Endothelial and Vascular Health: A Tale of Honey, H2O2 and Calcium
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Cell Culture and Reagents
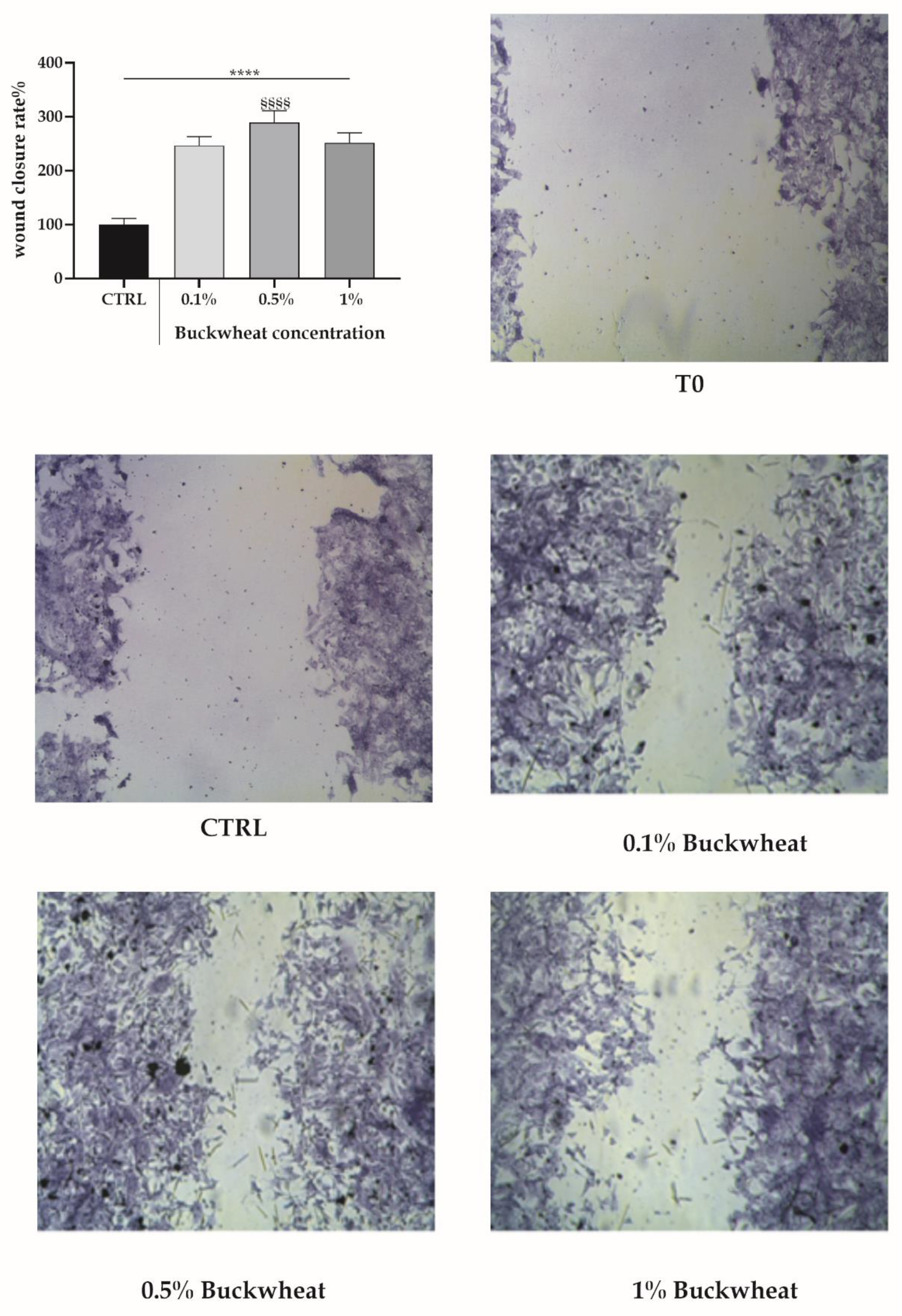
2.3. Scratch Wound Test
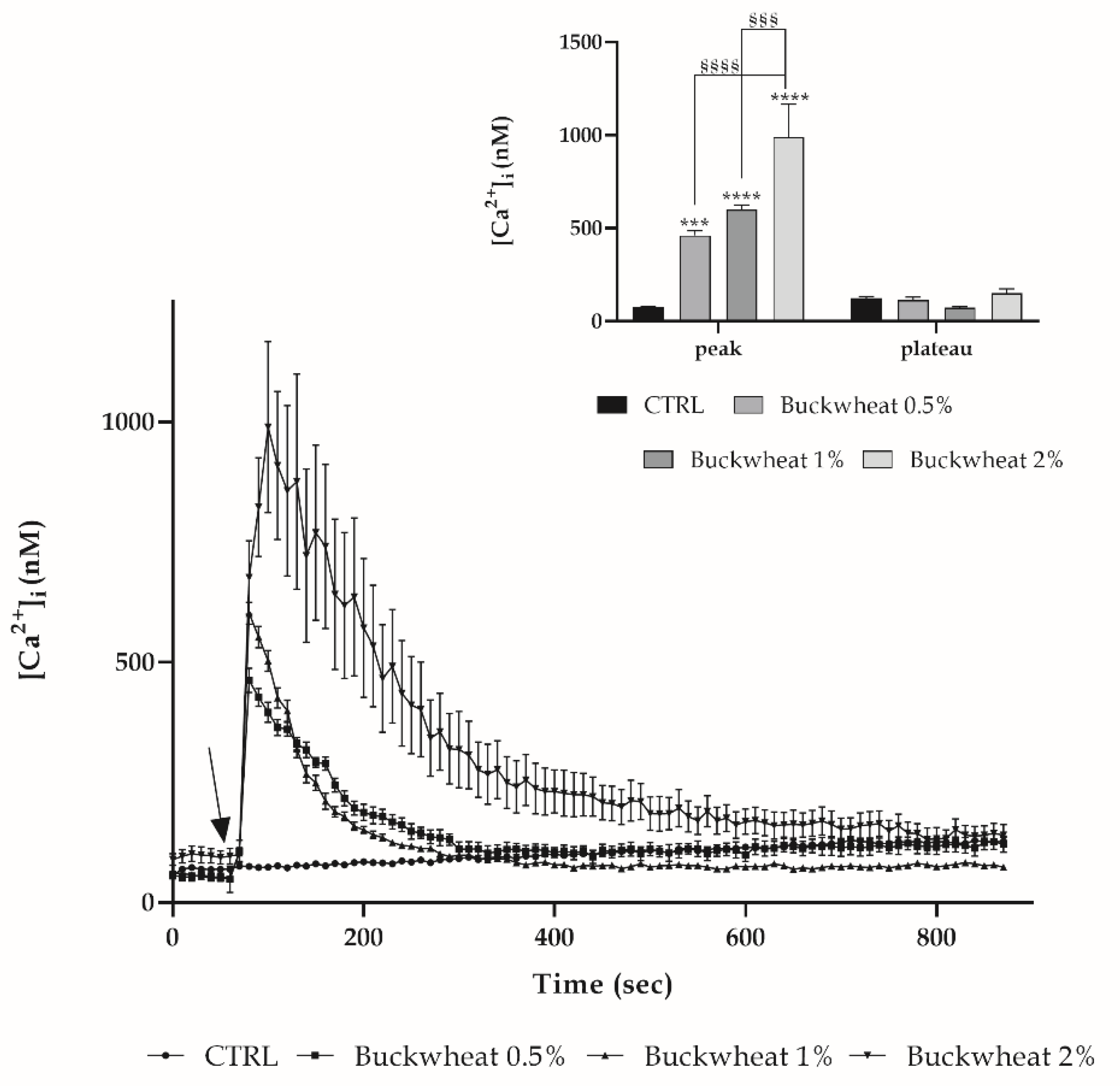
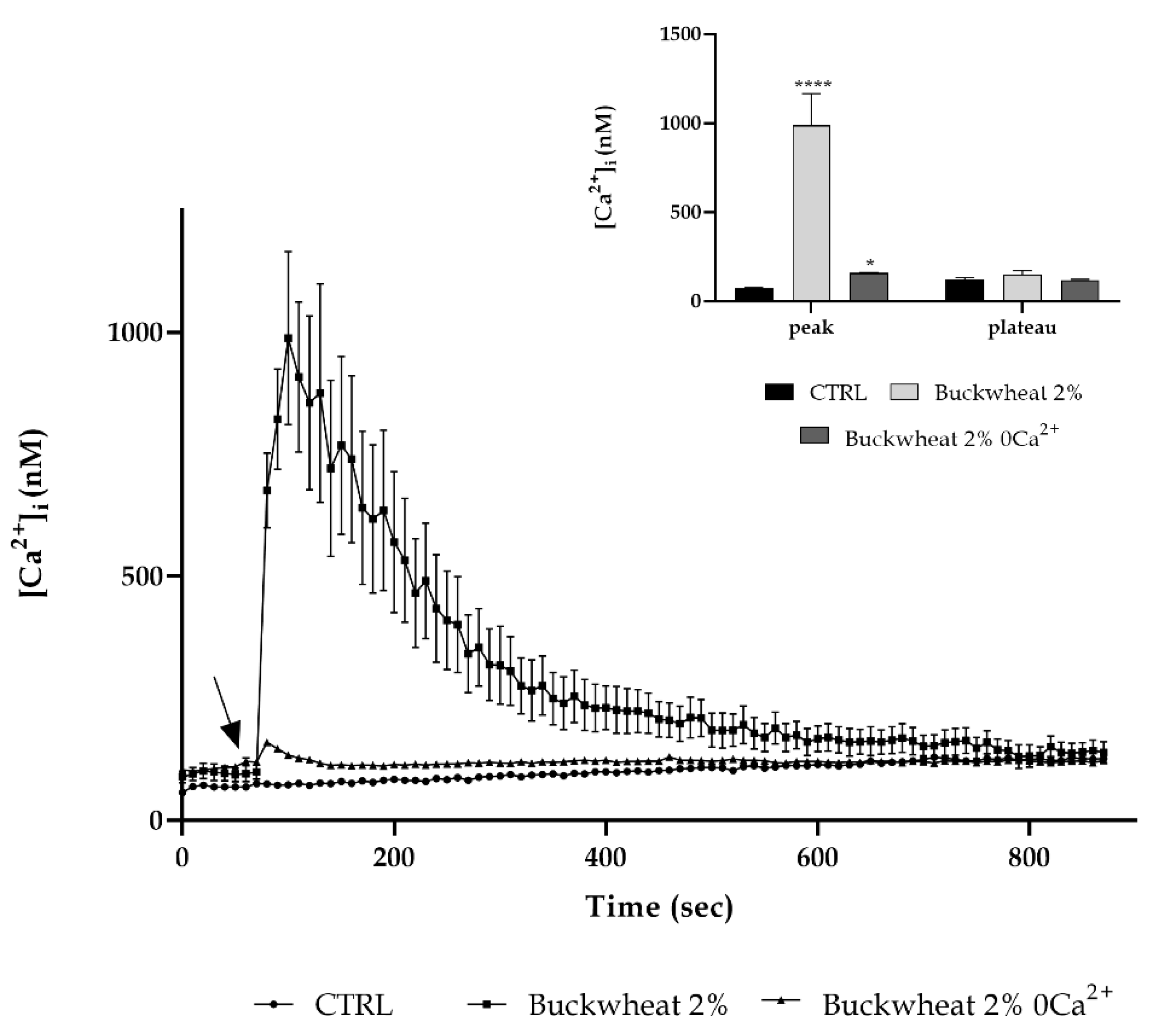
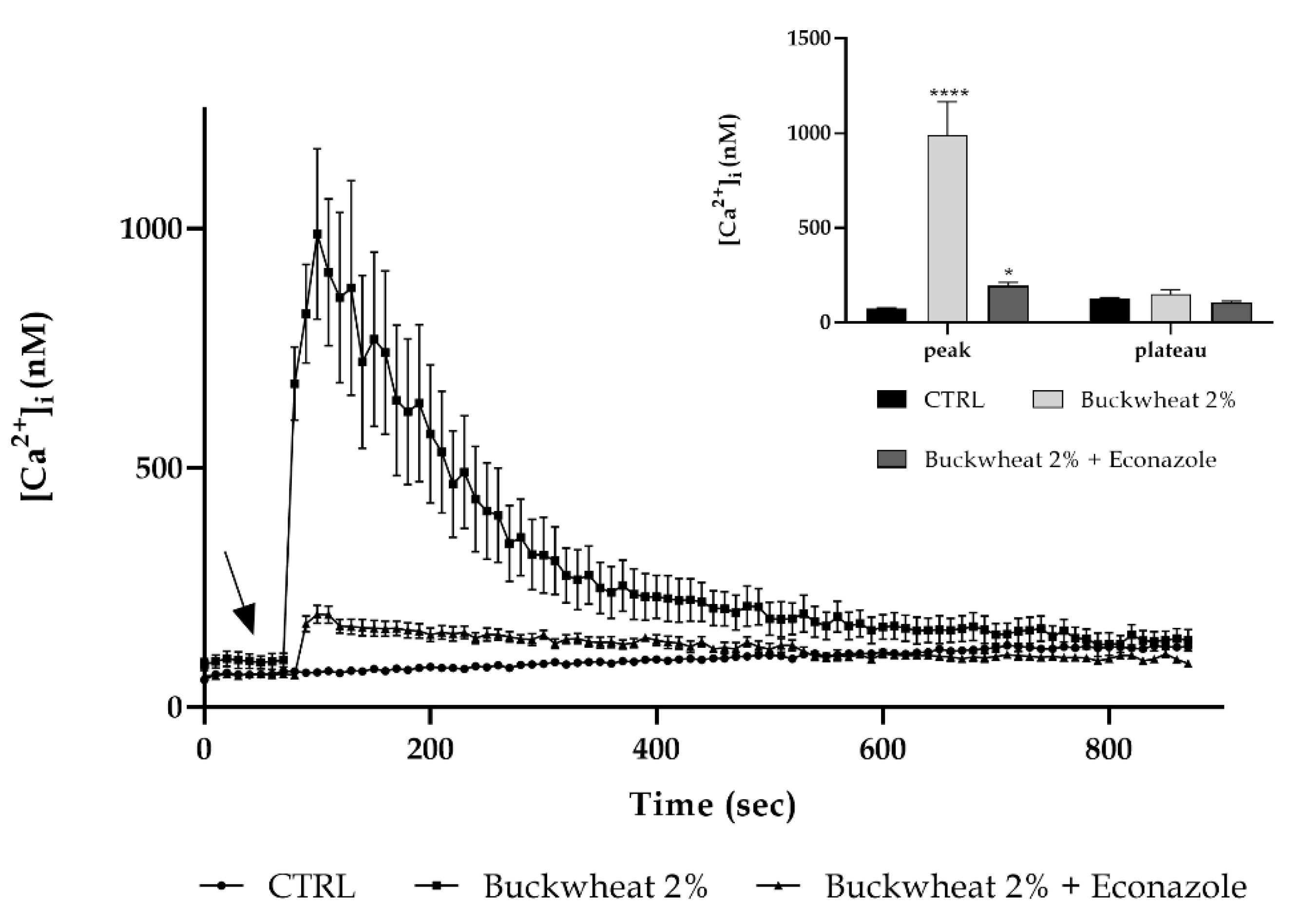
2.4. Measurements of Free Cytosolic Ca2+ Concentration ([Ca2+]i)
2.5. RNA Interference (siRNA)
2.6. Statistical Analysis
3. Results
3.1. Cells Viability Assay
3.2. Scratch Wound Assay
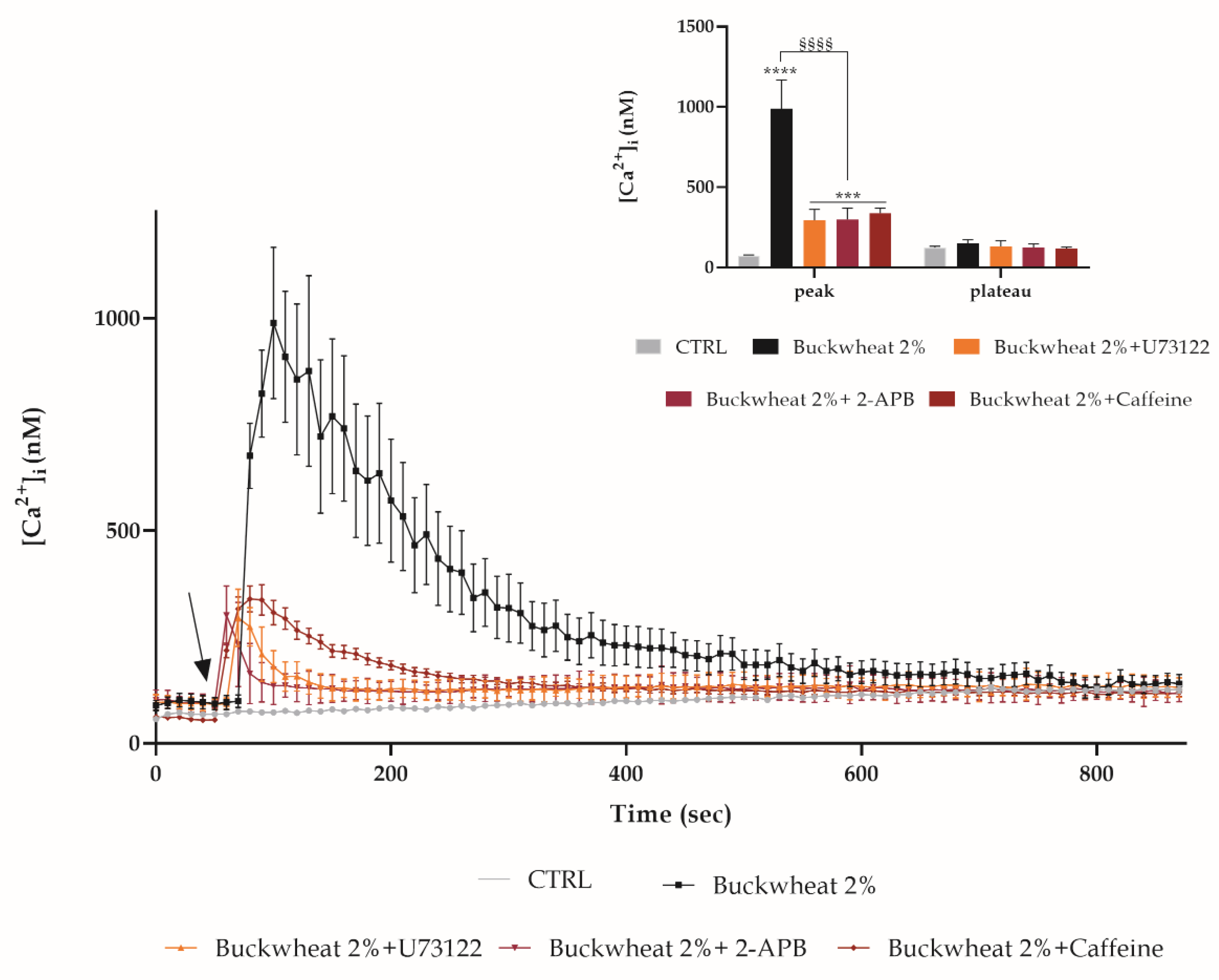
3.3. Evaluation of Free Cytosolic Ca2+ Concentration ([Ca2+]I) Variation
3.4. Effect of H2O2 on the [Ca2+]i Variation
3.5. Extracellular Ca2+ Involvement
3.6. Involvement of the Calcium Toolkit in the [Ca2+]i Variation
3.7. Role of Ca2+ Toolkit in the Endothelial Wound Closure Response
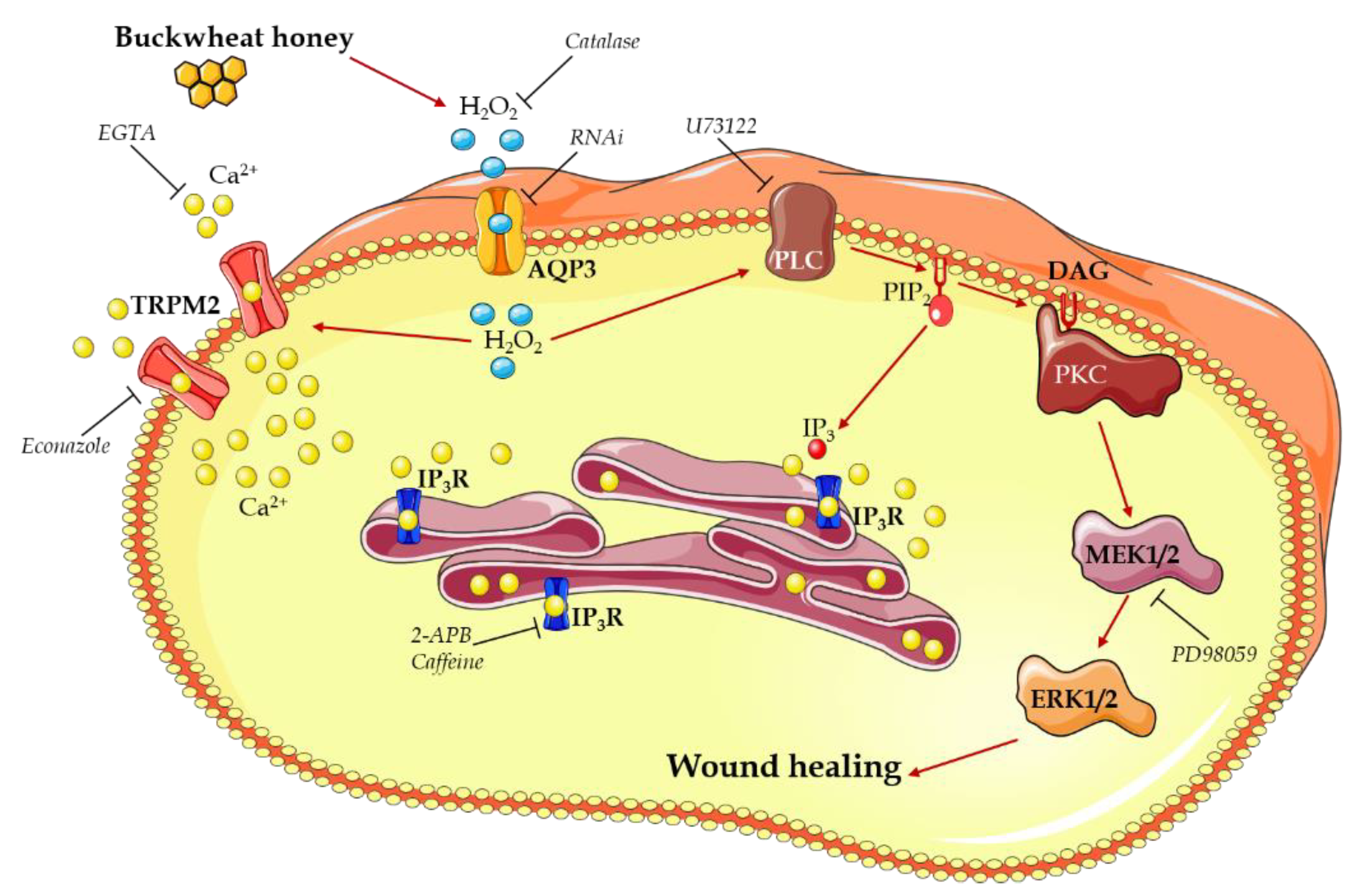
4. Discussion
- Buckwheat honey treatment results in the production of H2O2 outside the cells,
- H2O2 enters the cells through AQP3,
- H2O2 in the cytoplasm starts two different ways of action to increase the [Ca2+]i:
- o
- The activation of a TRPM2 channel determining calcium entry from the extracellular space,
- o
- The activation of a PLC-IP3 pathway leading to a calcium release from the ER,
- The [Ca2+]i increase contributes to the activation of PLC,
- PLC activation begins the MAPK pathway that results in the enhancing of the wound closure rate.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moccia, F.; Tanzi, F.; Munaron, L. Endothelial remodelling and intracellular calcium machinery. Curr. Mol. Med. 2014, 14, 457–480. [Google Scholar] [CrossRef]
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef]
- Munaron, L.; Fiorio Pla, A. Endothelial calcium machinery and angiogenesis: Understanding physiology to interfere with pathology. Curr. Med. Chem. 2009, 16, 4691–4703. [Google Scholar] [CrossRef]
- Moccia, F.; Berra-Romani, R.; Tanzi, F. Update on vascular endothelial Ca2+ signalling: A tale of ion channels, pumps and transporters. World J. Biol. Chem. 2012, 3, 127–158. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Berra-Romani, R.; Baruffi, S.; Spaggiari, S.; Signorelli, S.; Castelli, L.; Magistretti, J.; Taglietti, V.; Tanzi, F. Ca2+ uptake by the endoplasmic reticulum Ca2+-ATPase in rat microvascular endothelial cells. Biochem. J. 2002, 364, 235–244. [Google Scholar] [CrossRef]
- Martinotti, S.; Bucekova, M.; Majtan, J.; Ranzato, E. Honey: An effective regenerative medicine product in wound management. Curr. Med. Chem. 2019, 26, 5230–5240. [Google Scholar] [CrossRef]
- Martinotti, S.; Laforenza, U.; Patrone, M.; Moccia, F.; Ranzato, E. Honey-Mediated wound healing: H₂O₂ entry through AQP3 determines extracellular Ca. Int. J. Mol. Sci. 2019, 20, 764. [Google Scholar] [CrossRef]
- van den Berg, A.J.; van den Worm, E.; van Ufford, H.C.; Halkes, S.B.; Hoekstra, M.J.; Beukelman, C.J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J. Wound Care 2008, 17, 172–174, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Pellavio, G.; Laforenza, U.; Ranzato, E. Propolis induces AQP3 expression: A possible way of action in wound healing. Molecules 2019, 24, 1544. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Scratch wound healing assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar] [CrossRef]
- Martinotti, S.; Pellavio, G.; Patrone, M.; Laforenza, U.; Ranzato, E. Manuka honey induces apoptosis of epithelial cancer cells through aquaporin-3 and calcium signaling. Life 2020, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Hecquet, C.M.; Ahmmed, G.U.; Vogel, S.M.; Malik, A.B. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ. Res. 2008, 102, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Patrone, M.; Balbo, V.; Mazzucco, L.; Ranzato, E. Endothelial response boosted by platelet lysate: The involvement of calcium toolkit. Int. J. Mol. Sci. 2020, 21, 808. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Uchida, K.; Wang, X.; Lee, J.; Shimada, Y.; Tominaga, M.; Kadowaki, M. TRPM2 contributes to antigen-stimulated Ca²⁺ influx in mucosal mast cells. Pflug. Arch. 2013, 465, 1023–1030. [Google Scholar] [CrossRef]
- Hill, K.; McNulty, S.; Randall, A.D. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch. Pharm. 2004, 370, 227–237. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, S.; Yu, J.; Yang, W.; Liu, Z.; Zhang, L. Medicinal chemistry perspective of TRPM2 channel inhibitors: Where we are and where we might be heading? Drug Discov. Today 2020, 25, 2326–2334. [Google Scholar] [CrossRef]
- Hill, K.; Benham, C.D.; McNulty, S.; Randall, A.D. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology 2004, 47, 450–460. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shimizu, S. Targeting TRPM2 in ROS-Coupled Diseases. Pharmaceuticals 2016, 9, 57. [Google Scholar] [CrossRef]
- Maruyama, T.; Kanaji, T.; Nakade, S.; Kanno, T.; Mikoshiba, K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997, 122, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Missiaen, L.; Callewaert, G.; De Smedt, H.; Parys, J.B. 2-Aminoethoxydiphenyl borate affects the inositol 1,4,5-trisphosphate receptor, the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium 2001, 29, 111–116. [Google Scholar] [CrossRef]
- Iwasaki, H.; Mori, Y.; Hara, Y.; Uchida, K.; Zhou, H.; Mikoshiba, K. 2-Aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Recept Channels 2001, 7, 429–439. [Google Scholar] [PubMed]
- Bilmen, J.G.; Michelangeli, F. Inhibition of the type 1 inositol 1,4,5-trisphosphate receptor by 2-aminoethoxydiphenylborate. Cell Signal. 2002, 14, 955–960. [Google Scholar] [CrossRef]
- Wilsher, N.E.; Court, W.J.; Ruddle, R.; Newbatt, Y.M.; Aherne, W.; Sheldrake, P.W.; Jones, N.P.; Katan, M.; Eccles, S.A.; Raynaud, F.I. The phosphoinositide-specific phospholipase C inhibitor U73122 (1-(6-((17beta-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione) spontaneously forms conjugates with common components of cell culture medium. Drug Metab. Dispos. 2007, 35, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, C.; Schäfer, U.; Richardt, G.; Kurz, T. U73122, an aminosteroid phospholipase C inhibitor, is a potent inhibitor of cardiac phospholipase D by a PIP2-dependent mechanism. J. Cardiovasc. Pharmacol. 2010, 55, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Kim, K.S.; Kim, S.H.; Kim, D.K.; Park, H.S. Caffeine and 2-Aminoethoxydiphenyl Borate (2-APB) have different ability to inhibit intracellular calcium mobilization in pancreatic acinar cell. Korean J. Physiol. Pharmacol. 2010, 14, 105–111. [Google Scholar] [CrossRef]
- Ranzato, E.; Mazzucco, L.; Patrone, M.; Burlando, B. Platelet lysate promotes in vitro wound scratch closure of human dermal fibroblasts: Different roles of cell calcium, P38, Erk, and Pi3k/Akt. J. Cell. Mol. Med. 2008. [Google Scholar] [CrossRef]
- Ranzato, E.; Boccafoschi, F.; Mazzucco, L.; Patrone, M.; Burlando, B. Role of ERK1/2 in platelet lysate-driven endothelial cell repair. J. Cell. Biochem. 2010, 110, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Martinotti, S.; Burlando, B. Epithelial mesenchymal transition traits in honey-driven keratinocyte wound healing: Comparison among different honeys. Wound Repair Regen. 2012, 20, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Ranzato, E.; Martinotti, S.; Burlando, B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burn. Trauma 2013, 1, 32–38. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Honey’s healing history. In Cellular and Molecular Mechanisms of Honey Wound Healing; Ranzato, S.M.a.E., Ed.; Nova Publishers Inc.: Hauppage, NY, USA, 2014. [Google Scholar]
- Martinotti, S.; Calabrese, G.; Ranzato, E. Honeydew honey: Biological effects on skin cells. Mol. Cell. Biochem. 2017, 435, 185–192. [Google Scholar] [CrossRef]
- Filipič, B.; Gradišnik, L.; Ružić-Sabljić, E.; Trtnik, B.; Pereyra, A.; Jaklič, D.; Kopinč, R.; Potokar, J.; Puzić, A.; Mazija, H. Water soluble propolis and royal jelly enhance the antimicrobial activity of honeys and promote the growth of human macrophage cell line. J. Agric. Sci. Technol. B 2016, 6, 35–47. [Google Scholar] [CrossRef][Green Version]
- Rossello, R.A.; Kohn, D.H. Cell communication and tissue engineering. Commun. Integr. Biol. 2010, 3, 53–56. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Barroso, M.; Moura, T.; Castro, R.; Soveral, G. Endothelial aquaporins and hypomethylation: Potential implications for atherosclerosis and cardiovascular disease. Int. J. Mol. Sci. 2018, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Berra-Romani, R.; Faris, P.; Negri, S.; Botta, L.; Genova, T.; Moccia, F. Arachidonic acid evokes an increase in intracellular Ca. Cells 2019, 8, 689. [Google Scholar] [CrossRef] [PubMed]
- Sei, Y.; Gallagher, K.L.; Daly, J.W. Multiple effects of caffeine on Ca2+ release and influx in human B lymphocytes. Cell Calcium 2001, 29, 149–160. [Google Scholar] [CrossRef]
- Qin, S.; Chock, P.B. Bruton’s tyrosine kinase is essential for hydrogen peroxide-induced calcium signaling. Biochemistry 2001, 40, 8085–8091. [Google Scholar] [CrossRef]
- Lee, K.; Esselman, W.J. cAMP potentiates H2O2-induced ERK1/2 phosphorylation without the requirement for MEK1/2 phosphorylation. Cell Signal. 2001, 13, 645–652. [Google Scholar] [CrossRef]
- Ranzato, E.; Patrone, M.; Mazzucco, L.; Burlando, B. Platelet lysate stimulates wound repair of HaCaT keratinocytes. Br. J. Derm. 2008, 159, 537–545. [Google Scholar] [CrossRef]


| Target Protein | Forward Sequence | Reverse Sequence |
|---|---|---|
| AQP3 | 5′-GAGCAGAUCUGAGUGGGCA-3′ | 5′-UGCCCACUCAGAUCUGCUC-3′ |
| EC05 | EC50 | |
|---|---|---|
| Buckwheat honey | 0.73 (0.35–1.53) | 4.72 (3.86–5.76) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranzato, E.; Bonsignore, G.; Patrone, M.; Martinotti, S. Endothelial and Vascular Health: A Tale of Honey, H2O2 and Calcium. Cells 2021, 10, 1071. https://doi.org/10.3390/cells10051071
Ranzato E, Bonsignore G, Patrone M, Martinotti S. Endothelial and Vascular Health: A Tale of Honey, H2O2 and Calcium. Cells. 2021; 10(5):1071. https://doi.org/10.3390/cells10051071
Chicago/Turabian StyleRanzato, Elia, Gregorio Bonsignore, Mauro Patrone, and Simona Martinotti. 2021. "Endothelial and Vascular Health: A Tale of Honey, H2O2 and Calcium" Cells 10, no. 5: 1071. https://doi.org/10.3390/cells10051071
APA StyleRanzato, E., Bonsignore, G., Patrone, M., & Martinotti, S. (2021). Endothelial and Vascular Health: A Tale of Honey, H2O2 and Calcium. Cells, 10(5), 1071. https://doi.org/10.3390/cells10051071








