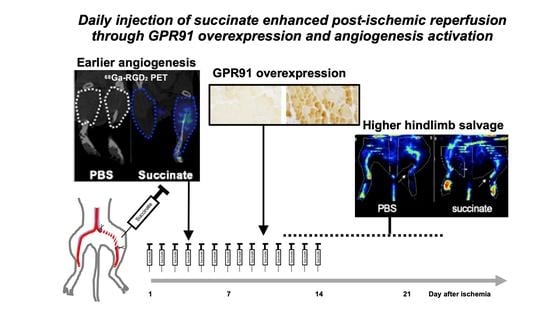
Succinate Injection Rescues Vasculature and Improves Functional Recovery Following Acute Peripheral Ischemia in Rodents: A Multimodal Imaging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mouse Model of Hindlimb Ischemia
2.3. Succinate Administration
2.4. Immunohistochemistry
2.5. Immunofluorescence
2.6. Western Blot
2.7. [68Ga]Ga-RGD2 Radiosynthesis
2.8. [68Ga]Ga-RGD2 MicroPET/CT Imaging
2.9. Hindlimb Perfusion
2.10. Motility Impairment Score
2.11. Statistical Analysis
3. Results
3.1. Ischemia Is Associated with an Increase of GPR91
3.2. GPR91 Colocalizes with Endothelial Cells in Ischemic Muscles Treated by Succinate
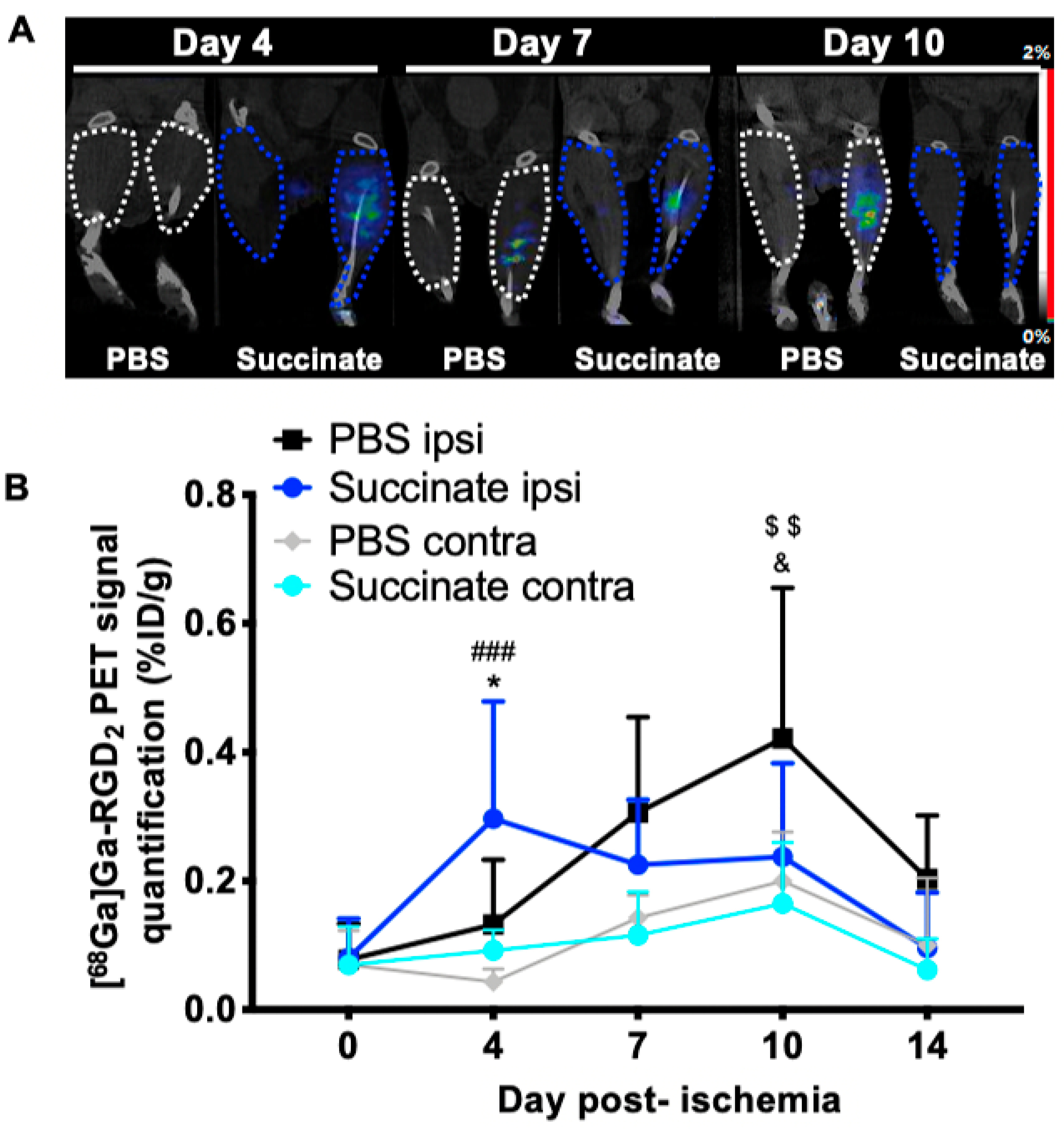
3.3. Succinate Injection Induces Earlier [68Ga]Ga-RGD2 Uptake Following Hindlimb Ischemia
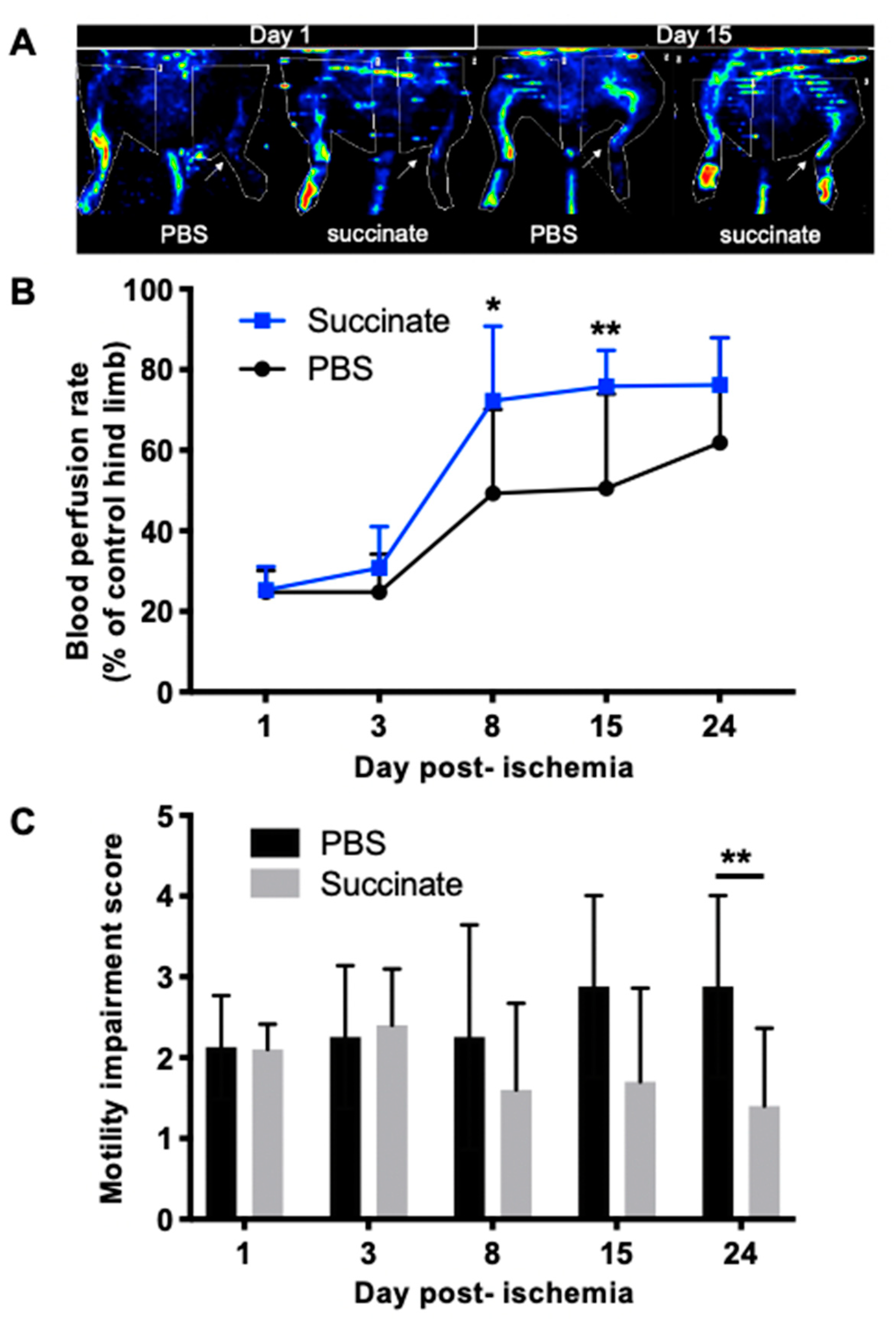
3.4. Succinate Injection Increases Blood Perfusion Recovery Following Hindlimb Ischemia
3.5. Succinate Injection Increases Clinical Recovery Score Following Hindlimb Ischemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fonseca, M.D.C.; Aguiar, C.J.; Franco, J.A.D.R.; Gingold, R.N.; Leite, M.F. GPR91: Expanding the frontiers of Krebs cycle intermediates. Cell Commun. Signal. 2016, 14, 1–9. [Google Scholar] [CrossRef]
- Kes, M.M.; Bossche, J.V.D.; Griffioen, A.W.; Huijbers, E.J. Oncometabolites lactate and succinate drive pro-angiogenic macrophage response in tumors. Biochim. Biophys. Acta (BBA) Bioenergy 2020, 1874, 188427. [Google Scholar] [CrossRef]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta (BBA) Bioenergy 2016, 1857, 1086–1101. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Miao, F.J.-P.; Lin, D.C.-H.; Schwandner, R.T.; Wang, Z.; Gao, J.; Chen, J.-L.; Tian, H.; Ling, L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nat. Cell Biol. 2004, 429, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Huang, T.-W.; Hsieh, Y.-T.; Wang, Y.-F.; Yen, C.-C.; Lee, G.-L.; Yeh, C.-C.; Peng, Y.-J.; Kuo, Y.-Y.; Wen, H.-T.; et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol. Cell 2020, 77, 213–227.e5. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhao, T.; Xu, C.; Shi, W.; Geng, B.; Shen, J.; Zhang, C.; Pan, J.; Yang, J.; Hu, S.; et al. Oncometabolite succinate promotes angiogenesis by upregulating VEGF expression through GPR91-mediated STAT3 and ERK activation. Oncotarget 2017, 8, 13174–13185. [Google Scholar] [CrossRef] [PubMed]
- Sapieha, P.; Sirinyan, M.; Hamel, D.; Zaniolo, K.; Joyal, J.-S.; Cho, J.-H.; Honoré, J.-C.; Kermorvant-Duchemin, E.; Varma, D.R.; Tremblay, S.; et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat. Med. 2008, 14, 1067–1076. [Google Scholar] [CrossRef]
- Aguiar, C.J.; Andrade, V.L.; Gomes, E.R.; Alves, M.N.; Ladeira, M.S.; Pinheiro, A.C.N.; Gomes, D.A.; Almeida, A.P.; Goes, A.M.; Resende, R.R.; et al. Succinate modulates Ca2+ transient and cardiomyocyte viability through PKA-dependent pathway. Cell Calcium 2010, 47, 37–46. [Google Scholar] [CrossRef]
- Diehl, J.; Gries, B.; Pfeil, U.; Goldenberg, A.; Mermer, P.; Kummer, W.; Paddenberg, R. Expression and localization of GPR91 and GPR99 in murine organs. Cell Tissue Res. 2015, 364, 245–262. [Google Scholar] [CrossRef]
- Toma, I.; Kang, J.J.; Sipos, A.; Vargas, S.; Bansal, E.; Hanner, F.; Meer, E.; Peti-Peterdi, J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Investig. 2008, 118, 2526–2534. [Google Scholar] [CrossRef]
- Garrigue, P.; Bodin-Hullin, A.; Balasse, L.; Fernandez, S.; Essamet, W.; Dignat-George, F.; Pacak, K.; Guillet, B.; Taieb, D. The Evolving Role of Succinate in Tumor Metabolism: An 18F-FDG-Based Study. J. Nucl. Med. 2017, 58, 1749–1755. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.; Gao, F.; Du, X.; Chen, Y.; Wu, Q. Transcription factors regulate GPR91-mediated expression of VEGF in hypoxia-induced retinopathy. Sci. Rep. 2017, 7, srep45807. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Wang, C.; Xia, W.-R.; Zheng, J.-Y.; Yang, J.; Liu, B.; Liu, J.-Q.; Liu, L.-F. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1α/VEGF axis. Free Radic. Biol. Med. 2018, 126, 1–14. [Google Scholar] [CrossRef]
- Moyon, A.; Garrigue, P.; Balasse, L.; Fernandez, S.; Brige, P.; Nollet, M.; Hache, G.; Blot-Chabaud, M.; Dignat-George, F.; Guillet, B. Early prediction of revascularisation by angiomotin-targeting positron emission tomography. Theranostics 2018, 8, 4985–4994. [Google Scholar] [CrossRef]
- Eliceiri, B.P.; Cheresh, D.A. The role of αv integrins during angiogenesis: Insights into potential mechanisms of action and clinical development. J. Clin. Investig. 1999, 103, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chakraborty, S.; Liu, S. Radiolabeled Cyclic RGD Peptides as Radiotracers for Imaging Tumors and Thrombosis by SPECT. Theranostics 2011, 1, 58–82. [Google Scholar] [CrossRef]
- Pichler, B.J.; Kneilling, M.; Haubner, R.; Braumüller, H.; Schwaiger, M.; Röcken, M.; Weber, W.A. Imaging of delayed-type hypersensitivity reaction by PET and 18F-galacto-RGD. J. Nucl. Med. 2005, 46, 184–189. [Google Scholar] [PubMed]
- Goldberg, E.J.; Schmidt, C.A.; Green, T.D.; Karnekar, R.; Yamaguchi, D.J.; Spangenberg, E.E.; McClung, J.M. Temporal Association Between Ischemic Muscle Perfusion Recovery and the Restoration of Muscle Contractile Function After Hindlimb Ischemia. Front. Physiol. 2019, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Hamel, D.; Sanchez, M.; Duhamel, F.; Roy, O.; Honoré, J.-C.; Noueihed, B.; Zhou, T.; Nadeau-Vallée, M.; Hou, X.; Lavoie, J.-C.; et al. G-Protein–Coupled Receptor 91 and Succinate Are Key Contributors in Neonatal Postcerebral Hypoxia-Ischemia Recovery. Arter. Thromb. Vasc. Biol. 2014, 34, 285–293. [Google Scholar] [CrossRef]
- Gilissen, J.; Jouret, F.; Pirotte, B.; Hanson, J. Insight into SUCNR1 (GPR91) structure and function. Pharmacol. Ther. 2016, 159, 56–65. [Google Scholar] [CrossRef]
- Mao, H.; Yang, A.; Zhao, Y.; Lei, L.; Li, H. Succinate Supplement Elicited “Pseudohypoxia” Condition to Promote Proliferation, Migration, and Osteogenesis of Periodontal Ligament Cells. Stem Cells Int. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Lei, W.; Ren, W.; Ohmoto, M.; Urban, J.F.; Matsumoto, I.; Margolskee, R.F.; Jiang, P. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl. Acad. Sci. USA 2018, 115, 5552–5557. [Google Scholar] [CrossRef]
- Martin, J.L.; Costa, A.S.H.; Gruszczyk, A.V.; Beach, T.E.; Allen, F.M.; Prag, H.A.; Hinchy, E.C.; Mahbubani, K.; Hamed, M.; Tronci, L.; et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat. Metab. 2019, 1, 966–974. [Google Scholar] [CrossRef]
- Kamarauskaite, J.; Baniene, R.; Trumbeckas, D.; Strazdauskas, A.; Trumbeckaite, S. Increased Succinate Accumulation Induces ROS Generation in In Vivo Ischemia/Reperfusion-Affected Rat Kidney Mitochondria. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Prag, H.A.; Gruszczyk, A.V.; Huang, M.M.; Beach, T.E.; Young, T.; Tronci, L.; Nikitopoulou, E.; Mulvey, J.F.; Ascione, R.; Hadjihambi, A.; et al. Mechanism of succinate efflux upon reperfusion of the ischaemic heart. Cardiovasc. Res. 2021, 117, 1188–1201. [Google Scholar] [CrossRef]
- Eariza, A.C.; Deen, P.M.T.; Robben, J.H. The Succinate Receptor as a Novel Therapeutic Target for Oxidative and Metabolic Stress-Related Conditions. Front. Endocrinol. 2012, 3, 22. [Google Scholar] [CrossRef]
- Xu, J.; Pan, H.; Xie, X.; Zhang, J.; Wang, Y.; Yang, G. Inhibiting Succinate Dehydrogenase by Dimethyl Malonate Alleviates Brain Damage in a Rat Model of Cardiac Arrest. Neuroscience 2018, 393, 24–32. [Google Scholar] [CrossRef]
- Valls-Lacalle, L.; Barba, I.; Miró-Casas, E.; Ruiz-Meana, M.; Rodríguez-Sinovas, A.; Garcia-Dorado, D. Selective Inhibition of Succinate Dehydrogenase in Reperfused Myocardium with Intracoronary Malonate Reduces Infarct Size. Sci. Rep. 2018, 8, 2442. [Google Scholar] [CrossRef]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.; Thompson, C.B.; Gottlieb, E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: An updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyon, A.; Garrigue, P.; Balasse, L.; Fernandez, S.; Brige, P.; Bouhlel, A.; Hache, G.; Dignat-George, F.; Taïeb, D.; Guillet, B. Succinate Injection Rescues Vasculature and Improves Functional Recovery Following Acute Peripheral Ischemia in Rodents: A Multimodal Imaging Study. Cells 2021, 10, 795. https://doi.org/10.3390/cells10040795
Moyon A, Garrigue P, Balasse L, Fernandez S, Brige P, Bouhlel A, Hache G, Dignat-George F, Taïeb D, Guillet B. Succinate Injection Rescues Vasculature and Improves Functional Recovery Following Acute Peripheral Ischemia in Rodents: A Multimodal Imaging Study. Cells. 2021; 10(4):795. https://doi.org/10.3390/cells10040795
Chicago/Turabian StyleMoyon, Anaïs, Philippe Garrigue, Laure Balasse, Samantha Fernandez, Pauline Brige, Ahlem Bouhlel, Guillaume Hache, Françoise Dignat-George, David Taïeb, and Benjamin Guillet. 2021. "Succinate Injection Rescues Vasculature and Improves Functional Recovery Following Acute Peripheral Ischemia in Rodents: A Multimodal Imaging Study" Cells 10, no. 4: 795. https://doi.org/10.3390/cells10040795
APA StyleMoyon, A., Garrigue, P., Balasse, L., Fernandez, S., Brige, P., Bouhlel, A., Hache, G., Dignat-George, F., Taïeb, D., & Guillet, B. (2021). Succinate Injection Rescues Vasculature and Improves Functional Recovery Following Acute Peripheral Ischemia in Rodents: A Multimodal Imaging Study. Cells, 10(4), 795. https://doi.org/10.3390/cells10040795