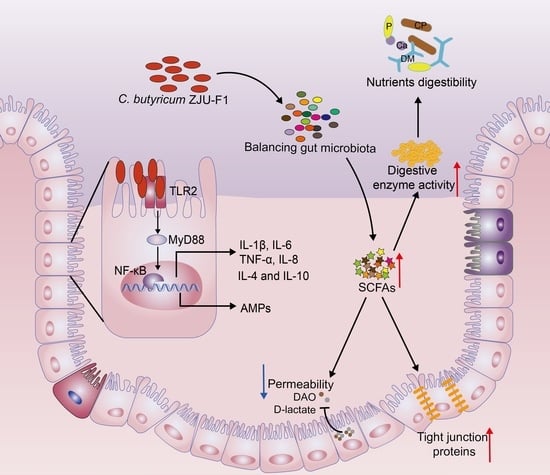
Clostridium Butyricum ZJU-F1 Benefits the Intestinal Barrier Function and Immune Response Associated with Its Modulation of Gut Microbiota in Weaned Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Experiment Design
2.3. Nutrient Digestibility, Digestive Enzyme Activity, and Intestinal Morphology Assessment
2.4. Western Blot Analysis and Antibodies
2.5. RNA Isolation and qRT-PCR
2.6. Plasma D-Lactate and DAO Levels
2.7. Cell Culture
2.8. Cell Damage and Adherence Assay
2.9. Diversity Analysis of Cecal Microorganisms
2.10. Determination of Short-Chain Fatty Acids (SCFAs)
2.11. Statistical Analysis
3. Results
3.1. Effects of C. Butyricum ZJU-F1 on Diarrhea Rate, Apparent Digestibility, and Digestive Enzymes Activity
3.2. Effects of C. Butyricum ZJU-F1 on Intestinal Morphology
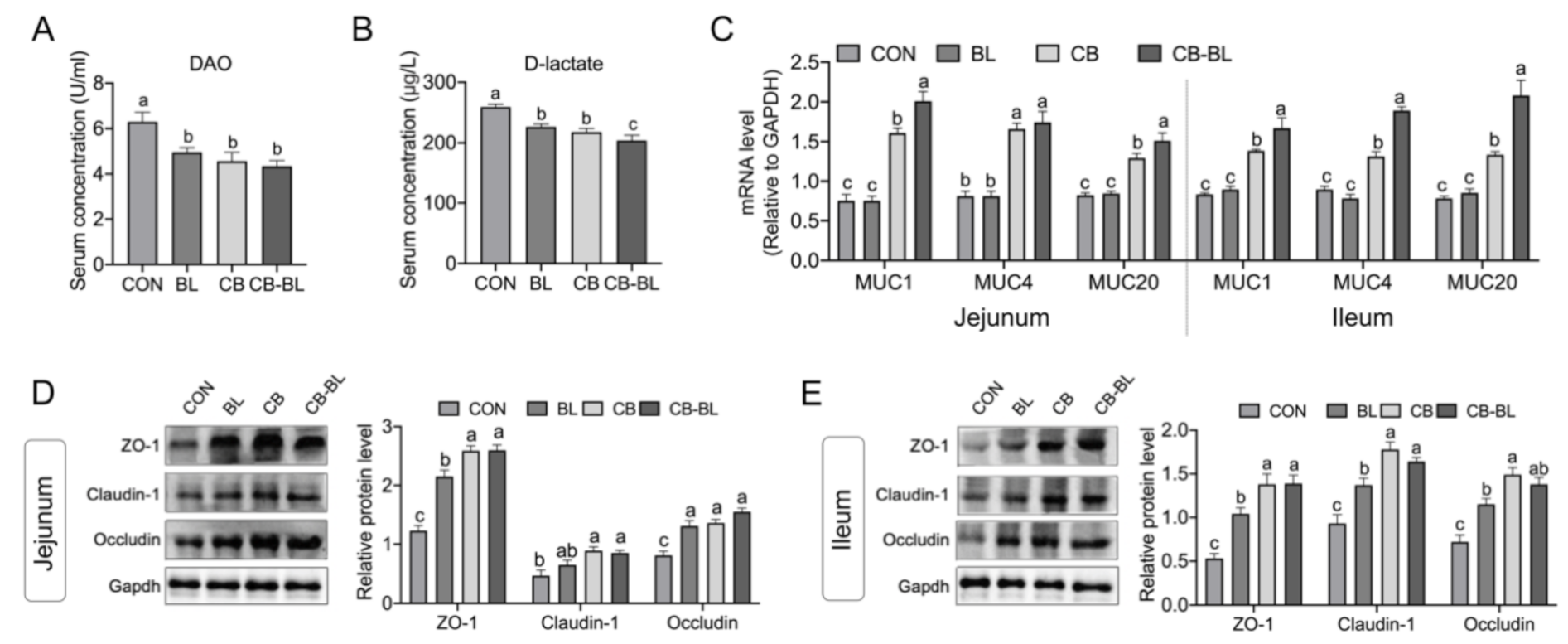
3.3. Effects of C. Butyricum ZJU-F1 on Intestinal Barrier Function
3.4. Effects of C. Butyricum ZJU-F1 on the Intestinal Immune Response
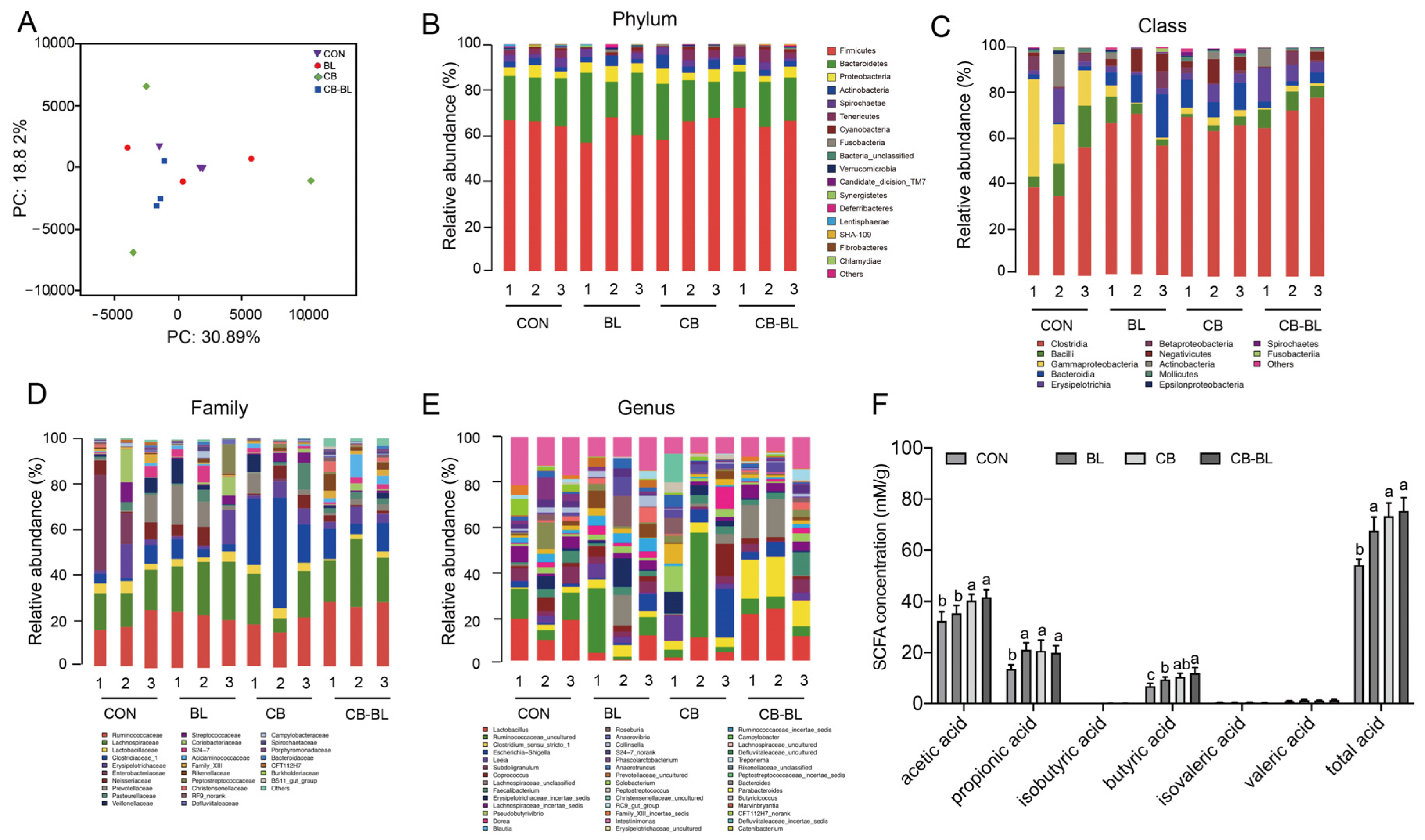
3.5. Effects of C. Butyricum ZJU-F1 on Intestinal Microbial Diversity
3.6. Effects of C. Butyricum ZJU-F1 on Cecal SCFAs
3.7. C. Butyricum ZJU-F1 Enhances the Intestinal Barrier Function and TLR-2-MyD88-NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef]
- Li, Z.; Xu, B.; Lu, Z.; Wang, Y. Effects of long-chain fatty acid supplementation on the growth performance of grower and finisher pigs: A meta-analysis. J. Anim. Sci. Biotechnol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Abraham, B.P.; Quigley, E.M.M. Probiotics in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 769–782. [Google Scholar] [CrossRef]
- Rembacken, B.J.; Snelling, A.M.; Hawkey, P.M.; Chalmers, D.M.; Axon, A. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: A randomised trial. Lancet 1999, 354, 635–639. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukás, M.; Fixa, B.; Kascák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Henker, J.; Müller, S.; Laass, M.W.; Schreiner, A.; Schulze, J. Probiotic Escherichia coli Nissle 1917 (EcN) for Successful Remission Maintenance of Ulcerative Colitis in Children and Adolescents: An Open-Label Pilot Study. Zeitschrift Gastroenterologie 2008, 46, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Bibiloni, R.; Fedorak, R.N.; Tannock, G.W.; Madsen, K.L.; Gionchetti, P.; Campieri, M.; De Simone, C.; Sartor, R.B. VSL#3 Probiotic-Mixture Induces Remission in Patients with Active Ulcerative Colitis. Am. J. Gastroenterol. 2005, 100, 1539–1546. [Google Scholar] [CrossRef]
- Geier, M.S.; Butler, R.N.; Giffard, P.M.; Howarth, G.S. Lactobacillus fermentum BR11, a potential new probiotic, alleviates symptoms of colitis induced by dextran sulfate sodium (DSS) in rats. Int. J. Food Microbiol. 2007, 114, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, M.; Kuitunen, M.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Savilahti, E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr Allergy Immunol. 2005, 16, 65–71. [Google Scholar] [CrossRef]
- Canani, R.B.; Cirillo, P.; Terrin, G.; Cesarano, L.; Spagnuolo, M.I.; De Vincenzo, A.; Albano, F.; Passariello, A.; De Marco, G.; Manguso, F.; et al. Probiotics for treatment of acute diarrhoea in children: Randomised clinical trial of five different preparations. Bmj 2007, 335, 340. [Google Scholar] [CrossRef]
- Henker, J.; Laass, M.W.; Blokhin, B.M.; Maydannik, V.G.; Bolbot, Y.K.; Elze, M.; Wolff, C.; Schreiner, A.; Schulze, J. Probiotic Escherichia coli Nissle 1917 Versus Placebo for Treating Diarrhea of Greater Than 4 Days Duration in Infants and Toddlers. Pediatr. Infect. Dis. J. 2008, 27, 494–499. [Google Scholar] [CrossRef]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; Macdonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef]
- Seki, H.; Shiohara, M.; Matsumura, T.; Miyagawa, N.; Tanaka, M.; Komiyama, A.; Kurata, S. Prevention of antibiotic-associated diarrhea in children by Clostridium butyricum MIYAIRI. Pediatr. Int. 2003, 45, 86–90. [Google Scholar] [CrossRef]
- Cassir, N.; Benamar, S.; La Scola, B. Clostridium butyricum: From beneficial to a new emerging pathogen. Clin. Microbiol. Infection. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Gao, Q.; Qi, L.; Wu, T.; Wang, J. Clostridium butyricum activates TLR2-mediated MyD88-independent signaling pathway in HT-29 cells. Mol. Cell. Biochem. 2011, 361, 31–37. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, D.; Yang, M.; Wen, B.; Lai, J.; Zhou, Y.; Sun, H.; Xiong, L.; Wang, J.; Lin, Y.; et al. Probiotic Clostridium butyricum Improves the Growth Performance, Immune Function, and Gut Microbiota of Weaning Rex Rabbits. Probiotics Antimicrob. Proteins 2018, 11, 1278–1292. [Google Scholar] [CrossRef]
- Huang, T.; Peng, X.-Y.; Gao, B.; Wei, Q.-L.; Xiang, R.; Yuan, M.-G.; Xu, Z.-H. The Effect of Clostridium butyricum on Gut Microbiota, Immune Response and Intestinal Barrier Function During the Development of Necrotic Enteritis in Chickens. Front. Microbiol. 2019, 10, 2309. [Google Scholar] [CrossRef]
- Zong, X.; Wang, T.; Lu, Z.; Song, D.; Zhao, J.; Wang, Y. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest. Sci. 2019, 220, 137–142. [Google Scholar] [CrossRef]
- Wang, C.; Lin, C.; Su, W.; Zhang, Y.; Wang, F.; Wang, Y.; Shi, C.; Lu, Z. Effects of supplementing sow diets with fermented corn and soybean meal mixed feed during lactation on the performance of sows and progeny. J Anim. Sci. 2018, 96, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Bleavins, K.; Perone, P.; Naik, M.; Rehman, M.; Aslam, M.N.; Dame, M.K.; Meshinchi, S.; Bhagavathula, N.; Varani, J. Stimulation of fibroblast proliferation by insoluble gadolinium salts. Biol. Trace Elem. Res. 2012, 145, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Zhao, J.; Wang, H.; Lu, Z.; Wang, F.; Du, H.; Wang, Y. Mettl3 Deficiency Sustains Long-Chain Fatty Acid Absorption through Suppressing Traf6-Dependent Inflammation Response. J. Immunol. 2019, 202, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, D.S.; Rault, L.; Berkova, N.; Le Loir, Y.; Even, S. Inhibition of Staphylococcus aureus Invasion into Bovine Mammary Epithelial Cells by Contact with Live Lactobacillus casei. Appl. Environ. Microbiol. 2012, 79, 877–885. [Google Scholar] [CrossRef]
- Suo, C.; Yin, Y.; Wang, X.; Lou, X.; Song, D.; Wang, X.; Gu, Q. Effects of lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet. Res. 2012, 8, 89. [Google Scholar] [CrossRef]
- Zhao, L.; Luo, L.; Jia, W.; Xiao, J.; Huang, G.; Tian, G.; Li, J.; Xiao, Y. Serum Diamine Oxidase as a Hemorrhagic Shock Biomarker in a Rabbit Model. PLoS ONE 2014, 9, e102285. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.I. Physiological changes in the gastrointestinal tract and host protective immunity: Learning from the mouse-Trichinella spiralis model. Parasitology 2008, 135, 671–682. [Google Scholar] [CrossRef]
- Patel, K.K.; Miyoshi, H.; Beatty, W.L.; Head, R.D.; Malvin, N.P.; Cadwell, K.; Guan, J.-L.; Saitoh, T.; Akira, S.; O Seglen, P.; et al. Autophagy proteins control goblet cell function by potentiating reactive oxygen species production. EMBO J. 2013, 32, 3130–3144. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.-S.; Xu, Z.-H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Podolsky, D.K.V. Innate mechanisms of mucosal defense and repair: The best offense is a good defense. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 277, G495–G499. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Li, Y.-P.; Zhu, Q.; Qiao, J.-Y.; Wang, W.-J. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. J. Appl. Microbiol. 2018, 125, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Zhan, X.; Zeng, X.; Zhou, L.; Cao, G.; Chen, A.; Yang, C. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J. Anim. Sci. Biotechnol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Liu, P.; Zhao, J.; Sun, J.; Guan, W.; Johnston, L.J.; Levesque, C.L.; Fan, P.; He, T.; et al. Dietary Clostridium butyricum Induces a Phased Shift in Fecal Microbiota Structure and Increases the Acetic Acid-Producing Bacteria in a Weaned Piglet Model. J. Agric. Food Chem. 2018, 66, 5157–5166. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Lynch, J.B.; Hsiao, E.Y. Microbiomes as sources of emergent host phenotypes. Science 2019, 365, 1405–1409. [Google Scholar] [CrossRef]
- Wang, K.; Cao, G.; Zhang, H.; Li, Q.; Yang, C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019, 10, 7844–7854. [Google Scholar] [CrossRef] [PubMed]
- Gyles, C.L.; Prescott, J.F.; Songer, J.G.; Thoen, C.O. Pathogenesis of Bacterial Infections in Animals; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rostami, K.; Nejad, M.R.; Asadzadeh, H.; Zali, M.R. PTU-199 Proinflammatory Cytokine (Il-8) in Microscopic Enteritis. Gut 2013, 62, A130. [Google Scholar] [CrossRef]
- Chen, L.; Li, S.; Zheng, J.; Li, W.; Jiang, X.; Zhao, X.; Li, J.; Che, L.; Lin, Y.; Xu, S.; et al. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. Biotechnol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Liu, X.-L.; Yang, R.-X.; Chen, Y.-W.; Liu, C.; Fan, J.-G. Clostridium butyricum B1 alleviates high-fat diet-induced steatohepatitis in mice via enterohepatic immunoregulation. J. Gastroenterol. Hepatol. 2017, 32, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.-J.; Tian, Z.-B.; Wang, Q.-C.; Chen, R.; Nie, J.; Li, J.-S.; Wei, L.-Z. Clostridium butyricum promotes intestinal motility by regulation of TLR2 in interstitial cells of Cajal. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 4730–4738. [Google Scholar] [PubMed]



| Item | Control | BL | CB | CB-BL |
|---|---|---|---|---|
| Zinc (mg Zn (ZnO)/kg) | 1.125 | 1.125 | 1.125 | 1.125 |
| Antibiotics a (mg/kg) | 170 | - | - | - |
| C. butyricum ZJU-F1 (CFU/kg) | - | - | 108 | 108 |
| Bacillus licheniformis (CFU/kg) | - | 109 | - | 109 |
| Item (Ingredient (g/kg)) | Content | Item (Nutrient Levels) b | Content |
|---|---|---|---|
| Maize | 567 | Digestible energy (MJ/kg) | 13.98 |
| Extruded soybean | 130 | Crude protein (g/kg) | 191 |
| Soybean meal | 155 | Moisture (g/kg) | 110.3 |
| Sucrose | 10 | Lys (g/kg) | 13.5 |
| Fish meal | 30 | Met (g/kg) | 3.0 |
| Dried whey | 30 | Ca (g/kg) | 9.3 |
| Spay-dried plasma protein | 10 | P (g/kg) | 6.5 |
| Monocalcium phosphate | 10 | ||
| Limestone | 8 | ||
| Soybean oil | 10 | ||
| Vitamin-trace mineral premix a | 40 |
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| MUC1 | ACACCCATGGGCGCTATGT | GCCTGCAGAAACCTGCTXAT |
| MUC4 | GATGCCCTGGCCACAGAA | TGATTCAAGGTAGCATTCATTTGC |
| MUC20 | AGGCAGTTACAACATCCACAGAAG | CTGTAGACCATGGCCGAGAAC |
| TNF-α | CCAATGGCAGAGTGGGTATG | TGAAGAGGACCTGGGAGTAG |
| IL-6 | TGGCTACTGCCTTCCCTACC | CAGAGATTTTGCCGAGGATG |
| IL-1β | ACAAAAGCCCGTCTTCCTG | ATGTGGACCTCTGGGTATGG |
| IL-4 | GGACACAAGTGCGACATCA | GCACGTGTGGTGTCTGTA |
| IL-8 | TTCGATGCCAGTGCATAAATA | CTGTACAACCTTCTGCACCCA |
| IL-10 | CAGATGGGCGACTTGTTG | ACAGGGCAGAAATTGATGAC |
| PR-39 | CAAGGCCACCTCCGTTTT | CCACTCCATCACCGTTTTCC |
| pBD1 | TTCCTCCTCATGGTCCTGTT | AGGTGCCGATCTGTTTCATC |
| pBD2 | TGTCTGCCTCCTCTCTTCC | AACAGGTCCCTTCAATCCTG |
| pBD3 | CCTTCTCTTTGCCTTGCTCTT | GCCACTCACAGAACAGCTACC |
| NF-κB | CTCGCACAAGGAGACATGAA | ACTCAGCCGGAAGGCATTAT |
| MyD88 | TGGTAGTGGTTGTCTCTGATGA | TGGAGAGAGGCTGAGTGCAA |
| TLR-2 | TCACTTGTCTAACTTATCATCCTCTTG | TCAGCGAAGGTGTCATTATTGC |
| Gapdh | CGGAGTGAACGGATTTGGC | TGCCGTGGGTGGAATCATAC |
| Item | Con | BL | CB | CB-BL |
|---|---|---|---|---|
| Actinobacillus | 2.78 ± 0.32 | 1.55 ± 1.12 | 0.63 ± 0.56 | 1.39 ± 1.16 |
| Alloprevotella | 1.04 ± 0.55 | 3.10 ± 1.95 | 0.29 ± 0.22 | 1.41 ± 0.93 |
| Anaerotruncus | 1.68 ± 0.48 | 2.03 ± 1.20 | 2.06 ± 1.75 | 1.01 ± 0.37 |
| Anaerovibrio | 0.89 ± 0.57 | 4.70 ± 2.38 | 1.30 ± 1.14 | 0.78 ± 0.40 |
| Blautia | 1.76 ± 0.33 | 1.90 ± 0.93 | 4.27 ± 2.47 | 1.32 ± 0.29 |
| Clostridium_sensu_stricto_1 | 10.07 ± 2.82 | 12.79 ± 8.34 | 17.71 ± 12.80 | 5.67 ± 0.64 |
| Coprococcus | 4.94 ± 1.63 | 3.56 ± 0.89 | 1.70 ± 0.95 | 3.37 ± 1.55 |
| Dorea | 4.23 ± 3.87 | 1.65 ± 0.83 | 1.31 ± 2.08 | 1.47 ± 0.96 |
| Erysipelotrichaceae_incertae_sedis | 2.20 ± 1.70 | 4.67 ± 3.98 | 5.49 ± 2.13 | 0.68 ± 0.08 |
| Escherichia-Shigella | 0.18 ± 0.12 | 4.62 ± 4.45 | 1.87 ± 1.32 | 10.75 ± 5.27 |
| Faecalibacterium | 1.33 ± 1.07 b | 3.48 ± 1.68 ab | 4.53 ± 3.36 ab | 1.20 ± 1.12 b |
| Lachnospiraceae_incertae_sedis | 1.54 ± 0.61 | 2.24 ± 0.56 | 5.52 ± 3.12 | 1.87 ± 1.04 |
| Lachnospiraceae_unclassified | 4.66 ± 1.87 | 2.28 ± 1.06 | 1.29 ± 0.55 | 4.69 ± 0.69 |
| Lactobacillus | 1.78 ± 0.62 b | 3.95 ± 0.81 b | 4.32 ± 0.17 b | 15.55 ± 2.13 a |
| Leeia | 1.61 ± 0.51 | 2.94 ± 2.27 | 9.27 ± 6.61 | 3.49 ± 1.76 |
| Phascolarctobacterium | 5.18 ± 2.56 a | 1.05 ± 0.54 b | 0.28 ± 0.23 b | 0.60 ± 0.29 b |
| Pseudobutyrivibrio | 3.01 ± 1.57 | 3.22 ± 0.80 | 4.87 ± 4.70 | 1.85 ± 0.36 |
| Roseburia | 1.13 ± 0.68 | 2.72 ± 0.78 | 3.33 ± 3.26 | 1.27 ± 0.53 |
| Ruminococcaceae_unclassified | 2.44 ± 0.66 | 4.94 ± 0.50 | 0.73 ± 0.06 | 1.23 ± 0.27 |
| Ruminococcaceae_uncultured | 15.34 ± 3.04 | 5.03 ± 3.27 | 4.95 ± 2.61 | 18.16 ± 3.77 |
| Streptococcus | 0.72 ± 0.21 | 3.10 ± 2.76 | 3.15 ± 0.58 | 1.91 ± 1.32 |
| Subdoligranulum | 3.49 ± 1.29 | 2.45 ± 0.90 | 2.49 ± 1.08 | 5.23 ± 2.60 |
| Other | 27.94 ± 4.53 | 21.99 ± 3.56 | 18.59 ± 7.35 | 15.04 ± 7.35 |
| Total | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Wang, T.; Xiao, X.; Cheng, Y.; Wang, F.; Jin, M.; Wang, Y.; Zong, X. Clostridium Butyricum ZJU-F1 Benefits the Intestinal Barrier Function and Immune Response Associated with Its Modulation of Gut Microbiota in Weaned Piglets. Cells 2021, 10, 527. https://doi.org/10.3390/cells10030527
Fu J, Wang T, Xiao X, Cheng Y, Wang F, Jin M, Wang Y, Zong X. Clostridium Butyricum ZJU-F1 Benefits the Intestinal Barrier Function and Immune Response Associated with Its Modulation of Gut Microbiota in Weaned Piglets. Cells. 2021; 10(3):527. https://doi.org/10.3390/cells10030527
Chicago/Turabian StyleFu, Jie, Tenghao Wang, Xiao Xiao, Yuanzhi Cheng, Fengqin Wang, Mingliang Jin, Yizhen Wang, and Xin Zong. 2021. "Clostridium Butyricum ZJU-F1 Benefits the Intestinal Barrier Function and Immune Response Associated with Its Modulation of Gut Microbiota in Weaned Piglets" Cells 10, no. 3: 527. https://doi.org/10.3390/cells10030527
APA StyleFu, J., Wang, T., Xiao, X., Cheng, Y., Wang, F., Jin, M., Wang, Y., & Zong, X. (2021). Clostridium Butyricum ZJU-F1 Benefits the Intestinal Barrier Function and Immune Response Associated with Its Modulation of Gut Microbiota in Weaned Piglets. Cells, 10(3), 527. https://doi.org/10.3390/cells10030527