell death [3,5,42,56].
It was previously found that deregulation of SA synthesis in the lsd1 background reverts the cell death phenotype that occurs in response to biotic stress [57]. Notwithstanding, there is little information about the role of SA in response to short events of abiotic stresses such as high light, high temperature, or UV irradiation. Therefore, in the current work we focused on the role of SA accumulation in LSD1-regulated cell death triggered by UV. We obtained sid2/lsd1 and NahG/lsd1 plants in which the SA synthesis and metabolism were deregulated [24,27].
As expected [24,27,36,38], the dysfunctional mutation in ICS1 or the expression of bacterial nahG in Arabidopsis strongly reduced SA accumulation in the double sid2/lsd1 mutant and in the NahG/lsd1 line both before stress and after UV irradiation. This result allowed us to continue our study of the role of SA in lsd1-specific cell death in response to UV stress.
UV-A, UV-B, and also UV-C irradiation were found to affect plants [58,59] and induce cell death [60,61]. The lsd1 mutant is very susceptible to many abiotic stresses, such as high light [43], chilling [19], or UV-C [5,42].
In this study, we used UV A+B to induce cell death in Arabidopsis plants. It was demonstrated that UV A+B cause DNA damage, membrane disruption, protein crosslinking, and ROS formation [62]. Our results proved that before stress there was no difference in the phenotype of tested genotypes. These plants were grown in permissive short day and low light conditions that did not induce cell death in the lsd1 mutant [15,43]. After UV irradiation, wild-type plants exhibited some visible changes, such as twisted leaves, and this effect was much more clear in the lsd1 mutant, which is in line with previous studies [5,42]. What is particularly important is that sid2/lsd1 and NahG/lsd1 plants were in better shape than lsd1 or even Col-0 after UV treatment.
The integrity of the cell membranes, tested using ion leakage, showed that the deregulation in SA synthesis/metabolism reverts the cell death phenotype of the lsd1 mutant. Moreover, using TB staining we showed that after UV irradiation there were significantly more dead cells in the lsd1 mutant than in Col-0 or sid2/lsd1 and NahG/lsd1. It was shown previously that the mutation in EDS1 or PAD4 gene reverts the cell death phenotype in the lsd1 mutant [5,17,42,55], but here we clearly show that SA acts as a crucial molecule in LSD1-dependent cell death signaling in response to abiotic stress.
The phenomenon of cell death phenotype reversal in the lsd1 mutant via the deregulation in SA metabolism/synthesis can be caused by a significant reduction in PR-genes expression. PR proteins are necessary in hypersensitive response (HR) regulation, and both EDS1 and PAD4 are crucial in this pathway [63,64,65]. It was shown that the expression of bacterial nahG reduced PR1 expression in response to biotic stress [66]. The eds1/lsd1 or pad4/lsd1 mutants lacking functional EDS1 or PAD4 proteins are not able to induce HR [20,67]. In the lsd1 mutant, we found very high expression of PR1, PR2, and PR5, while in sid2/lsd1 and NahG/lsd1 plants, the PR-genes expression was similar to Col-0 or even lower. Higher expression of PR2 in sid2 may be related to the fact that PR2 is not related to SA as much as PR1 [68,69]. These results prove that SA is a crucial signaling molecule during abiotic stress response, that SA is necessary for the induction of PR-genes expression [70], and that the LSD1/EDS1/PAD4 hub [20,71] acts downstream of SA synthesis.
During PCD, not only does SA act as a signaling molecule, but ROS are also involved in this process [10,72,73,74,75]. Recently, a correlation between ROS, glutathione, ethylene, and SA content in plants has been found [3,5,55]. Moreover, it was proved that SA can affect the efficiency of the antioxidant system [1,53]. Therefore, the content of one of the ROS forms (H2O2) and the activity of enzymes involved in H2O2 scavenging (APX) were tested. In response to UV stress, all genotypes used in this study increased the content of H2O2 in their tissues. This effect was most significant in the lsd1 mutant. It may be at least partially caused by higher SA level in the lsd1 mutant since SA can inhibit the APX activity. APX is an important enzyme decomposing H2O2 into water [76,77] and is one of the most important enzymes in plant defense against oxidative stress [76]. The action of ROS during PCD is twofold—they induce cell damage but also act as signaling molecules [78]. Another ROS form, superoxide anion radical (O2•−), generated by respiratory burst oxidase homologs D and F (RBOHD/F), may antagonize pro-death signals induced during abiotic stress in Arabidopsis, since cell death was enhanced in lsd1/rbohD and lsd1/rbohF double mutants in comparison with the lsd1 single mutant, implying that RBOHD/F function as suppressors of cell death in neighboring cells around sites of abiotic stress [74]. Taking into account that the lack of SA accumulation strongly correlates with the reversal of the lsd1-specific cell death phenotype in sid2/lsd1 and NahG/lsd1 plants, we postulate that SA induces H2O2 levels and acts as a negative regulator of the antioxidant system, which enhances PCD propagation.
In conclusion, our results show that SA is crucial in LSD1-dependent regulation of PCD and that deregulation of SA synthesis or metabolism inhibits the cell death phenotype in the lsd1 background in response to abiotic stress.
Author Contributions
M.J.B., W.C., and S.K. formulated the hypothesis, planed experiments, and wrote the manuscript. M.J.B. performed the plant crossing, relative electrolyte leakage measurement, TB staining, RNA isolation, cDNA synthesis, and real time PCR analysis. A.R. was involved in SA, ROS, and APX activity measurements. W.C. designed the primers. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by “Maestro 6” project (2014/14/A/NZ1/00218) and by the “OPUS 15” project (UMO-2018/29/B/NZ3/01198) granted to Stanisław Karpiński by the National Science Centre.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Karpiński, S.; Szechyńska-Hebda, M.; Wituszyńska, W.; Burdiak, P. Light Acclimation, Retrograde Signalling, Cell Death and Immune Defences in Plants. Plant Cell Environ. 2013, 36, 736–744. [Google Scholar] [CrossRef]
- Mühlenbock, P.; Szechynska-Hebda, M.; Plaszczyca, M.; Baudo, M.; Mateo, A.; Mullineaux, P.M.; Parker, J.E.; Karpinska, B.; Karpinski, S. Chloroplast Signaling and LESION SIMULATING DISEASE1 Regulate Crosstalk between Light Acclimation and Immunity in Arabidopsis. Plant Cell 2008, 20, 2339–2356. [Google Scholar] [CrossRef]
- Mühlenbock, P.; Plaszczyca, M.; Plaszczyca, M.; Mellerowicz, E.; Karpinski, S. Lysigenous Aerenchyma Formation in Arabidopsis Is Controlled by LESION SIMULATING DISEASE1. Plant Cell 2007, 19, 3819–3830. [Google Scholar] [CrossRef]
- Wituszyńska, W.; Ślesak, I.; Vanderauwera, S.; Szechyńska-Hebda, M.; Kornaś, A.; Kelen, K.V.D.; Mühlenbock, P.; Karpińska, B.; Maćkowski, S.; Breusegem, F.V.; et al. LESION SIMULATING DISEASE1, ENHANCED DISEASE SUSCEPTIBILITY1, and PHYTOALEXIN DEFICIENT4 Conditionally Regulate Cellular Signaling Homeostasis, Photosynthesis, Water Use Efficiency, and Seed Yield in Arabidopsis. Plant Physiol. 2013, 161, 1795–1805. [Google Scholar] [CrossRef]
- Bernacki, M.J.; Czarnocka, W.; Rusaczonek, A.; Witoń, D.; Kęska, S.; Czyż, J.; Szechyńska-Hebda, M.; Karpiński, S. LSD1, EDS1 and PAD4-Dependent Conditional Correlation among Salicylic Acid, Hydrogen Peroxide, Water Use Efficiency, and Seed Yield in Arabidopsis Thaliana. Physiol. Plant. 2018. [Google Scholar] [CrossRef]
- Wituszynska, W.; Karpinski, S. Programmed Cell Death as a Response to High Light, UV and Drought Stress in Plants. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1024-8. [Google Scholar]
- Fuchs, Y.; Steller, H. Live to Die Another Way: Modes of Programmed Cell Death and the Signals Emanating from Dying Cells. Nat. Rev. Mol. Cell Biol. 2015, 16, 329–344. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Sychta, K.; Słomka, A.; Kuta, E. Insights into Plant Programmed Cell Death Induced by Heavy Metals-Discovering a Terra Incognita. Cells 2021, 10, 65. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or Foe? Reactive Oxygen Species Production, Scavenging and Signaling in Plant Response to Environmental Stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Woltering, E.J. Many Ways to Exit? Cell Death Categories in Plants. Trends Plant Sci. 2005, 10, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Dong, X. How Do Plants Achieve Immunity? Defence without Specialized Immune Cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Szechyńska-Hebda, M.; Kruk, J.; Górecka, M.; Karpińska, B.; Karpiński, S. Evidence for Light Wavelength-Specific Photoelectrophysiological Signaling and Memory of Excess Light Episodes in Arabidopsis. Plant Cell 2010, 22, 2201–2218. [Google Scholar] [CrossRef] [PubMed]
- Górecka, M.; Lewandowska, M.; Dąbrowska-Bronk, J.; Białasek, M.; Barczak-Brzyżek, A.; Kulasek, M.; Mielecki, J.; Kozłowska-Makulska, A.; Gawroński, P.; Karpiński, S. Photosystem II 22kDa Protein Level—A Prerequisite for Excess Light-Inducible Memory, Cross-Tolerance to UV-C and Regulation of Electrical Signalling. Plant Cell Environ. 2020, 43, 649–661. [Google Scholar] [CrossRef]
- Dietrich, R.A.; Richberg, M.H.; Schmidt, R.; Dean, C.; Dangl, J.L. A Novel Zinc Finger Protein Is Encoded by the Arabidopsis LSD1 Gene and Functions as a Negative Regulator of Plant Cell Death. Cell 1997, 88, 685–694. [Google Scholar] [CrossRef]
- Czarnocka, W.; Van Der Kelen, K.; Willems, P.; Szechyńska-Hebda, M.; Shahnejat-Bushehri, S.; Balazadeh, S.; Rusaczonek, A.; Mueller-Roeber, B.; Van Breusegem, F.; Karpiński, S. The Dual Role of LESION SIMULATING DISEASE 1 as a Condition-Dependent Scaffold Protein and Transcription Regulator. Plant Cell Environ. 2017, 40, 2644–2662. [Google Scholar] [CrossRef] [PubMed]
- Rustérucci, C.; Aviv, D.H.; Holt, B.F.; Dangl, J.L.; Parker, J.E. The Disease Resistance Signaling Components EDS1 and PAD4 Are Essential Regulators of the Cell Death Pathway Controlled by LSD1 in Arabidopsis. Plant Cell 2001, 13, 2211–2224. [Google Scholar] [CrossRef]
- Chai, T.; Zhou, J.; Liu, J.; Xing, D. LSD1 and HY5 Antagonistically Regulate Red Light Induced-Programmed Cell Death in Arabidopsis. Front Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Zhang, X.; Zuo, J.; Yang, S. The Arabidopsis LSD1 Gene Plays an Important Role in the Regulation of Low Temperature-Dependent Cell Death. New Phytol. 2010, 187, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Feys, B.J.; Moisan, L.J.; Newman, M.-A.; Parker, J.E. Direct Interaction between the Arabidopsis Disease Resistance Signaling Proteins, EDS1 and PAD4. EMBO J. 2001, 20, 5400–5411. [Google Scholar] [CrossRef]
- Shah, J. The Salicylic Acid Loop in Plant Defense. Curr. Opin. Plant Biol. 2003, 6, 365–371. [Google Scholar] [CrossRef]
- Wiermer, M.; Feys, B.J.; Parker, J.E. Plant Immunity: The EDS1 Regulatory Node. Curr. Opin. Plant Biol. 2005, 8, 383–389. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic Acid, a Multifaceted Hormone to Combat Disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate Synthase Is Required to Synthesize Salicylic Acid for Plant Defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Duan, C.; Yu, J.; Bai, J.; Zhu, Z.; Wang, X. Induced Defense Responses in Rice Plants against Small Brown Planthopper Infestation. Crop J. 2014, 2, 55–62. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front Plant Sci 2020, 11. [Google Scholar] [CrossRef]
- Abreu, M.E.; Munné-Bosch, S. Salicylic Acid Deficiency in NahG Transgenic Lines and Sid2 Mutants Increases Seed Yield in the Annual Plant Arabidopsis Thaliana. J. Exp. Bot. 2009, 60, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Wang, W.; Han, X.; Wu, J.; Lyu, B.; Chen, F.; Caplan, A.; Li, C.; Wu, J.; Wang, W.; et al. Isochorismate-Based Salicylic Acid Biosynthesis Confers Basal Resistance to Fusarium Graminearum in Barley. Mol. Plant Pathol. 2018. [Google Scholar] [CrossRef]
- Catinot, J.; Buchala, A.; Abou-Mansour, E.; Métraux, J.-P. Salicylic Acid Production in Response to Biotic and Abiotic Stress Depends on Isochorismate in Nicotiana Benthamiana. FEBS Lett. 2008, 582, 473–478. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; van der Drift, K.M.G.M.; Olsson, P.E.; Thomas-Oates, J.E.; van Loon, L.C.; Bakker, P.A.H.M. Analysis of the PmsCEAB Gene Cluster Involved in Biosynthesis of Salicylic Acid and the Siderophore Pseudomonine in the Biocontrol Strain Pseudomonas Fluorescens WCS374. J. Bacteriol. 2001, 183, 1909–1920. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.-K. PBS3 and EPS1 Complete Salicylic Acid Biosynthesis from Isochorismate in Arabidopsis. Mol. Plant 2019, 12, 1577–1586. [Google Scholar] [CrossRef]
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-Derived Biosynthesis of the Plant Stress Hormone Salicylic Acid. Science 2019, 365, 498–502. [Google Scholar] [CrossRef]
- Nawrath, C.; Métraux, J.P. Salicylic Acid Induction-Deficient Mutants of Arabidopsis Express PR-2 and PR-5 and Accumulate High Levels of Camalexin after Pathogen Inoculation. Plant Cell 1999, 11, 1393–1404. [Google Scholar] [CrossRef]
- Yokoo, S.; Inoue, S.; Suzuki, N.; Amakawa, N.; Matsui, H.; Nakagami, H.; Takahashi, A.; Arai, R.; Katou, S. Comparative Analysis of Plant Isochorismate Synthases Reveals Structural Mechanisms Underlying Their Distinct Biochemical Properties. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Garcion, C.; Lohmann, A.; Lamodière, E.; Catinot, J.; Buchala, A.; Doermann, P.; Métraux, J.-P. Characterization and Biological Function of the ISOCHORISMATE SYNTHASE2 Gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287. [Google Scholar] [CrossRef]
- Morse, A.M.; Tschaplinski, T.J.; Dervinis, C.; Pijut, P.M.; Schmelz, E.A.; Day, W.; Davis, J.M. Salicylate and Catechol Levels Are Maintained in NahG Transgenic Poplar. Phytochemistry 2007, 68, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Okamoto, H.; Okuma, E.; Shiba, H.; Kamada, H.; Hasegawa, P.M.; Murata, Y. SIZ1 Deficiency Causes Reduced Stomatal Aperture and Enhanced Drought Tolerance via Controlling Salicylic Acid-Induced Accumulation of Reactive Oxygen Species in Arabidopsis. Plant J. 2013, 73, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-Activated Protein Kinase 4 Is a Salicylic Acid-Independent Regulator of Growth but Not of Photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef]
- Devadas, S.K.; Raina, R. Preexisting Systemic Acquired Resistance Suppresses Hypersensitive Response-Associated Cell Death in Arabidopsishrl1 Mutant. Plant Physiol. 2002, 128, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Nawrath, C.; Heck, S.; Parinthawong, N.; Métraux, J.-P. EDS5, an Essential Component of Salicylic Acid–Dependent Signaling for Disease Resistance in Arabidopsis, Is a Member of the MATE Transporter Family. Plant Cell 2002, 14, 275–286. [Google Scholar] [CrossRef]
- Radojičić, A.; Li, X.; Zhang, Y. Salicylic Acid: A Double-Edged Sword for Programed Cell Death in Plants. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Wituszyńska, W.; Szechyńska-Hebda, M.; Sobczak, M.; Rusaczonek, A.; Kozłowska-Makulska, A.; Witoń, D.; Karpiński, S. Lesion Simulating Disease 1 and Enhanced Disease Susceptibility 1 Differentially Regulate UV-C-Induced Photooxidative Stress Signalling and Programmed Cell Death in Arabidopsis Thaliana. Plant Cell Environ. 2015, 38, 315–330. [Google Scholar] [CrossRef]
- Mateo, A.; Mühlenbock, P.; Rustérucci, C.; Chang, C.C.-C.; Miszalski, Z.; Karpinska, B.; Parker, J.E.; Mullineaux, P.M.; Karpinski, S. LESION SIMULATING DISEASE 1 Is Required for Acclimation to Conditions That Promote Excess Excitation Energy. Plant Physiol. 2004, 136, 2818–2830. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, L.; Vernooij, B.; Gaffney, T.; Morse, A.; Ryals, J. Characterization of Tobacco Plants Expressing a Bacterial Salicylate Hydroxylase Gene. Plant Mol. Biol. 1995, 29, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Witoń, D.; Gawroński, P.; Czarnocka, W.; Ślesak, I.; Rusaczonek, A.; Sujkowska-Rybkowska, M.; Bernacki, M.J.; Dąbrowska-Bronk, J.; Tomsia, N.; Szechyńska-Hebda, M.; et al. Mitogen Activated Protein Kinase 4 (MPK4) Influences Growth in Populus tremula L. × tremuloides. Environ. Exp. Bot. 2016, 130, 189–205. [Google Scholar] [CrossRef]
- Rusaczonek, A.; Czarnocka, W.; Kacprzak, S.; Witoń, D.; Ślesak, I.; Szechyńska-Hebda, M.; Gawroński, P.; Karpiński, S. Role of Phytochromes A and B in the Regulation of Cell Death and Acclimatory Responses to UV Stress in Arabidopsis Thaliana. J. Exp. Bot. 2015, erv375. [Google Scholar] [CrossRef]
- Bernacki, M.J.; Czarnocka, W.; Witoń, D.; Rusaczonek, A.; Szechyńska-Hebda, M.; Ślesak, I.; Dąbrowska-Bronk, J.; Karpiński, S. ENHANCED DISEASE SUSCEPTIBILITY 1 (EDS1) Affects Development, Photosynthesis, and Hormonal Homeostasis in Hybrid Aspen (Populus tremula L. × P. tremuloides). J. Plant Physiol. 2018, 226, 91–102. [Google Scholar] [CrossRef]
- Bhar, A.; Chatterjee, M.; Gupta, S.; Das, S. Salicylic Acid Regulates Systemic Defense Signaling in Chickpea During Fusarium Oxysporum f. Sp. Ciceri Race 1 Infection. Plant Mol. Biol. Rep. 2018, 36, 162–175. [Google Scholar] [CrossRef]
- Delaney, T.P.; Uknes, S.; Vernooij, B.; Friedrich, L.; Weymann, K.; Negrotto, D.; Gaffney, T.; Gut-Rella, M.; Kessmann, H.; Ward, E.; et al. A Central Role of Salicylic Acid in Plant Disease Resistance. Science 1994, 266, 1247–1250. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Pennell, R.I.; Meijer, P.J.; Ishikawa, A.; Dixon, R.A.; Lamb, C. Reactive Oxygen Intermediates Mediate a Systemic Signal Network in the Establishment of Plant Immunity. Cell 1998, 92, 773–784. [Google Scholar] [CrossRef]
- Czarnocka, W.; Fichman, Y.; Bernacki, M.; Różańska, E.; Sańko-Sawczenko, I.; Mittler, R.; Karpiński, S. FMO1 Is Involved in Excess Light Stress-Induced Signal Transduction and Cell Death Signaling. Cells 2020, 9, 2163. [Google Scholar] [CrossRef]
- Morris, K.; Mackerness, S.A.H.; Page, T.; John, C.F.; Murphy, A.M.; Carr, J.P.; Buchanan-Wollaston, V. Salicylic Acid Has a Role in Regulating Gene Expression during Leaf Senescence. Plant J. 2000, 23, 677–685. [Google Scholar] [CrossRef]
- Durner, J.; Klessig, D.F. Inhibition of Ascorbate Peroxidase by Salicylic Acid and 2,6-Dichloroisonicotinic Acid, Two Inducers of Plant Defense Responses. Proc. Natl. Acad. Sci. USA 1995, 92, 11312–11316. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.E. Salicylic acid in the machinery of hypersensitive cell death and disease resistance. In Programmed Cell Death in Higher Plants; Lam, E., Fukuda, H., Greenberg, J., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 185–198. ISBN 978-94-010-0934-8. [Google Scholar]
- Mateo, A.; Funck, D.; Mühlenbock, P.; Kular, B.; Mullineaux, P.M.; Karpinski, S. Controlled Levels of Salicylic Acid Are Required for Optimal Photosynthesis and Redox Homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef]
- Karpinski, S.; Wingsle, G.; Karpinska, B.; Hällgren, J.-E. Redox Sensing of Photooxidative Stress and Acclimatory Mechanisms in Plants. In Regulation of Photosynthesis; Aro, E.-M., Andersson, B., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2001; pp. 469–486. ISBN 978-0-306-48148-2. [Google Scholar]
- Aviv, D.H.; Rustérucci, C.; Iii, B.F.H.; Dietrich, R.A.; Parker, J.E.; Dangl, J.L. Runaway Cell Death, but Not Basal Disease Resistance, in Lsd1 Is SA- and NIM1/NPR1-Dependent. Plant J. 2002, 29, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Kalbina, I.; Li, S.; Kalbin, G.; Björn, L.O.; Strid, Å. Two Separate UV-B Radiation Wavelength Regions Control Expression of Different Molecular Markers in Arabidopsis Thaliana. Funct. Plant Biol. 2008, 35, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Britt, A.B. Repair of DNA Damage Induced by Solar UV. Photosynth. Res. 2004, 81, 105–112. [Google Scholar] [CrossRef]
- Rusaczonek, A.; Czarnocka, W.; Willems, P.; Sujkowska-Rybkowska, M.; Van Breusegem, F.; Karpiński, S. Phototropin 1 and 2 Influence Photosynthesis, UV-C Induced Photooxidative Stress Responses, and Cell Death. Cells 2021, 10, 200. [Google Scholar] [CrossRef]
- Nawkar, G.M.; Maibam, P.; Park, J.H.; Sahi, V.P.; Lee, S.Y.; Kang, C.H. UV-Induced Cell Death in Plants. Int. J. Mol. Sci. 2013, 14, 1608–1628. [Google Scholar] [CrossRef]
- Dotto, M.; Casati, P. Developmental Reprogramming by UV-B Radiation in Plants. Plant Sci. 2017, 264, 96–101. [Google Scholar] [CrossRef]
- Morel, J.-B.; Dangl, J.L. The Hypersensitive Response and the Induction of Cell Death in Plants. Cell Death Differ. 1997, 4, 671–683. [Google Scholar] [CrossRef]
- Cui, H.; Gobbato, E.; Kracher, B.; Qiu, J.; Bautor, J.; Parker, J.E. A Core Function of EDS1 with PAD4 Is to Protect the Salicylic Acid Defense Sector in Arabidopsis Immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef]
- Falk, A.; Feys, B.J.; Frost, L.N.; Jones, J.D.G.; Daniels, M.J.; Parker, J.E. EDS1, an Essential Component of R Gene-Mediated Disease Resistance in Arabidopsis Has Homology to Eukaryotic Lipases. Proc. Natl. Acad. Sci. USA 1999, 96, 3292–3297. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Jia, Z.; Tian, S.; Wang, X.; Gou, Z.; Lü, B.; Dong, H. AtMYB44 Positively Modulates Disease Resistance to Pseudomonas Syringae through the Salicylic Acid Signalling Pathway in Arabidopsis. Funct. Plant Biol. 2013, 40, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Bernacki, M.J.; Czarnocka, W.; Zaborowska, M.; Różańska, E.; Labudda, M.; Rusaczonek, A.; Witoń, D.; Karpiński, S. EDS1-Dependent Cell Death and the Antioxidant System in Arabidopsis Leaves Is Deregulated by the Mammalian Bax. Cells 2020, 9, 2454. [Google Scholar] [CrossRef]
- Reuber, T.L.; Plotnikova, J.M.; Dewdney, J.; Rogers, E.E.; Wood, W.; Ausubel, F.M. Correlation of Defense Gene Induction Defects with Powdery Mildew Susceptibility in Arabidopsis Enhanced Disease Susceptibility Mutants. Plant J. 1998, 16, 473–485. [Google Scholar] [CrossRef]
- Thibaud, M.-C.; Gineste, S.; Nussaume, L.; Robaglia, C. Sucrose Increases Pathogenesis-Related PR-2 Gene Expression in Arabidopsis Thaliana through an SA-Dependent but NPR1-Independent Signaling Pathway. Plant Physiol. Biochem. 2004, 42, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Mullineaux, P.M.; Rausch, T. Glutathione, Photosynthesis and the Redox Regulation of Stress-Responsive Gene Expression. Photosynth Res. 2005, 86, 459–474. [Google Scholar] [CrossRef]
- Feys, B.J.; Wiermer, M.; Bhat, R.A.; Moisan, L.J.; Medina-Escobar, N.; Neu, C.; Cabral, A.; Parker, J.E. Arabidopsis SENESCENCE-ASSOCIATED GENE101 Stabilizes and Signals within an ENHANCED DISEASE SUSCEPTIBILITY1 Complex in Plant Innate Immunity. Plant Cell 2005, 17, 2601–2613. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M.; Sweetlove, L.J. ROS Signalling—Specificity Is Required. Trends Plant Sci. 2010, 15, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Mittler, R. The Roles of Reactive Oxygen Species in Plant Cells. Plant Physiol. 2006, 141, 311. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Pathogen-Induced, NADPH Oxidase–Derived Reactive Oxygen Intermediates Suppress Spread of Cell Death in Arabidopsis Thaliana. Nat. Genet. 2005, 37, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Apel, K. Singlet Oxygen-Mediated Signaling in Plants: Moving from Flu to Wild Type Reveals an Increasing Complexity. Photosynth Res. 2013, 116, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid. Redox. Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Elazar, Z. Regulation of Autophagy by ROS: Physiology and Pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).