Aromatic-Turmerone Analogs Protect Dopaminergic Neurons in Midbrain Slice Cultures through Their Neuroprotective Activities
Abstract
1. Introduction
2. Materials and Methods
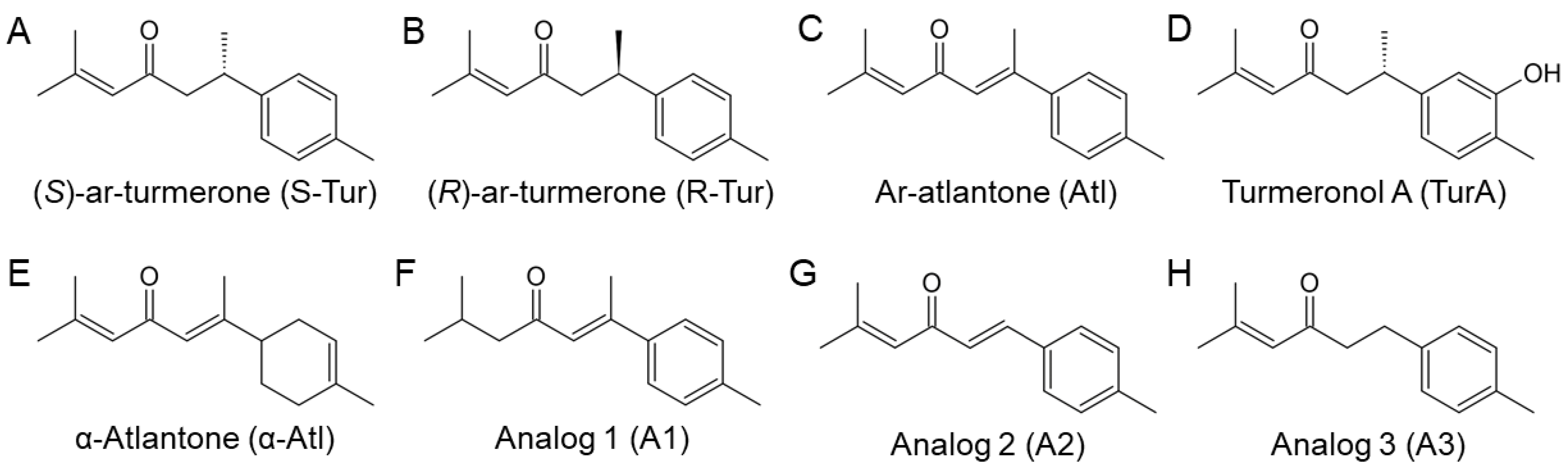
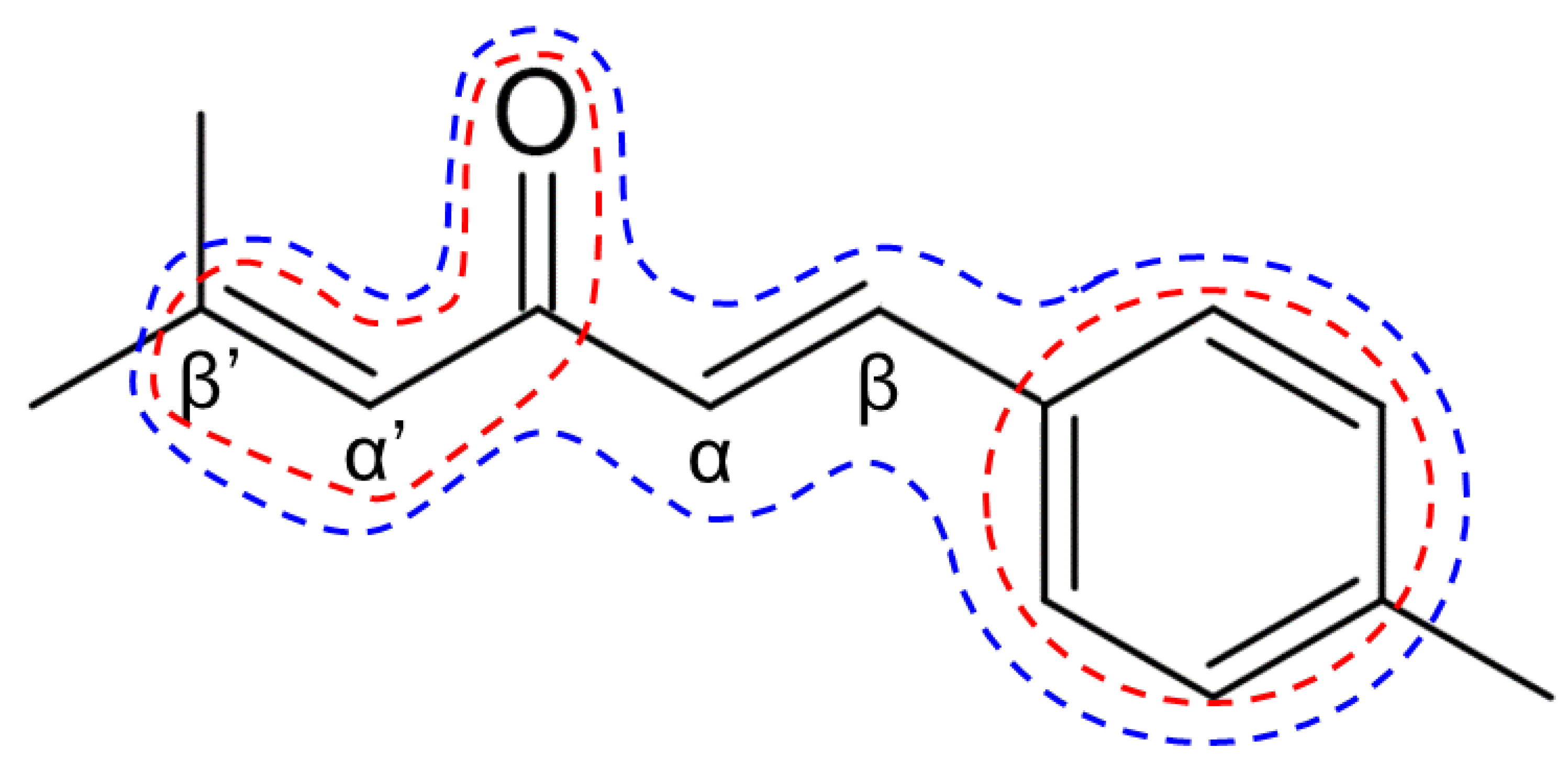
2.1. Chemical Synthesis of Ar-Turmerone Analogs
2.2. Cultivation of Cells and Slices and Drug Treatment
2.3. Isolation of RNA and Reverse Transcription–Quantitative Polymerase Chain Reaction
2.4. Immunohistochemistry
2.5. Nitrite Quantification
2.6. Immunoblotting
2.7. Statistical Analyses
3. Results
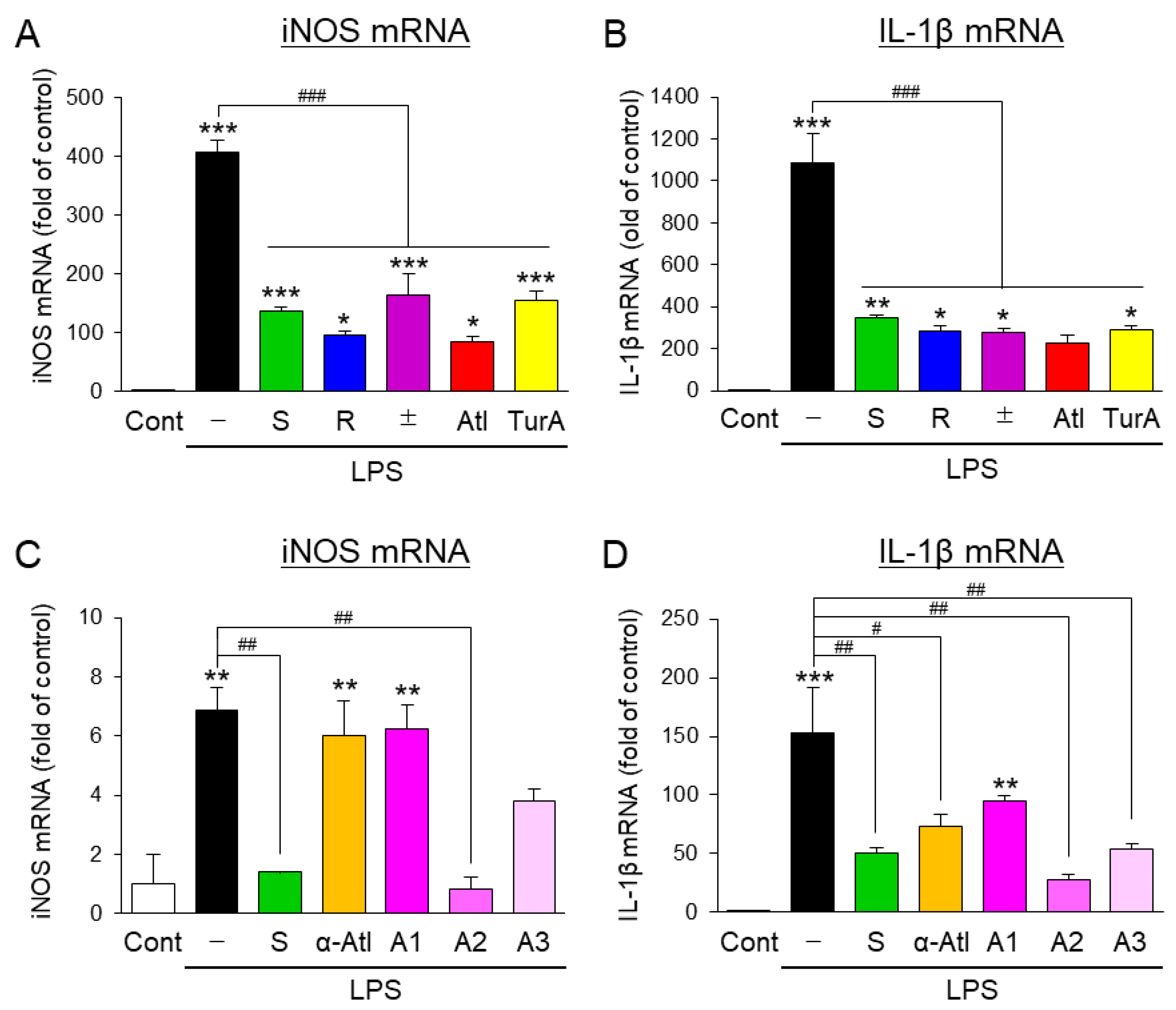
3.1. Effects of Aromatic-Turmerone Analogs on the Inflammatory Activation of Microglial BV2 Cells
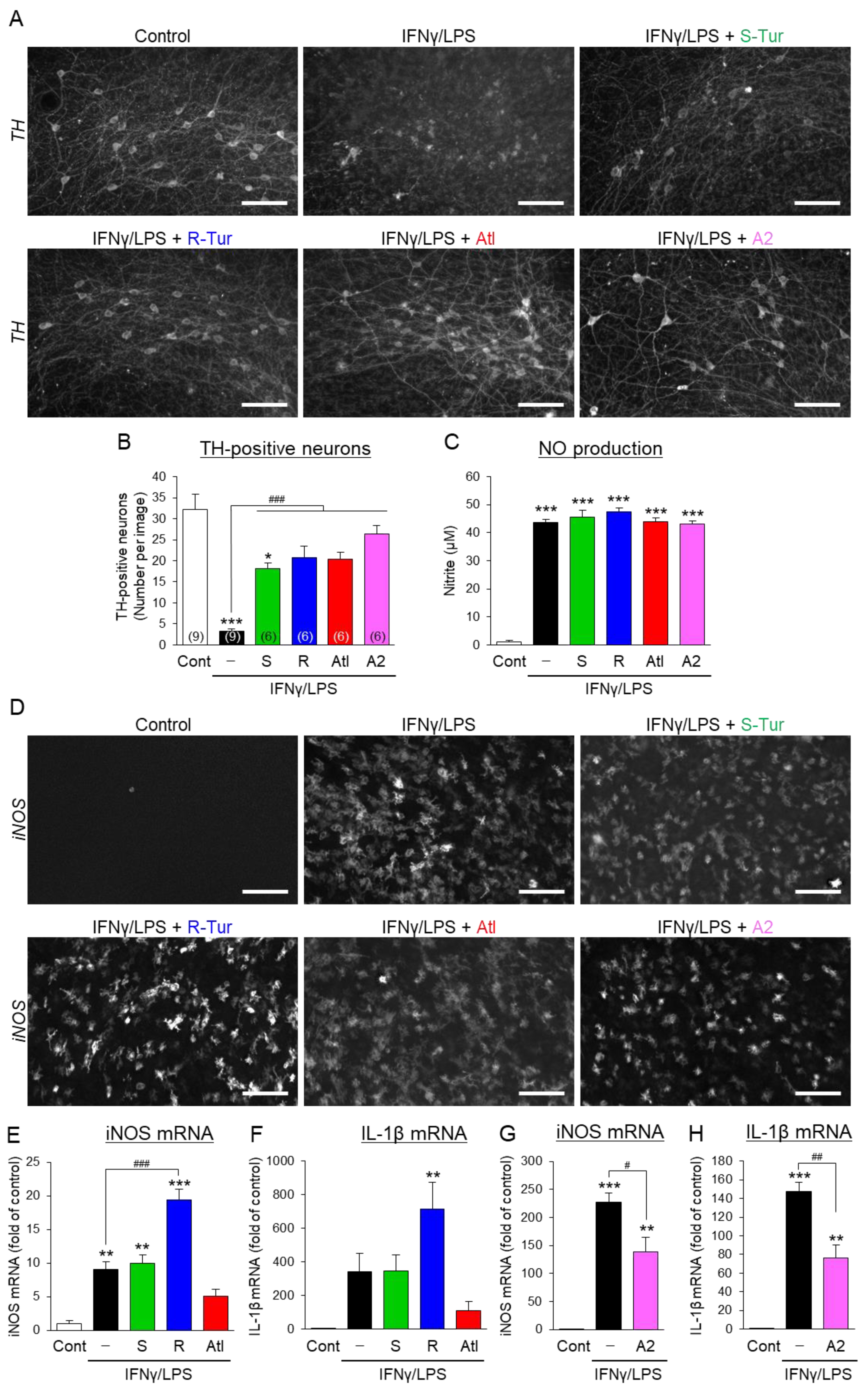
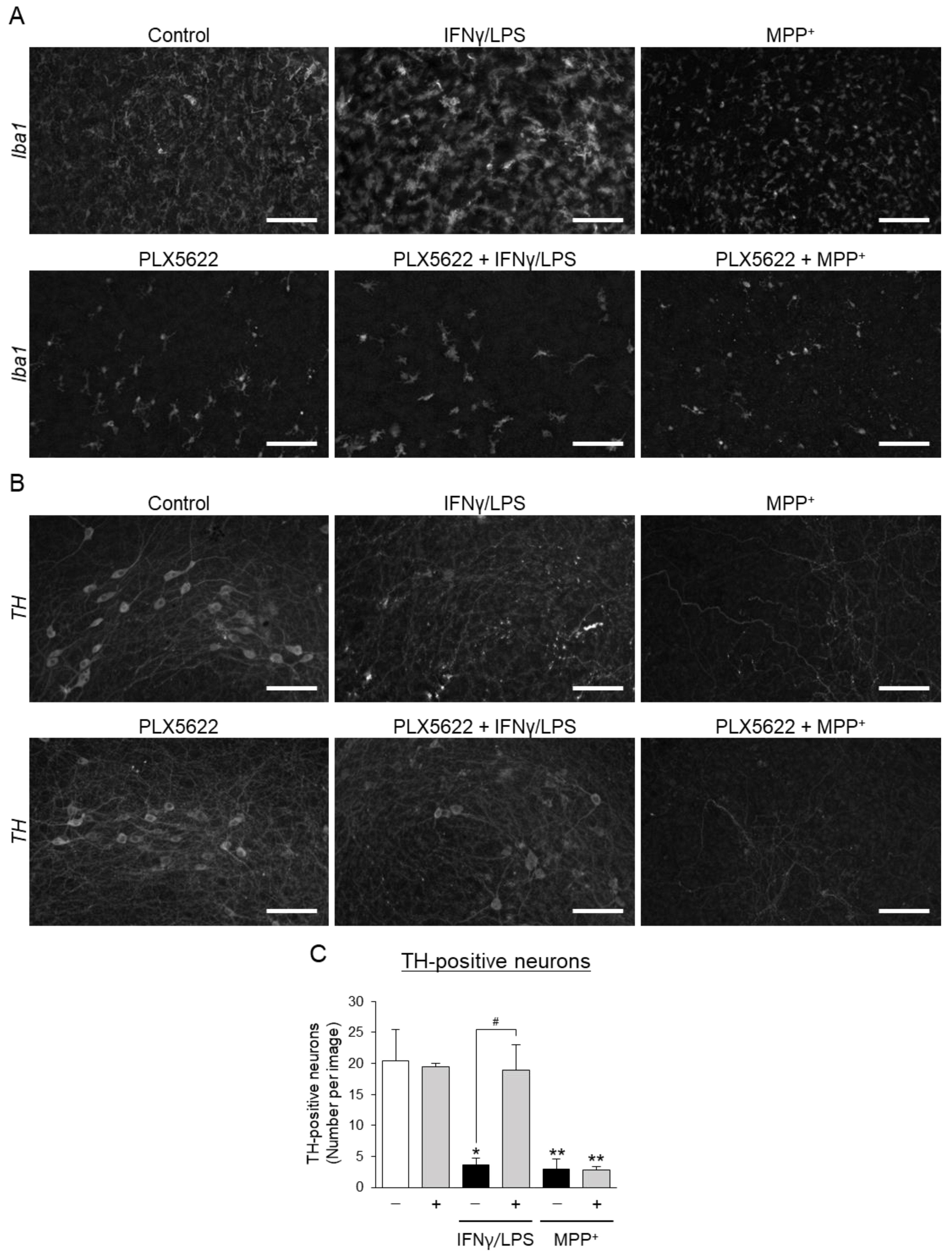
3.2. Effects of Aromatic-Turmerone Analogs on Dopaminergic Neurodegeneration Triggered by Microglial Activation in Midbrain Slice Cultures
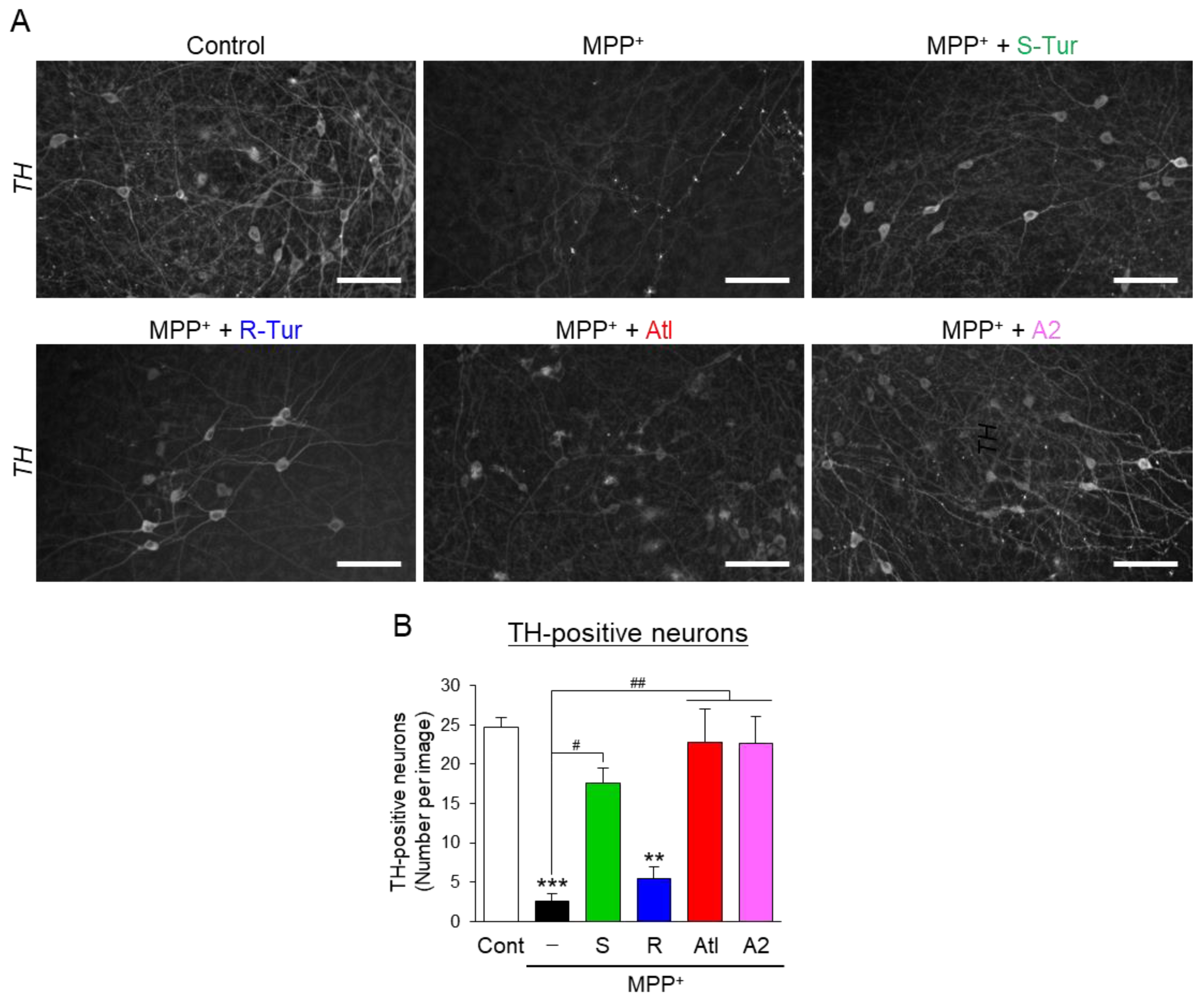
3.3. Effects of Aromatic-Turmerone Analogs on Dopaminergic Neurodegeneration Triggered by 1-Methyl-4-Phenylpyridinium in Midbrain Slice Cultures
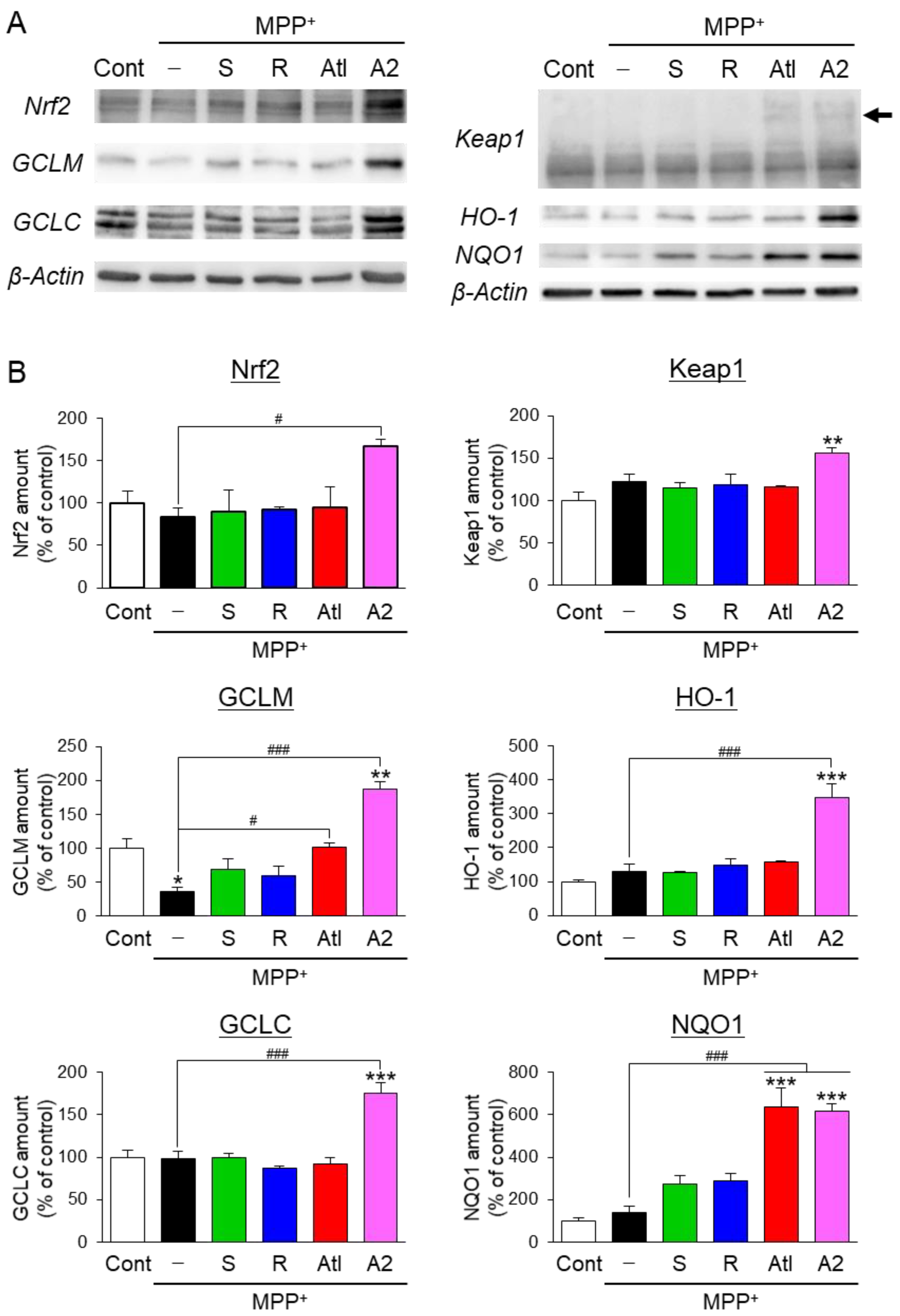
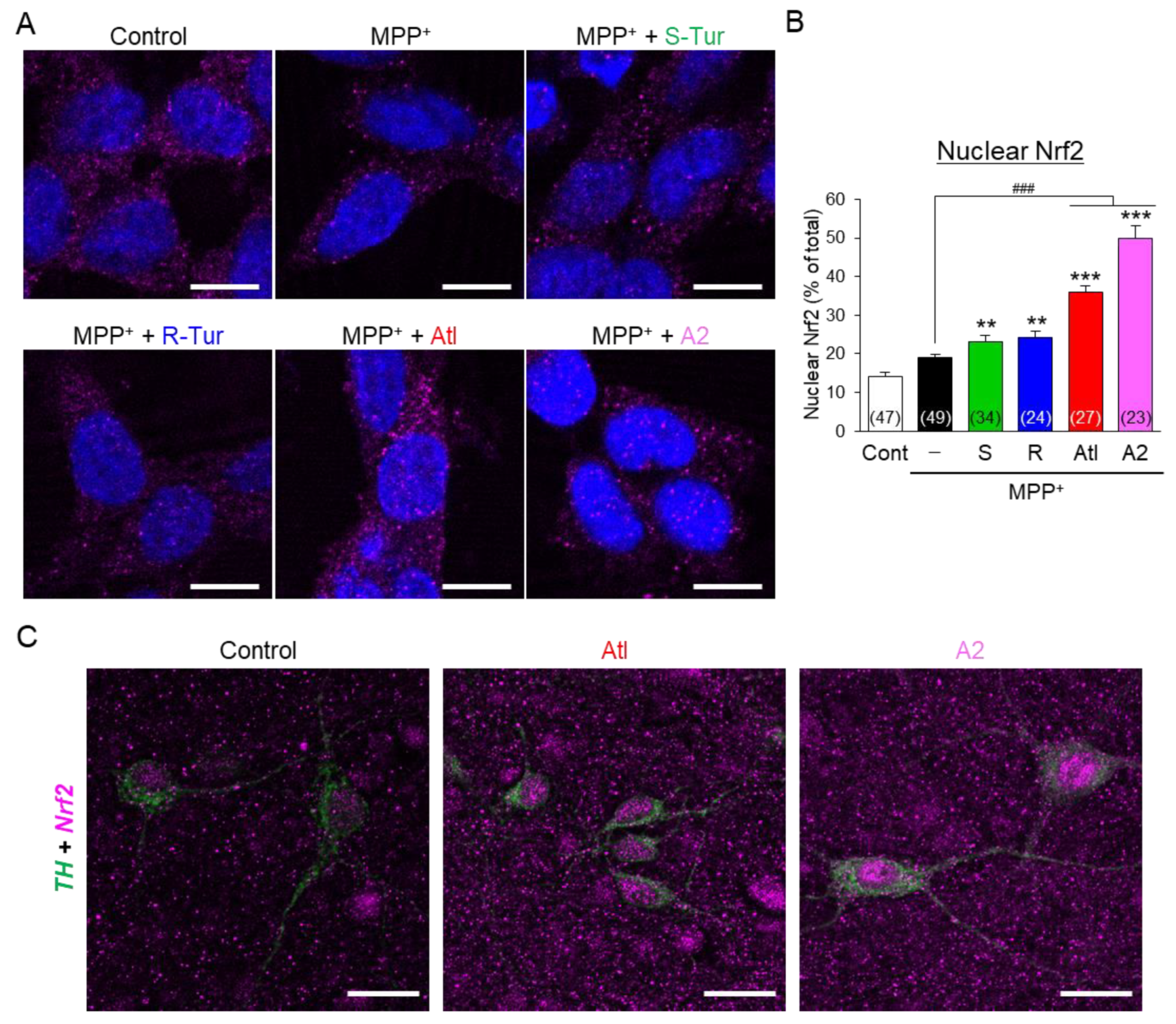
3.4. Effects of Aromatic-Turmerone Analogs on Anti-Oxidative Responses in Dopaminergic Neuronal Precursor Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahn, S.; Sulzer, D. Neurodegeneration and Neuroprotection in Parkinson Disease. NeuroRx 2004, 1, 139–154. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Thomas, B.; Flint Beal, M. Parkinson’s disease. Hum. Mol. Genet. 2007, 16, 183–194. [Google Scholar] [CrossRef]
- Collins, L.M.; Toulouse, A.; Connor, T.J.; Nolan, Y.M. Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology 2012, 62, 2153–2167. [Google Scholar] [CrossRef] [PubMed]
- Doorn, K.J.; Moors, T.; Drukarch, B.; Berg, W.; Lucassen, P.J.; Dam, A.M. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson inverted question marks disease patients. Acta Neuropathol. Commun. 2014, 2, 1–17. [Google Scholar]
- Kurauchi, Y.; Hisatsune, A.; Isohama, Y.; Mishima, S.; Katsuki, H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving haem oxygenase-1 and brain-derived neurotrophic factor. Br. J. Pharmacol. 2012, 166, 1151–1168. [Google Scholar] [CrossRef]
- Shibata, H.; Katsuki, H.; Nishiwaki, M.; Kume, T.; Kaneko, S.; Akaike, A. Lipopolysaccharide-induced dopaminergic cell death in rat midbrain slice cultures: Role of inducible nitric oxide synthase and protection by indomethacin. J. Neurochem. 2003, 86, 1201–1212. [Google Scholar] [CrossRef]
- Shibata, H.; Katsuki, H.; Okawara, M.; Kume, T.; Akaike, A. C-Jun N-terminal kinase inhibition and α-tocopherol protect midbrain dopaminergic neurons from interferon-γ/lipopolysaccharide-induced injury without affecting nitric oxide production. J. Neurosci. Res. 2006, 83, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rupe, H.; Clar, G.; St. Pfau, A.; Plattner, P. Zur Kenntnis der flüchtigen Pflanzenstoffe II. Über Turmeron, den Riechstoff des Curcumaöls. Helv. Chim. Acta 1934, 17, 372–389. [Google Scholar] [CrossRef]
- Roth, G.N.; Chandra, A.; Nair, M.G. Novel bioactivities of Curcuma longa constituents. J. Nat. Prod. 1998, 61, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Choi, J.; Lee, J.; Lee, Y. Induction of apoptosis by ar-turmerone on various cell lines. Int. J. Mol. Med. 2004, 14, 253–256. [Google Scholar] [CrossRef]
- Kim, D.; Suh, Y.; Lee, H.; Lee, Y. Immune activation and antitumor response of ar-turmerone on P388D1 lymphoblast cell implanted tumors. Int. J. Mol. Med. 2013, 31, 386–392. [Google Scholar] [CrossRef]
- Li, Y.L.; Du, Z.Y.; Li, P.H.; Yan, L.; Zhou, W.; Tang, Y.D.; Liu, G.R.; Fang, Y.X.; Zhang, K.; Dong, C.Z.; et al. Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 2018, 64, 319–325. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone’s anti-inflammatory effects in microglial cells are mediated by protein kinase A and heme oxygenase-1 signaling. Neurochem. Int. 2012, 61, 767–777. [Google Scholar] [CrossRef]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Anti-inflammatory effects of aromatic-turmerone through blocking of NF-κB, JNK, and p38 MAPK signaling pathways in amyloid β-stimulated microglia. Int. Immunopharmacol. 2012, 14, 13–20. [Google Scholar] [CrossRef]
- Chen, M.; Chang, Y.Y.; Huang, S.; Xiao, L.H.; Zhou, W.; Zhang, L.Y.; Li, C.; Zhou, R.P.; Tang, J.; Lin, L.; et al. Aromatic-Turmerone Attenuates LPS-Induced Neuroinflammation and Consequent Memory Impairment by Targeting TLR4-Dependent Signaling Pathway. Mol. Nutr. Food Res. 2018, 62, 1–9. [Google Scholar] [CrossRef]
- Hucklenbroich, J.; Klein, R.; Neumaier, B.; Graf, R.; Fink, G.R.; Schroeter, M.; Rueger, M.A. Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Res. Ther. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Honwad, V.K.; Rao, A.S. Terpenoids-LX. Absolute configuration of ar-turmerone. Tetrahedron 1964, 20, 2921–2925. [Google Scholar] [CrossRef]
- Sugiura, M.; Ashikari, Y.; Nakajima, M. One-Pot Synthesis of β,β-Disubstituted α,β-Unsaturated Carbonyl Compounds. J. Org. Chem. 2015, 80, 8830–8835. [Google Scholar] [CrossRef]
- Sugiura, M.; Ashikari, Y.; Takahashi, Y.; Yamaguchi, K.; Kotani, S.; Nakajima, M. Lewis Base-Catalyzed Enantioselective Conjugate Reduction of β,β-Disubstituted α,β-Unsaturated Ketones with Trichlorosilane: E/ Z-Isomerization, Regioselectivity, and Synthetic Applications. J. Org. Chem. 2019, 84, 11458–11473. [Google Scholar] [CrossRef]
- Kohler, E.P.; Chadwell, H.M. Benzalacetophenone. Org. Synth. 1922, 2, 1. [Google Scholar] [CrossRef]
- Takahashi, S.; Hisatsune, A.; Kurauchi, Y.; Seki, T.; Katsuki, H. Polysulfide protects midbrain dopaminergic neurons from MPP+-induced degeneration via enhancement of glutathione biosynthesis. J. Pharmacol. Sci. 2018, 137, 47–54. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Hori, Y.; Seki, T.; Kurauchi, Y.; Sato, M.; Oshima, M.; Hisatsune, A.; Katsuki, H. Involvement of exosomes in dopaminergic neurodegeneration by microglial activation in midbrain slice cultures. Biochem. Biophys. Res. Commun. 2019, 511, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Lotharius, J.; Barg, S.; Wiekop, P.; Lundberg, C.; Raymon, H.K.; Brundin, P. Effect of mutant α-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J. Biol. Chem. 2002, 277, 38884–38894. [Google Scholar] [CrossRef]
- Takaoka, Y.; Takahashi, M.; Kurauchi, Y.; Hisatsune, A.; Seki, T.; Shudo, K.; Katsuki, H. Retinoic acid receptor agonist Am80 inhibits CXCL2 production from microglial BV-2 cells via attenuation of NF-κB signaling. Int. Immunopharmacol. 2016, 38, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Sato, M.; Kibe, Y.; Ohta, T.; Oshima, M.; Konno, A.; Hirai, H.; Kurauchi, Y.; Hisatsune, A.; Katsuki, H. Lysosomal dysfunction and early glial activation are involved in the pathogenesis of spinocerebellar ataxia type 21 caused by mutant transmembrane protein 240. Neurobiol. Dis. 2018, 120, 34–50. [Google Scholar] [CrossRef]
- Seki, T.; Adachi, N.; Ono, Y.; Mochizuki, H.; Hiramoto, K.; Amano, T.; Matsubayashi, H.; Matsumoto, M.; Kawakami, H.; Saito, N.; et al. Mutant protein kinase Cγ found in spinocerebellar ataxia type 14 is susceptible to aggregation and causes cell death. J. Biol. Chem. 2005, 280, 29096–29106. [Google Scholar] [CrossRef]
- Peng, Z.; Luchtman, D.W.; Wang, X.; Zhang, Y.; Song, C. Activation of microglia synergistically enhances neurodegeneration caused by MPP+ in human SH-SY5Y cells. Eur. J. Pharmacol. 2019, 850, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Hwang, I.; Park, S.; Hong, S.; Hwang, B.; Cho, Y.; Son, J.; Yu, J.W. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 2019, 26, 213–228. [Google Scholar] [CrossRef]
- Liu, Y.; Given, K.S.; Dickson, E.L.; Owens, G.P.; Macklin, W.B.; Bennett, J.L. Concentration-dependent effects of CSF1R inhibitors on oligodendrocyte progenitor cells ex vivo and in vivo. Exp. Neurol. 2019, 318, 32–41. [Google Scholar] [CrossRef]
- Abed, D.A.; Goldstein, M.; Albanyan, H.; Jin, H.; Hu, L. Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents. Acta Pharm. Sin. B 2015, 5, 285–299. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Kiyofuji, K.; Kurauchi, Y.; Hisatsune, A.; Seki, T.; Mishima, S.; Katsuki, H. A natural compound macelignan protects midbrain dopaminergic neurons from inflammatory degeneration via microglial arginase-1 expression. Eur. J. Pharmacol. 2015, 760, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Zhang, X.; Dong, M. Puerarin suppresses MPP+/MPTP-induced oxidative stress through an Nrf2-dependent mechanism. Food Chem. Toxicol. 2020, 144, 111644. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Shih, C.T.; Ching, C.H.; Huang, J.Y.; Jen, C.J.; Yu, L.; Kuo, Y.M.; Wu, F.S.; Chuang, J.I. Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP+ toxicity. Exp. Neurol. 2015, 263, 50–62. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V.; Canning, P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017, 617, 84–93. [Google Scholar] [CrossRef]
- Hong, F.; Sekhar, K.E.; Freeman, M.L.; Liebler, D.C. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J. Biol. Chem. 2005, 280, 31768–31775. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, D.; Li, R.; Xu, J.; Gu, Q.; Xu, J. Discovery of new inhibitors against both NF-κB and osteoclastogenesis from in-house library with α, β-unsaturated-enone fragment. Bioorg. Chem. 2019, 87, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Furuno, A.; Watari, K.; Mamiyo, N.; Yuichi, F.; Jung, J.H.; Ono, M. A natural anti-inflammatory enone fatty acid inhibits angiogenesis by attenuating nuclear factor-κB signaling in vascular endothelial cells. Int. J. Oncol. 2011, 38, 493–501. [Google Scholar] [CrossRef]
- Boyd, G.V. Synthetic uses of enones. In The Chemistry of Enones (1989), Part 1; John Wiley & Sons, Inc.: Chichester, UK, 1989; pp. 281–315. [Google Scholar]
- Duval, D.; Géribaldi, S. Nucleophilic attacks on enones. In The Cheistry of Enones (1989), Part 1; John Wiley & Sons, Inc.: Chichester, UK, 1989; pp. 355–469. [Google Scholar]
- Deck, L.M.; Hunsaker, L.A.; Vander Jagt, T.A.; Whalen, L.J.; Royer, R.E.; Vander Jagt, D.L. Activation of anti-oxidant Nrf2 signaling by enone analogues of curcumin. Eur. J. Med. Chem. 2018, 143, 854–865. [Google Scholar] [CrossRef]
- Bernasconi, C.F. Nucleophilic addition to olefins. Kinetics and mechanism. Tetrahedron 1989, 45, 4017–4090. [Google Scholar] [CrossRef]
- Nguyen, T.S.; Yang, M.S.; May, J.A. Experimental mechanistic insight into the BINOL-catalyzed enantioselective conjugate addition of boronates to enones. Tetrahedron Lett. 2015, 56, 3337–3341. [Google Scholar] [CrossRef]
- Saga, Y.; Hatakenaka, Y.; Matsumoto, M.; Yoshioka, Y.; Matsumura, S.; Zaima, N.; Konishi, Y. Neuroprotective effects of aromatic turmerone on activity deprivation-induced apoptosis in cerebellar granule neurons. Neuroreport 2020, 31, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.C.; Tsai, J.C.; Liu, C.Y.; Huang, H.C.; Wu, L.Y.; Peng, W.H. Antidepressant-like activity of turmerone in behavioral despair tests in mice. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| iNOS (mouse, rat) | TGCTTTGTGCGAAGTGTCAGT | CGGACCATCTCCTGCATTTCT |
| IL-1β (mouse) | TGAAGGGCTGCTTCCAAACC | TGTCCATTGAGGTGGAGAG |
| IL-1β (rat) | AAAGAAGAAGATGGAAAAGCGGTT | GGAACTGTGCAGACTCAAACTC |
| IL-10 (rat) | AAAGCAAGGCAGTGGAGCAG | TCAAACTCATTCATGGCCTTGT |
| Arginase-1 (rat) | CCAGTATTCACCCCGGCTAC | ACAAGACAAGGTCAACGCCA |
| GAPDH (mouse, rat) | ACCATCTTCCAGGAGCGAGA | CAGTCTTCTGGGTGGCAGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hori, Y.; Tsutsumi, R.; Nasu, K.; Boateng, A.; Ashikari, Y.; Sugiura, M.; Nakajima, M.; Kurauchi, Y.; Hisatsune, A.; Katsuki, H.; et al. Aromatic-Turmerone Analogs Protect Dopaminergic Neurons in Midbrain Slice Cultures through Their Neuroprotective Activities. Cells 2021, 10, 1090. https://doi.org/10.3390/cells10051090
Hori Y, Tsutsumi R, Nasu K, Boateng A, Ashikari Y, Sugiura M, Nakajima M, Kurauchi Y, Hisatsune A, Katsuki H, et al. Aromatic-Turmerone Analogs Protect Dopaminergic Neurons in Midbrain Slice Cultures through Their Neuroprotective Activities. Cells. 2021; 10(5):1090. https://doi.org/10.3390/cells10051090
Chicago/Turabian StyleHori, Yuria, Reiho Tsutsumi, Kento Nasu, Alex Boateng, Yasuhiko Ashikari, Masaharu Sugiura, Makoto Nakajima, Yuki Kurauchi, Akinori Hisatsune, Hiroshi Katsuki, and et al. 2021. "Aromatic-Turmerone Analogs Protect Dopaminergic Neurons in Midbrain Slice Cultures through Their Neuroprotective Activities" Cells 10, no. 5: 1090. https://doi.org/10.3390/cells10051090
APA StyleHori, Y., Tsutsumi, R., Nasu, K., Boateng, A., Ashikari, Y., Sugiura, M., Nakajima, M., Kurauchi, Y., Hisatsune, A., Katsuki, H., & Seki, T. (2021). Aromatic-Turmerone Analogs Protect Dopaminergic Neurons in Midbrain Slice Cultures through Their Neuroprotective Activities. Cells, 10(5), 1090. https://doi.org/10.3390/cells10051090







