Kappa Free Light Chains in the Context of Blood Contamination, and Other IgA- and IgM-Related Cerebrospinal Fluid Disease Pattern
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Analysis
With QFLC-k as the CSF/serum quotient of FLC-k
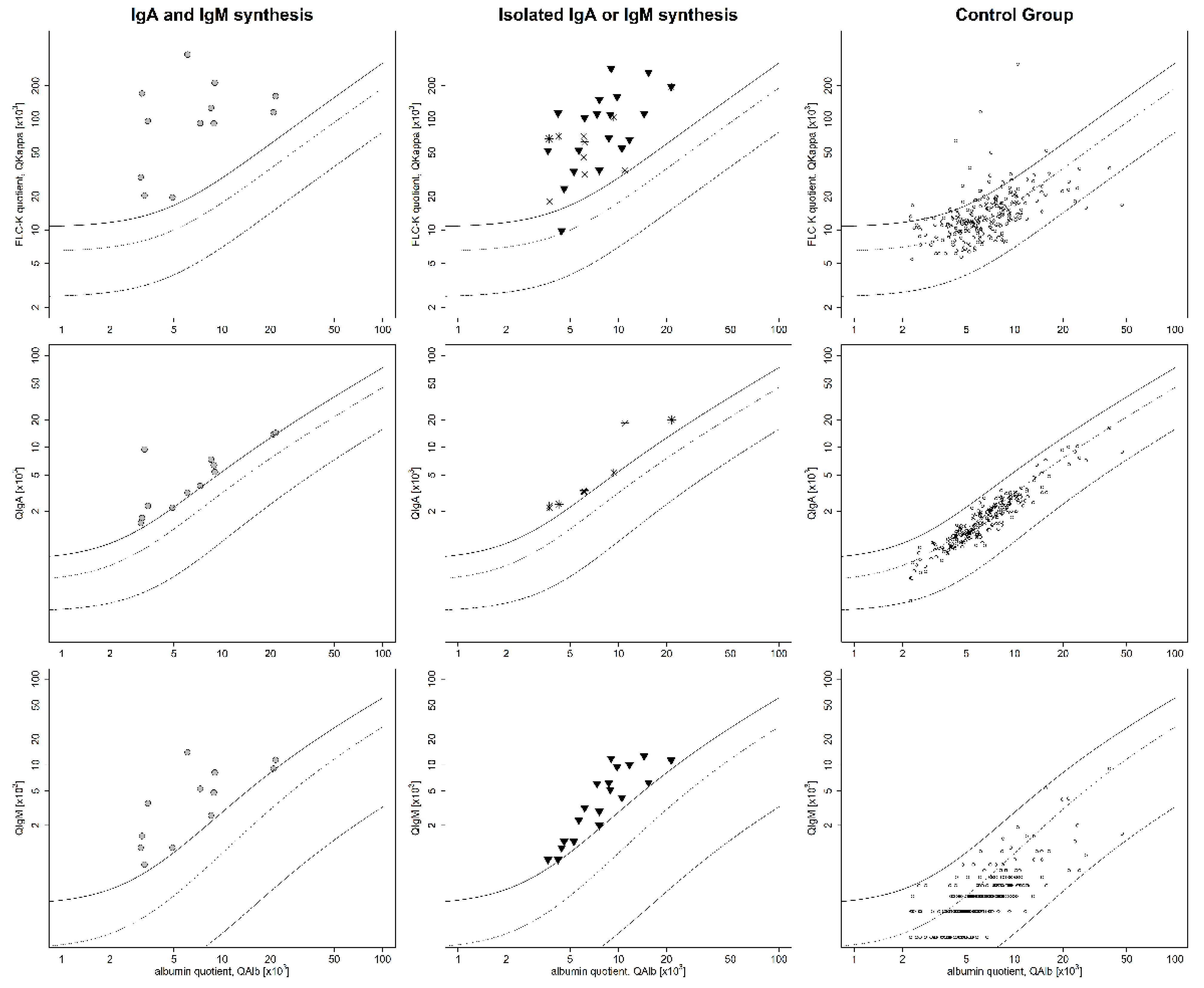
- Cohort I: isolated IgM or A synthesis (QIgM > Qlim-IgM, QIgA > Qlim-IgA) or combined IgA and IgM synthesis. No quantitative or qualitative IgG synthesis, and erythrocyte count <500/µL;
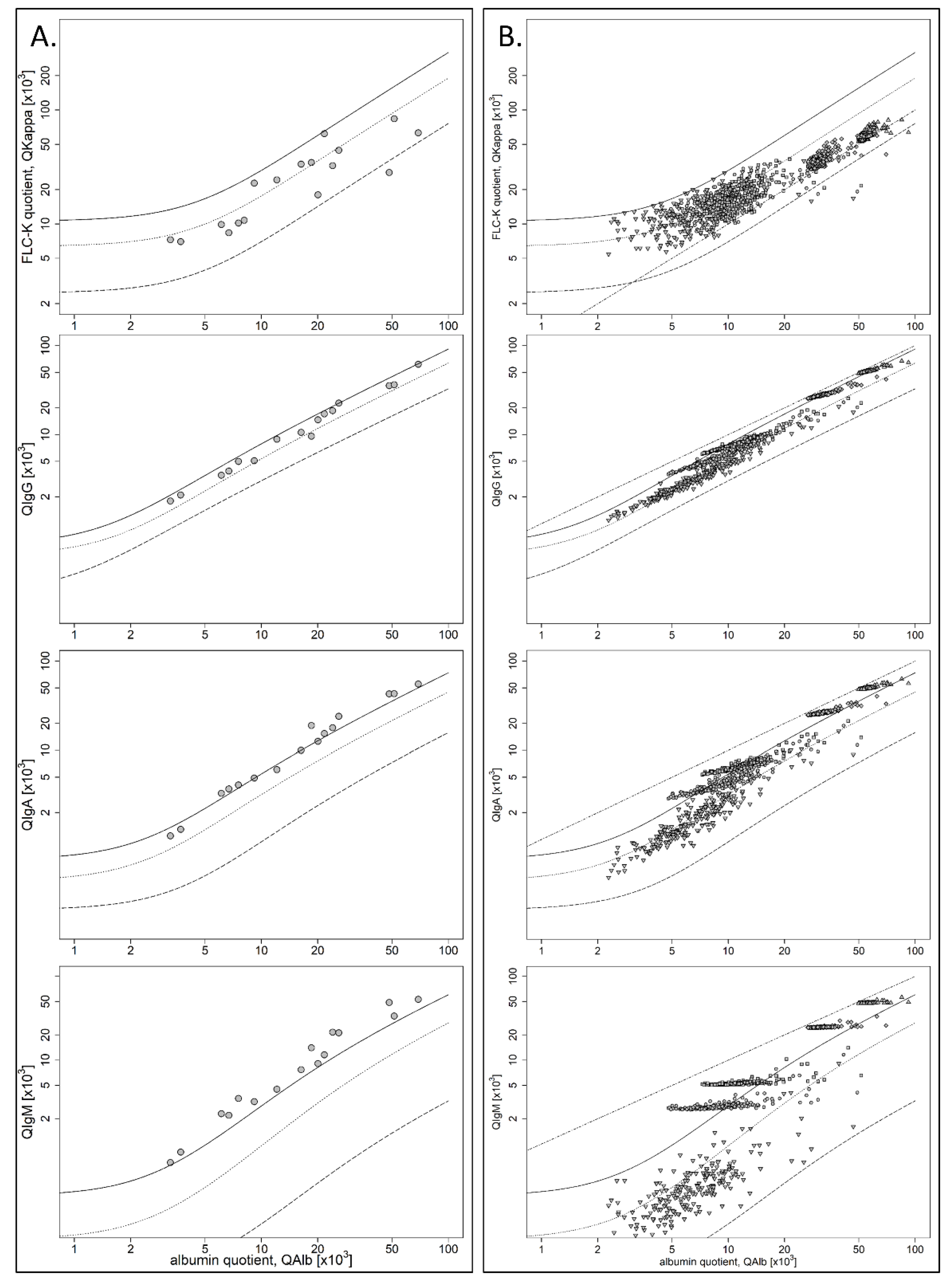
- Cohort II: blood contamination by traumatic lumbar puncture with QIgM > QlimIgM, and if present, QIgA > Qlim-IgA and QIgG > QLim-IgG but QIgM > QIgA > QIgG. Erythrocyte count was >500/µL and/or elevated ferritin, which was done in cases of suspected subarachnoid hemorrhage and always if the erythrocyte count was >100/µL. Ferritin is a very sensitive, while not a specific marker to detect intrathecal blood. The absence of elevated ferritin is a strong indicator of artificial blood contamination [13]. No patient sample had a quantitative or qualitative intrathecal IgG synthesis;
- Control group: no signs of inflammation in CSF profile; no pleocytosis or absence of intrathecal quantitative synthesis of IgM, IgA, IgG; and an absence of OCB in CSF.
2.2. Statistical Analysis
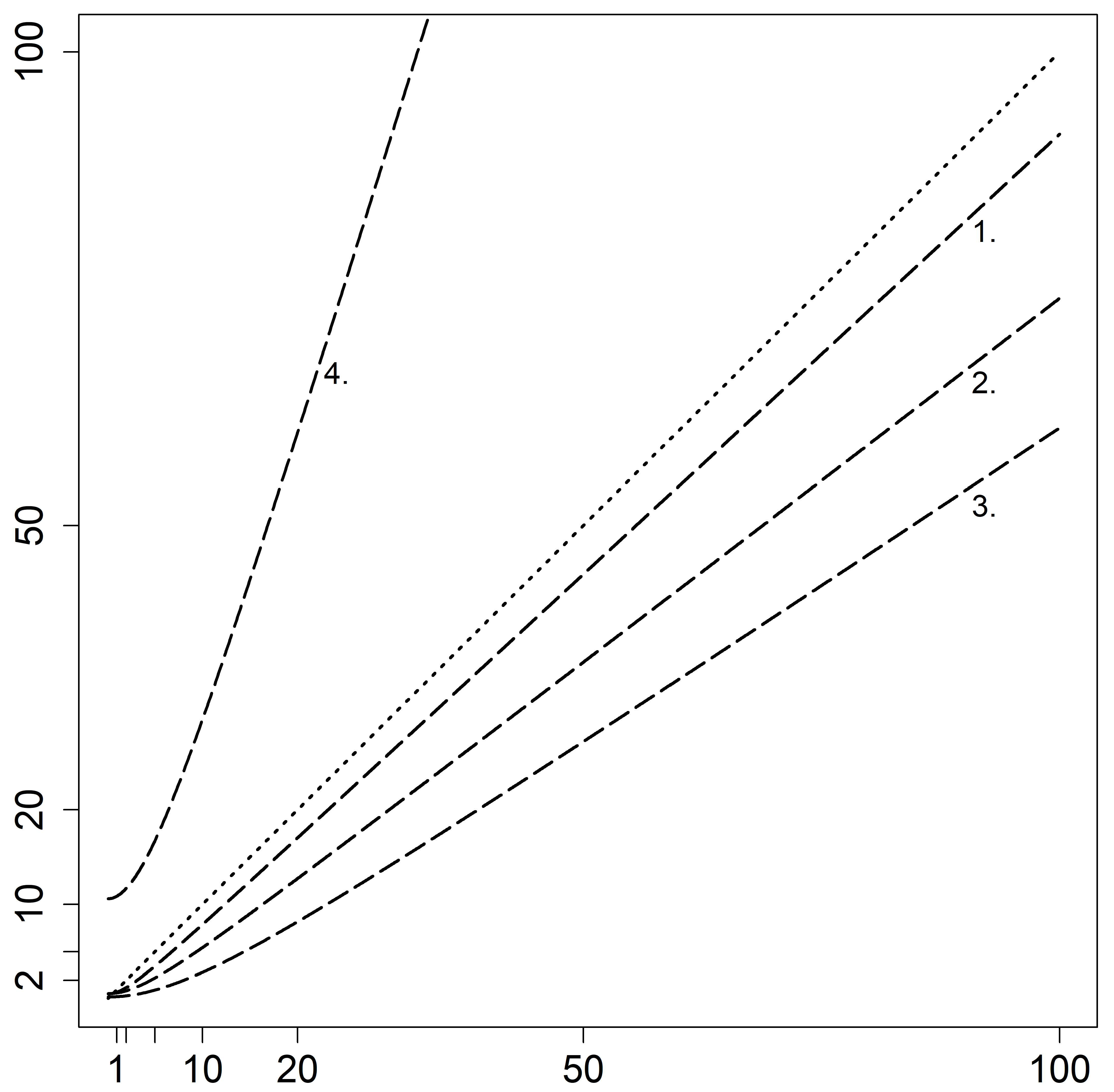
3. Results
4. Discussion
4.1. FLC-k as a CSF Biomarker for Inflammation
4.2. FLC-k in the Context of Artificial Blood Contamination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reiber, H. Flow rate of cerebrospinal fluid (CSF)—A concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J. Neurol. Sci. 1994, 122, 189–203. [Google Scholar] [CrossRef]
- Reiber, H.; Ungefehr, S.; Jacobi, C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult. Scler. 1998, 4, 111–117. [Google Scholar] [CrossRef]
- Reiber, H.; Zeman, D.; Kušnierová, P.; Mundwiler, E.; Bernasconi, L. Diagnostic relevance of free light chains in cerebrospinal fluid—The hyperbolic reference range for reliable data interpretation in quotient diagrams. Clin. Chim. Acta 2019, 497, 153–162. [Google Scholar] [CrossRef]
- Ramsden, D.B. Multiple sclerosis: Assay of free immunoglobulin light chains. Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 54, 5–13. [Google Scholar] [CrossRef]
- Senel, M.; Mojib-Yezdani, F.; Braisch, U.; Bachhuber, F.; Lewerenz, J.; Ludolph, A.C.; Otto, M.; Tumani, H. CSF Free Light Chains as a Marker of Intrathecal Immunoglobulin Synthesis in Multiple Sclerosis: A Blood-CSF Barrier Related Evaluation in a Large Cohort. Front. Immunol. 2019, 10, 641. [Google Scholar] [CrossRef]
- Link, H. Immunoglobulin G and low molecular weight proteins in human cerebrospinal fluid. Chemical and immunological characterisation with special reference to multiple sclerosis. Acta Neurol. Scand. 1967, 43 (Suppl. 28), 1–136. [Google Scholar]
- Süße, M.; Hannich, M.; Petersmann, A.; Zylla, S.; Pietzner, M.; Nauck, M.; Dressel, A. Kappa free light chains in cerebrospinal fluid to identify patients with oligoclonal bands. Eur. J. Neurol. 2018, 25, 1134–1139. [Google Scholar] [CrossRef]
- Nakano, T.; Matsui, M.; Inoue, I.; Awata, T.; Katayama, S.; Murakoshi, T. Free immunoglobulin light chain: Its biology and implications in diseases. Clin. Chim. Acta 2011, 412, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H.; Peter, J.B. Cerebrospinal fluid analysis: Disease-related data patterns and evaluation programs. J. Neurol. Sci. 2001, 184, 101–122. [Google Scholar] [CrossRef]
- Schwenkenbecher, P.; Janssen, T.; Wurster, U.; Konen, F.F.; Neyazi, A.; Ahlbrecht, J.; Puppe, W.; Bönig, L.; Sühs, K.-W.; Stangel, M.; et al. The Influence of Blood Contamination on Cerebrospinal Fluid Diagnostics. Front. Neurol. 2019, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Konen, F.F.; Wurster, U.; Witte, T.; Jendretzky, K.F.; Gingele, S.; Tumani, H.; Sühs, K.-W.; Stangel, M.; Schwenkenbecher, P.; Skripuletz, T. The Impact of Immunomodulatory Treatment on Kappa Free Light Chains as Biomarker in Neuroinflammation. Cells 2020, 9, 842. [Google Scholar] [CrossRef]
- Süße, M.; Reiber, H.; Grothe, M.; Petersmann, A.; Nauck, M.; Dressel, A.; Hannich, M.J. Free light chain kappa and the polyspecific immune response in MS and CIS—Application of the hyperbolic reference range for most reliable data interpretation. J. Neuroimmunol. 2020, 346, 577287. [Google Scholar] [CrossRef]
- Petzold, A.; Worthington, V.; Pritchard, C.; Appleby, I.; Kitchen, N.; Smith, M. The Longitudinal Profile of Bilirubin and Ferritin in the Cerebrospinal Fluid Following a Subarachnoid Hemorrhage: Diagnostic Implications. Neurocrit. Care 2009, 11, 398–402. [Google Scholar] [CrossRef]
- Schwenkenbecher, P.; Konen, F.F.; Wurster, U.; Jendretzky, K.F.; Gingele, S.; Sühs, K.-W.; Pul, R.; Witte, T.; Stangel, M.; Skripuletz, T. The Persisting Significance of Oligoclonal Bands in the Dawning Era of Kappa Free Light Chains for the Diagnosis of Multiple Sclerosis. Int. J. Mol. Sci. 2018, 19, 3796. [Google Scholar] [CrossRef]
- Leurs, C.E.; Twaalfhoven, H.; Lissenberg-Witte, B.I.; Van Pesch, V.; Dujmovic, I.; Drulovic, J.; Castellazzi, M.; Bellini, T.; Pugliatti, M.; Kuhle, J.; et al. Kappa free light chains is a valid tool in the diagnostics of MS: A large multicenter study. Mult. Scler. J. 2019, 26, 912–923. [Google Scholar] [CrossRef]
- Süße, M.; Feistner, F.; Holbe, C.; Grothe, M.; Nauck, M.; Dressel, A.; Hannich, M.J. Diagnostic value of kappa free light chains in patients with one isolated band in isoelectric focusing. Clin. Chim. Acta 2020, 507, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Tjernberg, I.; Johansson, M.; Henningsson, A.J. Diagnostic performance of cerebrospinal fluid free light chains in Lyme neuroborreliosis—A pilot study. Clin. Chem. Lab. Med. 2019, 57, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Gudowska-Sawczuk, M.; Mroczko, B. Free Light Chains as a Novel Diagnostic Biomarker of Immune System Abnormalities in Multiple Sclerosis and HIV Infection. BioMed Res. Int. 2019, 2019, 8382132. [Google Scholar] [CrossRef] [PubMed]
- Girard, L.-P.; Soekojo, C.Y.; Ooi, M.; Poon, L.M.; Chng, W.-J.; de Mel, S. Immunoglobulin M Paraproteinaemias. Cancers 2020, 12, 1688. [Google Scholar] [CrossRef]
- Reiber, H. Knowledge-base for interpretation of cerebrospinal fluid data patterns. Essentials in neurology and psychiatry. Arq. Neuro Psiquiatr. 2016, 74, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. Response to the letter of Villar et al. ‘Quantitative and qualitative IgM analysis in CSF’. Mult. Scler. J. 2011, 18, 252–253. [Google Scholar] [CrossRef]
- Stauch, C.; Reiber, H.; Rauchenzauner, M.; Strasak, A.; Pohl, D.; Hanefeld, F.; Gärtner, J.; Rostásy, K. Intrathecal IgM synthesis in pediatric MS is not a negative prognostic marker of disease progression: Quantitative versus qualitative IgM analysis. Mult. Scler. J. 2010, 17, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Sindic, C.J.; Magnusson, C.G.; Laterre, E.C.; Masson, P.L. IgE in the cerebrospinal fluid. J. Neuroimmunol. 1984, 6, 319–324. [Google Scholar] [CrossRef]
- Dincić, E.; Jovicić, A.; Dokić-Protić, V.; Popović, P.; Dordević, D.; Raicević, R. Significance of de-novo IgD and IgM synthesis in CSF of patients with relapsing-remitting form of disseminated demyelinating disease of CNS. Vojnosanit. Pregl. 2005, 57, 141–147. [Google Scholar]
- Tumani, H.; Petereit, H.-F. Lumbal-Punktion und Liquordiagnostik. Available online: https://dgn.org/leitlinien/ll-030-141-lumbalpunktion-und-liquordiagnostik-2019/ (accessed on 25 July 2019).
- Reiber, H. Proteins in cerebrospinal fluid and blood: Barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003, 21, 79–96. [Google Scholar]

| Patient Cohort I (Intrathecal Synthesis) | Patient Cohort II (Blood Contamination) | Control Group | p-Value | |
|---|---|---|---|---|
| n | 41 | 16 | 239 | |
| Age | 40 (28; 59) | 58 (45; 77) | 52 (37; 68) | |
| Female (%) | 44 | 62 | 52 | |
| CC (/µL) | 23 (3; 48) | 16 (6; 52) | 1 (1; 1) | <0.0001 |
| EC (/µL) | 9 (1; 60) | 4466 (2233; 28750) | 1 (0;4) | <0.0001 |
| Ferritin ng/mL | - | 11.3 (6.1; 26.8) | - | |
| QAlb | 7.3 (4.6; 9.3) | 17.4 (7.3; 24.5) | 6.1 (4.7; 8.6) | 0.02 |
| QIgM | 2.6 (1.1; 6.3) | 8.4 (2.9; 21.3) | 0.3 (0.2; 0.4) | <0.0001 |
| QIgA | 3.1 (2.2; 5.3) | 11.3 (4.0; 20.2) | 1.5 (1.1; 2.4) | <0.0001 |
| QIgG | 3.5 (2.7; 5.0) | 10.1 (4.7; 19.6) | 3.1 (2.3; 4.2) | <0.0001 |
| IF IgM (%) | 21 (4; 60) | 24 (18; 45) | 0 (0; 0) | <0.0001 |
| IF IgA (%) | 4 (0; 17) | 9 (0; 13) | 0 (0; 0) | <0.0001 |
| IF IgG (%) | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | <0.0001 |
| FLC-k serum mg/L | 15.30 (11.40; 18.90) | 16.80 (14.05; 23.07) | 12.40 (9.45; 16.10) | 0.001 |
| FLC-k CSF mg/L | 1.29 (0.65; 1.81) | 0.40 (0.17; 0.80) | 0.20 (0.11; 0.31) | <0.0001 |
| QFLC-k | 91.4 (45.1; 125.8) | 26.4 (10.12; 37.1) | 12.7 (10.2; 17.5) | <0.0001 |
| IF FLC-k (%) | 68 (47; 79) | 0 (0; 0) | 0 (0; 0) | <0.0001 |
| QFLC-k > Qlim–FLC-k (n (%)) | 40 (97.6) | 0 (0) | 19 (6.5) |
| Added Volume of Serum | QIgG > Qlim–IgG; n (%) | QIgA > Qlim–IgA; n (%) | QIgM > Qlim–IgM; n (%) | QFLC-k > Qlim–FLC-k |
|---|---|---|---|---|
| 0.005 mL | 14 (6.3) | 66 (30) | 142 (64.5) | 0 |
| 0.010 mL | 28 (12.7) | 127 (57.7) | 189 (85.9) | 0 |
| 0.050 mL | 138 (62.7) | 213 (96.8) | 215 (97.7) | 0 |
| 0.100 mL | 195 (88.6) | 218 (99) | 218 (99) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannich, M.J.; Dressel, A.; Budde, K.; Petersmann, A.; Nauck, M.; Süße, M. Kappa Free Light Chains in the Context of Blood Contamination, and Other IgA- and IgM-Related Cerebrospinal Fluid Disease Pattern. Cells 2021, 10, 616. https://doi.org/10.3390/cells10030616
Hannich MJ, Dressel A, Budde K, Petersmann A, Nauck M, Süße M. Kappa Free Light Chains in the Context of Blood Contamination, and Other IgA- and IgM-Related Cerebrospinal Fluid Disease Pattern. Cells. 2021; 10(3):616. https://doi.org/10.3390/cells10030616
Chicago/Turabian StyleHannich, Malte Johannes, Alexander Dressel, Kathrin Budde, Astrid Petersmann, Matthias Nauck, and Marie Süße. 2021. "Kappa Free Light Chains in the Context of Blood Contamination, and Other IgA- and IgM-Related Cerebrospinal Fluid Disease Pattern" Cells 10, no. 3: 616. https://doi.org/10.3390/cells10030616
APA StyleHannich, M. J., Dressel, A., Budde, K., Petersmann, A., Nauck, M., & Süße, M. (2021). Kappa Free Light Chains in the Context of Blood Contamination, and Other IgA- and IgM-Related Cerebrospinal Fluid Disease Pattern. Cells, 10(3), 616. https://doi.org/10.3390/cells10030616






