Cardiac GRK2 Protein Levels Show Sexual Dimorphism during Aging and Are Regulated by Ovarian Hormones
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Protocols
2.2. Ovariectomy Model
2.3. Western Blotting
2.4. RT-qPCR from Rat and Mouse Tissues
2.5. Statistical Analysis
3. Results
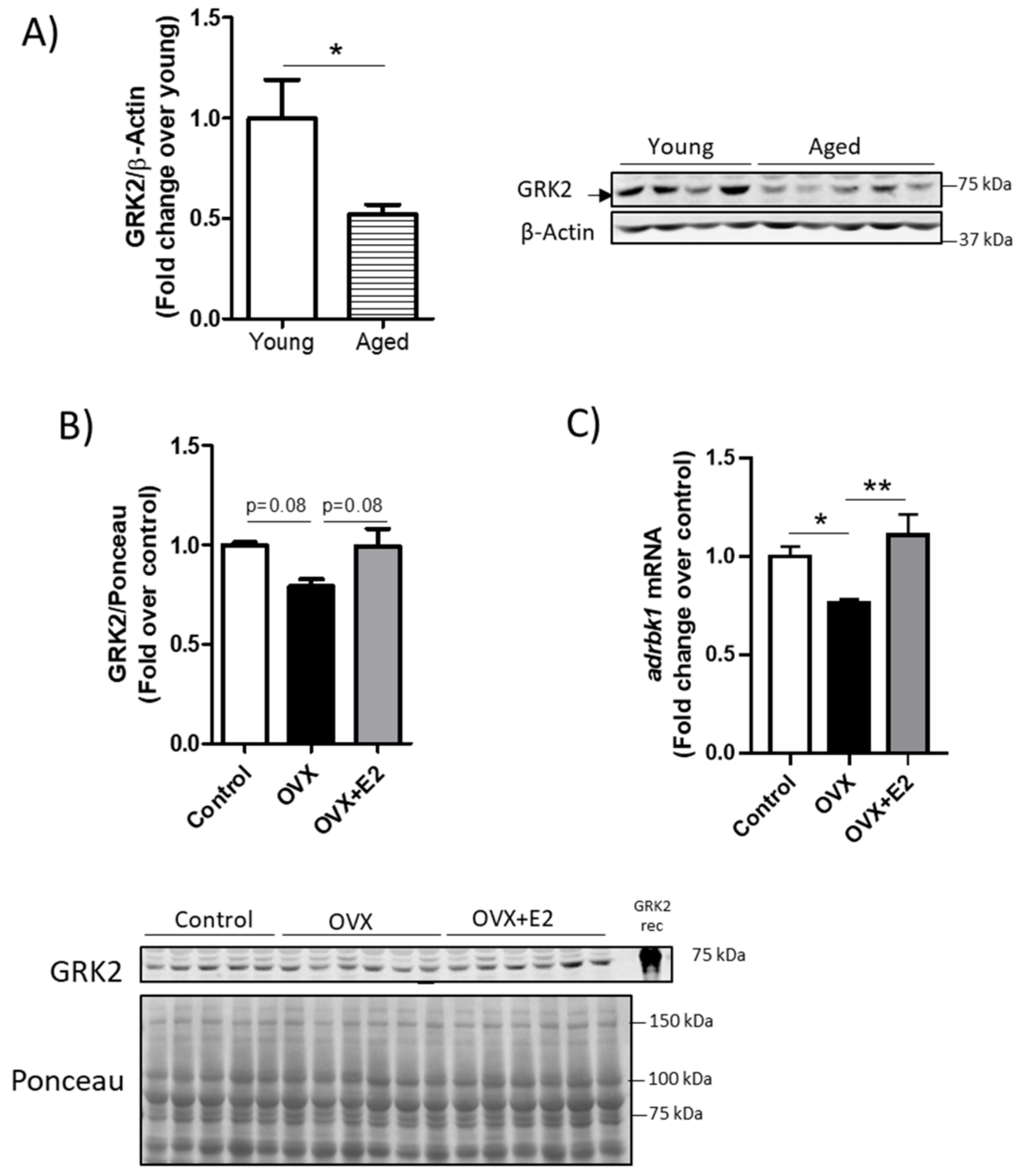
3.1. GRK2 Levels Change with Age in a Sex-Dependent Manner in the Mouse Heart and Show an Inverse Correlation with Mitochondrial Fusion Markers
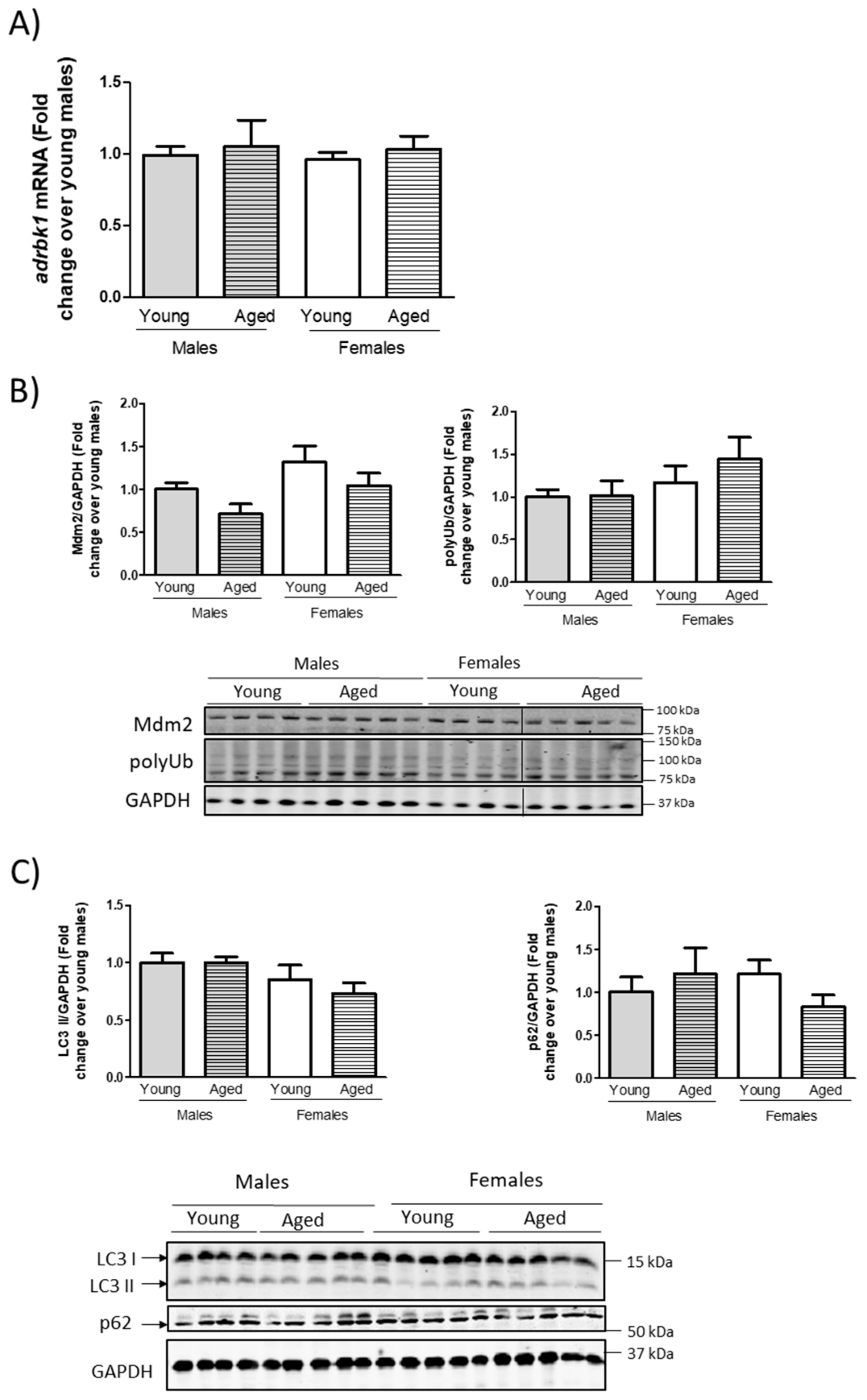
3.2. Modulation of GRK2 Levels in the Heart Does Not Seem to Be Mediated by Transcriptional Modulation or by Overall Changes in the Proteasomal or Autophagic Machinery
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nonstandard Abbreviations
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Deaton, C.; Froelicher, E.S.; Wu, L.H.; Ho, C.; Shishani, K.; Jaarsma, T. The Global Burden of Cardiovascular Disease. Eur. J. Cardiovasc. Nurs. 2011, 10, S5–S13. [Google Scholar] [CrossRef]
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.B.; Buring, J.E.; Manson, J.E. Cardiovascular Disease in Women. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.; Hevener, A.L.; Moreau, K.L.; Morselli, E.; Criollo, A.; Van Pelt, R.E.; Vieira-Potter, V.J. Sex Hormones and Cardiometabolic Health: Role of Estrogen and Estrogen Receptors. Endocrinology 2017, 158, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Huebschmann, A.G.; Huxley, R.R.; Kohrt, W.M.; Zeitler, P.; Regensteiner, J.G.; Reusch, J.E.B. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetology 2019, 62, 1761–1772. [Google Scholar] [CrossRef]
- Krishnan, K.C.; Mehrabian, M.; Lusis, A.J. Sex differences in metabolism and cardiometabolic disorders. Curr. Opin. Lipidol. 2018, 29, 404–410. [Google Scholar] [CrossRef]
- Patten, R.D. Models of gender differences in cardiovascular disease. Drug Discov. Today Dis. Model. 2007, 4, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Umar, S.; Ruffenach, G.; Aryan, L.; Li, J.; Sharma, S.; Motayagheni, N.; Nadadur, R.D.; Bopassa, J.C.; Eghbali, M. Estrogen rescues heart failure through estrogen receptor Beta activation. Biol. Sex Differ. 2018, 9, 48. [Google Scholar] [CrossRef]
- Wang, H.; Sun, X.; Lin, M.S.; Ferrario, C.M.; Van Remmen, H.; Groban, L. G protein-coupled estrogen receptor (GPER) deficiency induces cardiac remodeling through oxidative stress. Transl. Res. 2018, 199, 39–51. [Google Scholar] [CrossRef]
- Sbert-Roig, M.; Bauzá-Thorbrügge, M.; Galmés-Pascual, B.M.; Capllonch-Amer, G.; García-Palmer, F.J.; Lladó, I.; Proenza, A.M.; Gianotti, M. GPER mediates the effects of 17β-estradiol in cardiac mitochondrial biogenesis and function. Mol. Cell. Endocrinol. 2016, 420, 116–124. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.; Tan, Y.; Jiang, L.; Zhao, M.; Gao, E.; Yu, S.; Liu, J. GPR 30 reduces myocardial infarct area and fibrosis in female ovariectomized mice by activating the PI3K/AKT pathway. Life Sci. 2019, 226, 22–32. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.L.; Pacheco, C.; Tweet, M.S.; Miller, V.M. Sex-Specific Physiology and Cardiovascular Disease. Adv. Exp. Med. Biol. 2018, 1065, 433–454. [Google Scholar] [CrossRef]
- Speth, R.C.; D’Ambra, M.; Ji, H.; Sandberg, K. A heartfelt message, estrogen replacement therapy: Use it or lose it. Am. J. Physiol. Circ. Physiol. 2018, 315, H1765–H1778. [Google Scholar] [CrossRef]
- Giordano, S.; Xing, D.; Chen, Y.-F.; Allon, S.; Chen, C.-J.; Oparil, S.; Hage, F.G. Estrogen and Cardiovascular Disease: Is Timing Everything? Am. J. Med. Sci. 2015, 350, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Schirone, L.; Ameri, P.; Basso, C.; Catalucci, D.; Modica, J.; Chimenti, C.; Crotti, L.; Frati, G.; Rubattu, S.; et al. The role of mitochondrial dynamics in cardiovascular diseases. Br. J. Pharmacol. 2020, 15068. [Google Scholar] [CrossRef] [PubMed]
- Nan, J.; Zhu, W.; Rahman, M.; Liu, M.; Li, D.; Su, S.; Zhang, N.; Hu, X.; Yu, H.; Gupta, M.P.; et al. Molecular regulation of mitochondrial dynamics in cardiac disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W. Mitochondrial dynamics in heart disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.R.M.; Abdel-Rahman, E.A.; Mahmoud, A.M.; Ali, M.H.; Noureldin, M.; Saber, S.H.; Mohsen, M.; Ali, S.S. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol. Rep. 2017, 5, e13125. [Google Scholar] [CrossRef] [PubMed]
- Maneechote, C.; Palee, S.; Kerdphoo, S.; Jaiwongkam, T.; Chattipakorn, S.C.; Chattipakorn, N. Balancing mitochondrial dynamics via increasing mitochondrial fusion attenuates infarct size and left ventricular dysfunction in rats with cardiac ischemia/reperfusion injury. Clin. Sci. 2019, 133, 497–513. [Google Scholar] [CrossRef]
- Ding, M.; Liu, C.; Shi, R.; Yu, M.; Zeng, K.; Kang, J.; Fu, F.; Mi, M. Mitochondrial fusion promoter restores mitochondrial dynamics balance and ameliorates diabetic cardiomyopathy in an optic atrophy 1-dependent way. Acta Physiol. 2020, 229, e13428. [Google Scholar] [CrossRef] [PubMed]
- Arcones, A.C.; Murga, C.; Penela, P.; Inserte, J.; Mayor, F. G protein–coupled receptor kinase 2 at crossroads of metabolic and cardiovascular diseases. Curr. Opin. Endocr. Metab. Res. 2021, 16, 75–85. [Google Scholar] [CrossRef]
- Pfleger, J.; Gresham, K.; Koch, W.J. G protein-coupled receptor kinases as therapeutic targets in the heart. Nat. Rev. Cardiol. 2019, 16, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Murga, C.; Arcones, A.C.; Cruces-Sande, M.; Briones, A.M.; Salaices, M.; Mayor, F., Jr. G Protein-Coupled Receptor Kinase 2 (GRK2) as a Potential Therapeutic Target in Cardiovascular and Metabolic Diseases. Front. Pharmacol. 2019, 10, 112. [Google Scholar] [CrossRef]
- Woodall, M.C.; Ciccarelli, M.; Woodall, B.P.; Koch, W.J. G protein-coupled receptor kinase 2: A link between myocardial contractile function and cardiac metabolism. Circ. Res. 2014, 114, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Penela, P. Chapter Three-Ubiquitination and Protein Turnover of G-Protein-Coupled Receptor Kinases in GPCR Signaling and Cellular Regulation. Prog. Mol. Biol. Transl. Sci. 2016, 141, 85–140. [Google Scholar] [CrossRef] [PubMed]
- Sato, P.Y.; Chuprun, J.K.; Grisanti, L.A.; Woodall, M.C.; Brown, B.R.; Roy, R.; Traynham, C.J.; Ibetti, J.; Lucchese, A.M.; Yuan, A.; et al. Restricting mitochondrial GRK2 post-ischemia confers cardioprotection by reducing myocyte death and maintaining glucose oxidation. Sci. Signal. 2018, 11, eaau0144. [Google Scholar] [CrossRef]
- Sato, P.Y.; Chuprun, J.K.; Schwartz, M.; Koch, W.J. The Evolving Impact of G Protein-Coupled Receptor Kinases in Cardiac Health and Disease. Physiol. Rev. 2015, 95, 377–404. [Google Scholar] [CrossRef]
- Schumacher, S.M.; Gao, E.; Zhu, W.; Chen, X.; Chuprun, J.K.; Feldman, A.M.; Tesmer, J.J.G.; Koch, W.J. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci. Transl. Med. 2015, 7, 277ra31. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.; Jurado-Pueyo, M.; Fortuño, M.A.; Fernández-Veledo, S.; Vila-Bedmar, R.; Jiménez-Borreguero, L.J.; Lazcano, J.J.; Gao, E.; Gómez-Ambrosi, J.; Frühbeck, G.; et al. Downregulation of G protein-coupled receptor kinase 2 levels enhances cardiac insulin sensitivity and switches on cardioprotective gene expression patterns. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 2448–2456. [Google Scholar] [CrossRef]
- Lucas, E.; Vila-Bedmar, R.; Arcones, A.C.; Cruces-Sande, M.; Cachofeiro, V.; Mayor, F., Jr.; Murga, C. Obesity-induced cardiac lipid accumulation in adult mice is modulated by G protein-coupled receptor kinase 2 levels. Cardiovasc. Diabetol. 2016, 15, 155. [Google Scholar] [CrossRef]
- Rengo, G.; Pagano, G.; Filardi, P.P.; Femminella, G.D.; Parisi, V.; Cannavo, A.; Liccardo, D.; Komici, K.; Gambino, G.; D’Amico, M.L.; et al. Prognostic Value of Lymphocyte G Protein-Coupled Receptor Kinase-2 Protein Levels in Patients With Heart Failure. Circ. Res. 2016, 118, 1116–1124. [Google Scholar] [CrossRef]
- Penela, P.; Inserte, J.; Ramos, P.; Rodriguez-Sinovas, A.; Garcia-Dorado, D.; Mayor, F. Degradation of GRK2 and AKT is an early and detrimental event in myocardial ischemia/reperfusion. EBioMedicine 2019, 48, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Cruces-Sande, M.; Arcones, A.C.; Vila-Bedmar, R.; Val-Blasco, A.; Sharabi, K.; Díaz-Rodríguez, D.; Puigserver, P.; Mayor, F.; Murga, C. Autophagy mediates hepatic GRK2 degradation to facilitate glucagon-induced metabolic adaptation to fasting. FASEB J. 2020, 34, 399–409. [Google Scholar] [CrossRef]
- Arcones, A.C.; Cruces-Sande, M.; Ramos, P.; Mayor, J.F.; Murga, C. Sex Differences in High Fat Diet-Induced Metabolic Alterations Correlate with Changes in the Modulation of GRK2 Levels. Cells 2019, 8, 1464. [Google Scholar] [CrossRef] [PubMed]
- Sato, P.Y.; Chuprun, J.K.; Ibetti, J.; Cannavo, A.; Drosatos, K.; Elrod, J.W.; Koch, W.J. GRK2 compromises cardiomyocyte mitochondrial function by diminishing fatty acid-mediated oxygen consumption and increasing superoxide levels. J. Mol. Cell. Cardiol. 2015, 89, 360–364. [Google Scholar] [CrossRef]
- Wai, T.; García-Prieto, J.; Baker, M.J.; Merkwirth, C.; Benit, P.; Rustin, P.; Rupérez, F.J.; Barbas, C.; Ibañez, B.; Langer, T. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 2015, 350, aad0116. [Google Scholar] [CrossRef] [PubMed]
- Thacker, J.S.; Yeung, D.H.; Staines, W.R.; Mielke, J.G. Total protein or high-abundance protein: Which offers the best loading control for Western blotting? Anal. Biochem. 2016, 496, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Cruces-Sande, M.; Vila-Bedmar, R.; Arcones, A.C.; González-Rodríguez, Á.; Rada, P.; de-Juan, V.G.; Vargas-Castrillón, J.; Iruzubieta, P.; Sánchez-González, C.; Formentini, L.; et al. Involvement of G protein-coupled receptor kinase 2 (GRK2) in the development of non-alcoholic steatosis and steatohepatitis in mice and humans. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 3655–3667. [Google Scholar] [CrossRef]
- Salcedo, A.; Mayor, F., Jr.; Penela, P. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J. 2006, 25, 4752–4762. [Google Scholar] [CrossRef]
- De Lucia, C.; Eguchi, A.; Koch, W.J. New Insights in Cardiac β-Adrenergic Signaling During Heart Failure and Aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef]
- Knowlton, A.A.; Korzick, D.H. Estrogen and the female heart. Mol. Cell. Endocrinol. 2014, 389, 31–39. [Google Scholar] [CrossRef]
- Yang, X.-P.; Reckelhoff, J.F. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Adachi, Y.; Liu, P.; Fukuma, N.; Takimoto, E. Regulatory Actions of Estrogen Receptor Signaling in the Cardiovascular System. Front. Endocrinol. 2020, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Puglisi, R.; Mattia, G.; Carè, A.; Marano, G.; Malorni, W.; Matarrese, P. Non-genomic Effects of Estrogen on Cell Homeostasis and Remodeling with Special Focus on Cardiac Ischemia/Reperfusion Injury. Front. Endocrinol. 2019, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.D.; Schattauer, S.S.; Reichard, K.L.; Cohen, J.H.; Fontaine, H.M.; Song, A.J.; Johnson, S.D.; Land, B.B.; Chavkin, C. Estrogen Regulation of GRK2 Inactivates Kappa Opioid Receptor Signaling Mediating Analgesia, But Not Aversion. J. Neurosci. 2018, 38, 8031–8043. [Google Scholar] [CrossRef] [PubMed]
- Nogués, L.; Reglero, C.; Rivas, V.; Salcedo, A.; Lafarga, V.; Neves, M.; Ramos, P.; Mendiola, M.; Berjón, A.; Stamatakis, K.; et al. G Protein-coupled Receptor Kinase 2 (GRK2) Promotes Breast Tumorigenesis Through a HDAC6-Pin1 Axis. EBioMedicine 2016, 13, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Liu, X.; Ren, J.; Chen, K.; Wang, H.-L.; Miao, Y.-Y.; Qi, M.-M. How does estrogen work on autophagy? Autophagy 2019, 15, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Kondakova, I.V.; Shashova, E.E.; Sidenko, E.A.; Astakhova, T.M.; Zakharova, L.; Sharova, N.P. Estrogen Receptors and Ubiquitin Proteasome System: Mutual Regulation. Biomolecules 2020, 10, 500. [Google Scholar] [CrossRef]
- Avendaño, M.S.; Lucas, E.; Jurado-Pueyo, M.; Martínez-Revelles, S.; Vila-Bedmar, R.; MayorJr, F.; Salaices, M.; Briones, A.M.; Murga, C. Increased Nitric Oxide Bioavailability in Adult GRK2 Hemizygous Mice Protects Against Angiotensin II–Induced Hypertension. Hypertension 2014, 63, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Hullmann, J.; Traynham, C.J.; Coleman, R.C.; Koch, W.J. The expanding GRK interactome: Implications in cardiovascular disease and potential for therapeutic development. Pharmacol. Res. 2016, 110, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Ciccarelli, M.; Santulli, G.; Illario, M.; Trimarco, B.; Iaccarino, G. Trafficking GRK2: Cellular and Metabolic consequences of GRK2 subcellular localization. Transl. Med. UniSa 2014, 10, 3–7. [Google Scholar]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Serra, J.; Nadal-Serrano, M.; Pons, D.G.; Roca, P.; Oliver, J.O. Mitochondrial dynamics is affected by 17β-estradiol in the MCF-7 breast cancer cell line. Effects on fusion and fission related genes. Int. J. Biochem. Cell Biol. 2012, 44, 1901–1905. [Google Scholar] [CrossRef]
- Vona, R.; Ascione, B.; Malorni, W.; Straface, E. Mitochondria and Sex-Specific Cardiac Function. Regul. Mech. Striated Muscle Contraction 2018, 1065, 241–256. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W. Mitochondrial dynamism and heart disease: Changing shape and shaping change. EMBO Mol. Med. 2015, 7, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W. Mitochondrial fission/fusion and cardiomyopathy. Curr. Opin. Genet. Dev. 2016, 38, 38–44. [Google Scholar] [CrossRef]
- Ong, S.-B.; Kalkhoran, S.B.; Hernández-Reséndiz, S.; Samangouei, P.; Hausenloy, D.J. Mitochondrial-Shaping Proteins in Cardiac Health and Disease-the Long and the Short of It! Cardiovasc. Drugs Ther. 2017, 31, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.B.; Campos, J.C.; Qvit, N.; Qi, X.; Bozi, L.H.M.; Bechara, L.R.G.; Lima, V.M.; Queliconi, B.B.; Disatnik, M.-H.; Dourado, P.M.M.; et al. A selective inhibitor of mitofusin 1-βIIPKC association improves heart failure outcome in rats. Nat. Commun. 2019, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.J.; Carvalho, R.A.; Portincasa, P.; Bonfrate, L.; Sardao, V.A. Fatty Acid Oxidation and Cardiovascular Risk during Menopause: A Mitochondrial Connection? J. Lipids 2012, 2012, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Maning, J.; McCrink, K.A.; Pollard, C.M.; Desimine, V.L.; Ghandour, J.; Perez, A.; Cora, N.; Ferraino, K.E.; Parker, B.M.; Brill, A.R.; et al. Antagonistic Roles of GRK2 and GRK5 in Cardiac Aldosterone Signaling Reveal GRK5-Mediated Cardioprotection via Mineralocorticoid Receptor Inhibition. Int. J. Mol. Sci. 2020, 21, 2868. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcones, A.C.; Martínez-Cignoni, M.R.; Vila-Bedmar, R.; Yáñez, C.; Lladó, I.; Proenza, A.M.; Mayor, F., Jr.; Murga, C. Cardiac GRK2 Protein Levels Show Sexual Dimorphism during Aging and Are Regulated by Ovarian Hormones. Cells 2021, 10, 673. https://doi.org/10.3390/cells10030673
Arcones AC, Martínez-Cignoni MR, Vila-Bedmar R, Yáñez C, Lladó I, Proenza AM, Mayor F Jr., Murga C. Cardiac GRK2 Protein Levels Show Sexual Dimorphism during Aging and Are Regulated by Ovarian Hormones. Cells. 2021; 10(3):673. https://doi.org/10.3390/cells10030673
Chicago/Turabian StyleArcones, Alba C., Melanie Raquel Martínez-Cignoni, Rocío Vila-Bedmar, Claudia Yáñez, Isabel Lladó, Ana M. Proenza, Federico Mayor, Jr., and Cristina Murga. 2021. "Cardiac GRK2 Protein Levels Show Sexual Dimorphism during Aging and Are Regulated by Ovarian Hormones" Cells 10, no. 3: 673. https://doi.org/10.3390/cells10030673
APA StyleArcones, A. C., Martínez-Cignoni, M. R., Vila-Bedmar, R., Yáñez, C., Lladó, I., Proenza, A. M., Mayor, F., Jr., & Murga, C. (2021). Cardiac GRK2 Protein Levels Show Sexual Dimorphism during Aging and Are Regulated by Ovarian Hormones. Cells, 10(3), 673. https://doi.org/10.3390/cells10030673





