Comparison of Monoclonal Gammopathies Linked to Poliovirus or Coxsackievirus vs. Other Infectious Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Separation of Monoclonal and Non-Clonal Igs
2.3. Analysis of the Specificity of Recognition of Monoclonal Igs Using the GlcSph and MIAA Assays
2.4. Analysis of the Specificity of Recognition of Monoclonal Igs Using PEPperCHIP® Infectious Epitope Micro-Arrays
2.5. Confirmation of the Recognition of Enterovirus VP1 Coat Protein Sequences by Monoclonal Igs Using Dot Blotting Assays with Peptides
2.6. Statistics
3. Results
3.1. Characteristics of Patients and Monoclonal Igs
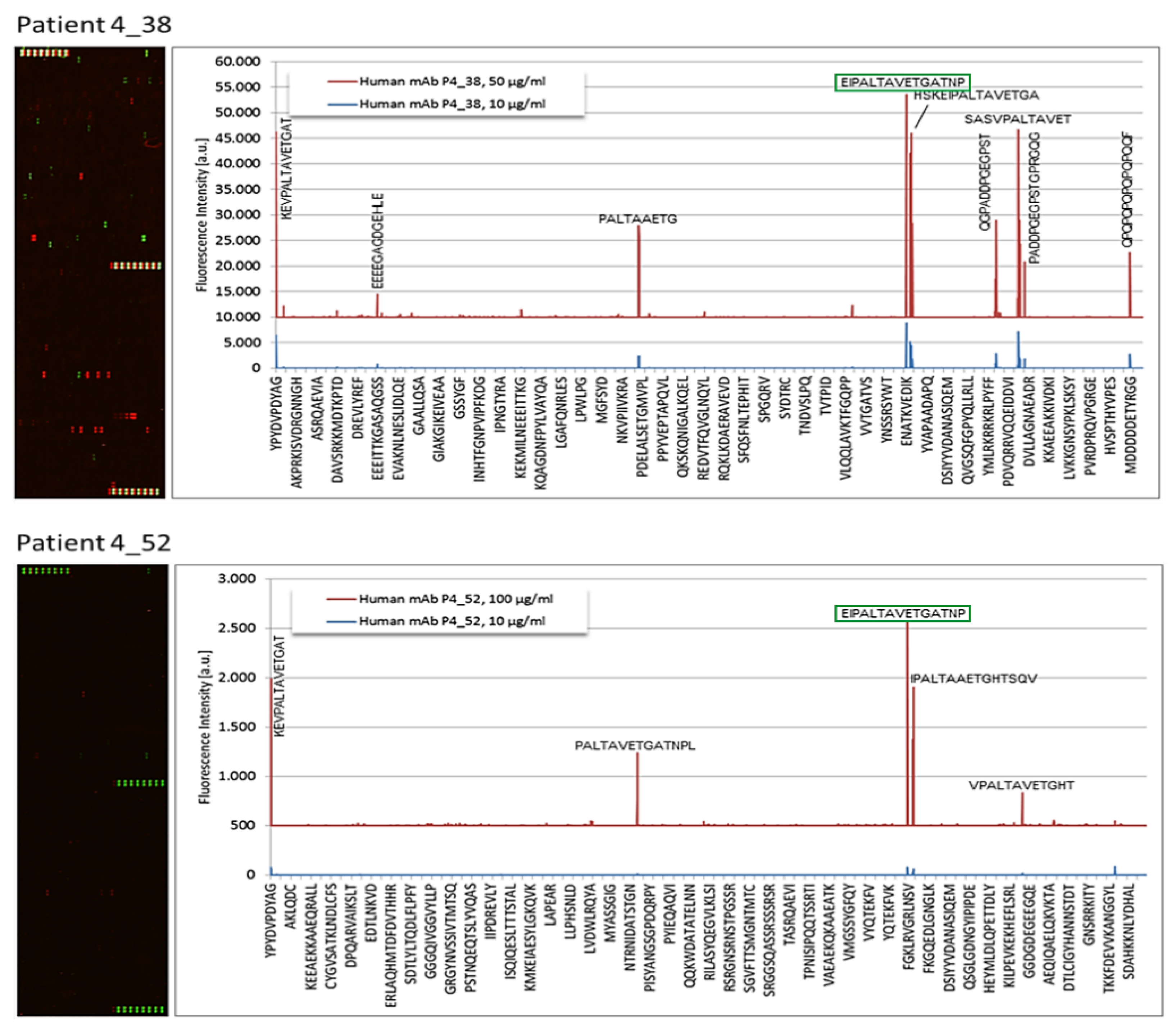
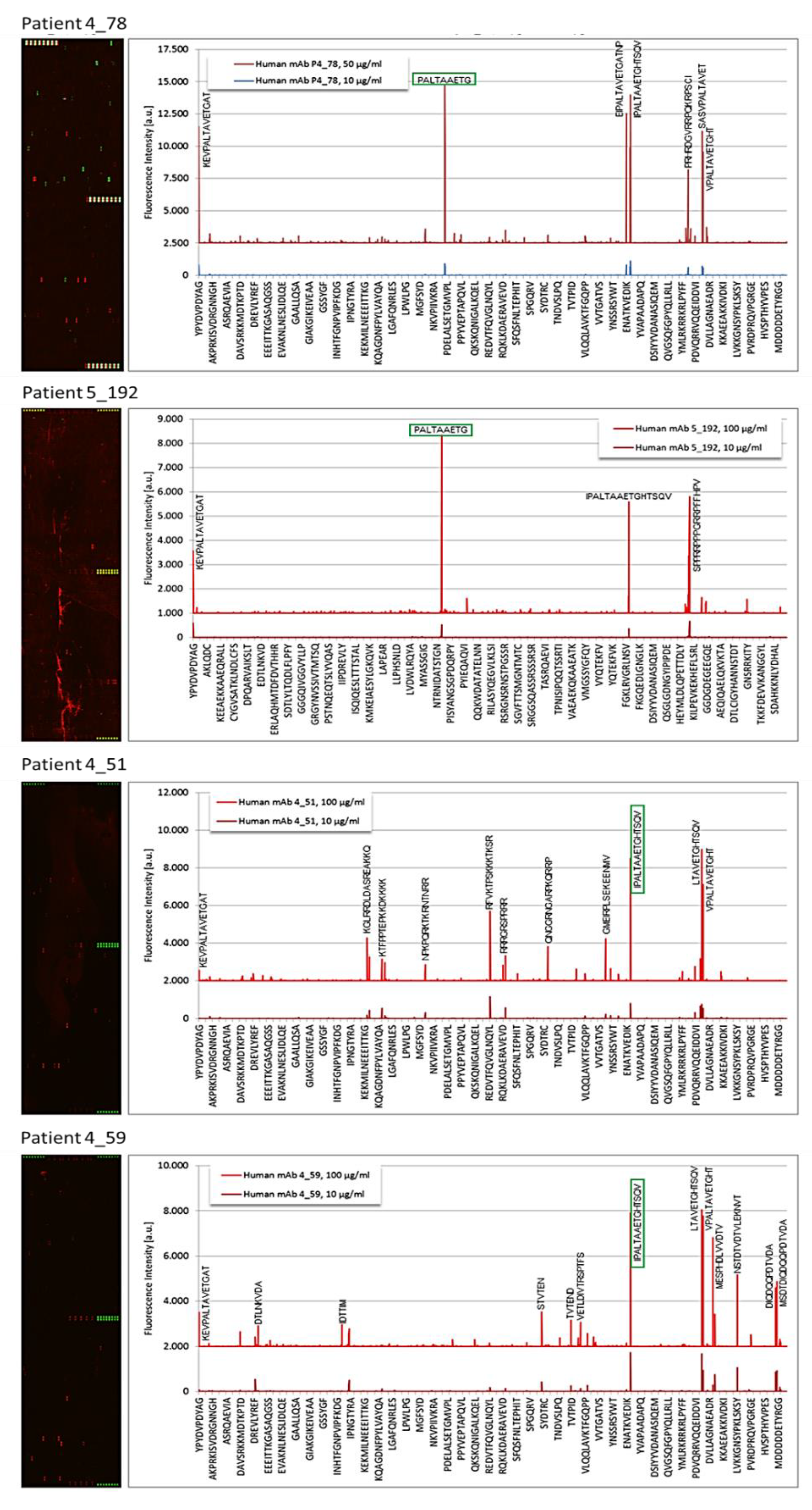
3.2. Epitope Recognition by Monoclonal Igs as Assessed by PEPperCHIP® Infectious Epitope MicroArrays
3.3. Confirmation of Poliovirus and Coxsackievirus VP1 Recognition by PALTAV/AETG-Specific Monoclonal Igs Using Dot Blotting Assays
3.4. Screening of MGUS/SM and MM Cohorts for Poliovirus and Coxsackievirus VP1 Recognition by Serum Antibodies and Purified Monoclonal Igs
3.5. Characteristics of Patients with a PALTAV/AETG-Specific Monoclonal Ig
3.6. Comparison of Monoclonal Gammopathies Linked to the PALTAV/AETG Epitopes vs. Monoclonal Gammopathies Linked to Infectious Pathogens Other Than Enteroviruses or Linked to GlcSph
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhodapkar, M.V. MGUS to myeloma: A mysterious gammopathy of underexplored significance. Blood 2016, 128, 2599–2606. [Google Scholar] [CrossRef]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Hermouet, S.; Corre, I.; Gassin, M.; Bigot-Corbel, E.; Sutton, C.A.; Casey, J.W. Hepatitis C virus, human herpesvirus 8 and the development of plasma-cell leukemia. N. Engl. J. Med. 2003, 348, 178–179. [Google Scholar] [CrossRef]
- Bigot-Corbel, E.; Gassin, M.; Corre, I.; Le Carrer, D.; Delaroche, O.; Hermouet, S. Hepatitis C virus (HCV) infection, monoclonal immunoglobulin specific for HCV core protein and plasma-cell malignancy. Blood 2008, 112, 4357–4358. [Google Scholar] [CrossRef][Green Version]
- Bosseboeuf, A.; Feron, D.; Tallet, A.; Rossi, C.; Charlier, C.; Garderet, L.; Caillot, D.; Moreau, P.; Cardo-Vila, M.; Pasqualini, R.; et al. Monoclonal IgG in MGUS and multiple myeloma target infectious pathogens. J. Clin Investig. Insight 2017, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Branagan, A.R.; Liu, J.; Boddupalli, C.S.; Mistry, P.K.; Dhodapkar, M.V. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N. Engl. J. Med. 2016, 374, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Sng, J.; Sekhar Boddupalli, C.; Seckinger, A.; Chesi, M.; Fulciniti, M.; Zhang, L.; Rauniyar, N.; Lopez, M.; Neparidze, N.; et al. Antigen-mediated regulation in monoclonal gammopathies and myeloma. J. Clin. Investig. Insight 2018, 3, e98259. [Google Scholar] [CrossRef] [PubMed]
- Bosseboeuf, A.; Mennesson, N.; Allain-Maillet, S.; Tallet, A.; Piver, E.; Moreau, C.; Moreau, P.; Lehours, P.; Mégraud, F.; Salle, V.; et al. Characteristics of MGUS and Multiple Myeloma According to the Target of Monoclonal Immunoglobulins, Glucosylsphingosine or Epstein-Barr Virus EBNA-1. Cancers 2020, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- Bosseboeuf, A.; Seillier, C.; Mennesson, N.; Allain-Maillet, S.; Fourny, M.; Tallet, A.; Piver, E.; Lehours, P.; Mégraud, F.; Berthelot, L.; et al. Analysis of the targets and glycosylation of monoclonal IgAs from MGUS and myeloma patients. Front. Immunol. 2020, 11, 854, eCollection 2020. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Bar, N.; Xu, M.L.; Dhodapkar, M.; Mistry, P.K. Glucosylsphingosine but not Saposin C, is the target antigen in Gaucher disease-associated gammopathy. Mol. Genet. Metab. 2020, 129, 286–291. [Google Scholar] [CrossRef]
- Panfilio, S.; D’Urso, P.; Annechini, G.; D’Elia, G.M.; De Angelis, F.; Stefanizzi, C.; Pulsoni, A. Regression of a case of Multiple Myeloma with antiviral treatment in a patient with chronic HCV infection. Leuk. Res. Rep. 2013, 2, 39–40. [Google Scholar] [CrossRef][Green Version]
- García, A.R.; Linares, M.; Mennesson, N.; Sanchez-Vega, B.; Sanchez, R.; Fernandez, R.A.; Bigot-Corbel, E.; Hermouet, S.; Lopez, J.M. The Role of Hepatitis C Virus in the Development of Multiple Myeloma: A Case Study. Blood 2018, 132, 5592. [Google Scholar] [CrossRef]
- Feron, D.; Charlier, C.; Gourain, V.; Garderet, L.; Coste-Burel, M.; Le Pape, P.; Weigel, P.; Jacques, Y.; Hermouet, S.; Bigot-Corbel, E. Multiplexed infectious protein microarray immunoassay suitable for the study of the specificity of monoclonal immunoglobulins. Anal. Biochem. 2013, 433, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Bosseboeuf, A.; Allain, S.; Mennesson, N.; Tallet, A.; Rossi, C.; Garderet, L.; Caillot, D.; Moreau, P.; Piver, E.; Girodon, F.; et al. Pro-inflammatory state in MGUS and Myeloma is characterized by low sialylation of pathogen-specific and other monoclonal and polyclonal immunoglobulin G. Front. Immunol. 2017, 8, 1347, eCollection 2017. [Google Scholar] [CrossRef]
- Nooij, F.J.; Van der Sluijs-Gelling, A.J.; Jol-Van der Zijde, C.M.; Van Tol, M.J.; Haas, H.; Radl, J. Immunoblotting techniques for the detection of low level homogeneous immunoglobulin components in serum. J. Immunol. Methods 1990, 134, 273–281. [Google Scholar] [CrossRef]
- Braun, W.; Abraham, R. Modified diffusion blotting for rapid and efficient protein transfer with PhastSystem. Electrophoresis 1989, 10, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Gorbalenya, A.E.; Harvala, H.; Hovi, T.; Knowles, N.J.; Lindberg, A.M.; Oberste, M.S.; Palmenberg, A.C.; Reuter, G.; Skern, T.; et al. Recommendations for the nomenclature of enteroviruses and rhinoviruses. Arch. Virol. 2020, 165, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Oberste, M.S.; Maher, K.; Kilpatrick, D.R.; Pallansch, M.A. Molecular evolution of the human enteroviruses: Correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 1999, 73, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Salk, J.E. Poliomyelitis vaccination in the fall of 1956. Am. J. Public Health Nations Health 1957, 47, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sabin, A.B.; Boulger, L.R. History of Sabin attenuated poliovirus oral live vaccine strains. J. Biol. Stand. 1973, 1, 115–118. [Google Scholar] [CrossRef]
- Sabin, A.B.; Ramos-Alvarez, M.; Alvarez-Amezquita, J.; Pelon, W.; Michaels, R.H.; Spigland, I.; Koch, M.A.; Barnes, J.M.; Rhim, J.S. Live, orally given poliovirus vaccine. Effects of rapid mass immunization on population under conditions of massive enteric infection with other viruses. JAMA 1960, 173, 1521–1526. [Google Scholar] [CrossRef]
- Kew, O.M.; Sutter, R.W.; de Gourville, E.M.; Dowdle, W.R.; Pallansch, M.A. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 2005, 59, 587–635. [Google Scholar] [CrossRef]
- Signorini, L.; Barbi, M.; Matteelli, A.; Binda, S.; Didò, P.; Caroppo, S.; Primache, V.; Pisani, S.; Messino, C.; Brunelli, M.; et al. Prevalence of anti-poliovirus type 1, 2 and 3 antibodies in unvaccinated Italian travelers. J. Travel. Med. 2004, 11, 34–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nijsten, D.; Carrillo-Santisteve, P.; Miglietta, A.; Ruitenberg, J.; Lopalco, P.L. Is EU/EEA population protected from polio? Hum. Vaccin Immunother 2015, 11, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Prevots, D.R.; Pascual, F.B.; Angellili, M.L.; Brayden, R.; Irigoyen, M.; Larussa, P.; Sawyer, M.; Baughman, A.L.; Pallansch, M.A. Population immunity to polioviruses among preschool children from four urban underserved low income communities, United States, 1997–2001. Pediatr. Infect. Dis. J. 2004, 23, 1130–1136. [Google Scholar] [PubMed]
- Sciandra, I.; Falasca, F.; Maida, P.; Tranquilli, G.; Di Carlo, D.; Mazzuti, L.; Melengu, T.; Giannelli, G.; Antonelli, G.; Turriziani, O. Seroprevalence of group B Coxsackieviruses: Retrospective study in an Italian population. J. Med. Virol. 2020, 92, 3138–3143. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Sexton, R.; Hoering, A.; Van Rhee, F.; Barlogie, B.; Orlowski, R. Race-Dependent Differences in Risk, Genomics and Epstein-Barr Virus Exposure in Monoclonal Gammopathies: Results of SWOG S0120. Clin. Cancer Res. 2020, 26, 5814–5819. [Google Scholar] [CrossRef]
- Manojlovic, Z.; Christofferson, A.; Liang, W.S.; Aldrich, J.; Washington, M.; Wong, S.; Rohrer, D.; Jewell, S.; Kittles, R.A.; Derome, M.; et al. Comprehensive molecular profiling of 718 Multiple Myelomas reveals significant differences in mutation frequencies between African and European descent cases. PLoS Genet. 2017, 13, e1007087. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D.; Hill, E.; Hultcrantz, M.; Rustad, E.H.; Yellapantula, V.; Akhlaghi, T.; Korde, N.; Mailankody, S.; Dew, A.; Papaemmanuil, E.; et al. Molecular underpinnings of clinical disparity patterns in African American vs. Caucasian American multiple myeloma patients. Blood Cancer J. 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Munoz, M.E.; Fuentes-Panana, E.M. Beta and gamma human herpesviruses: Agonistic and antagonistic interactions with the host immune system. Front. Microbiol. 2017, 8, 2521. [Google Scholar] [CrossRef] [PubMed]
- Bunce, C.M.; Drayson, M.T. Dissecting racial disparities in multiple myeloma: Clues from differential immunoglobulin levels. Blood Cancer J. 2020, 10, 44. [Google Scholar] [CrossRef] [PubMed]



| Patient | Pathogen | Protein | Sequence |
|---|---|---|---|
| 4_07 1 | EBV | EBNA-1 | PPRRPPPGRRPFFHPVG |
| 2_91 | HSV-2 | Envelope glycoprotein G | PEEFEGAGDGEPPED |
| 4_09 | HSV-1 | Envelope glycoprotein G | TPPMPSIGLEEEE |
| 4_11 | EBV | EBNA-1 | PPGRRPFFHPVGEADYF |
| 4_12 | HSV-1 | Envelope glycoprotein G | EGAGDGEH + LEGGD |
| 4_19 | EBV | EBNA-1 | GRRPFFHPVGEADYFEY |
| 4_38 | Enterovirus C | VP1 coat protein | EIPALTAVETGATNP |
| 4_51 | CVB1 | VP1 coat protein | IPALTAAETGHTSQV |
| 4_52 | Enterovirus C | VP1 coat protein | EIPALTAVETGATNP |
| 4_59 | CVB1 | VP1 coat protein | IPALTAAETGHTSQV |
| 4_78 | CVB3 | VP1 coat protein | PALTAAETG |
| 4_89 2 | HSV-1 | Envelope glycoprotein G | MPSIGLEEEEEEE |
| 4_98 | HSV-1 | Envelope glycoprotein G | LPQSPGPAFPLAE |
| 5_192 | CVB3 | VP1 coat protein | PALTAAETG |
| Patient Parameters | MGUS/SM | MM |
|---|---|---|
| Men/Women (% men) Age (years) Median (Min-Max) Amount of Mc Ig (g/L) Median (Min-Max) BM plasma cells (%) Median (Min-Max) β2-microglobulin (mg/L) Values Bone lesions Number with lesions (%) ISS Stage Stage I (%) DSS Stage Stage I (%) Anti-GlcSph antibodies Positive (%) | 4/4 (50%) n = 9 68.0 (38.2–84.9) n = 10 17.1 (8.0–37.4) n = 4 3.5 (1.0–5.0) n = 1 2.1 NA NA NA NA NA NA 4/10 (40.0%) | 0/3 (0%) n = 3 70.0 (65–71) n = 3 18.0 (12–25.2) n = 2 28.1 (26.5–29.6) n = 2 2.1-3.2 n = 3 0 (0%) n = 2 2 (100%) n = 3 3 (100%) 1/3 (33.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harb, J.; Mennesson, N.; Lepetit, C.; Fourny, M.; Louvois, M.; Bosseboeuf, A.; Allain-Maillet, S.; Decaux, O.; Moreau, C.; Tallet, A.; et al. Comparison of Monoclonal Gammopathies Linked to Poliovirus or Coxsackievirus vs. Other Infectious Pathogens. Cells 2021, 10, 438. https://doi.org/10.3390/cells10020438
Harb J, Mennesson N, Lepetit C, Fourny M, Louvois M, Bosseboeuf A, Allain-Maillet S, Decaux O, Moreau C, Tallet A, et al. Comparison of Monoclonal Gammopathies Linked to Poliovirus or Coxsackievirus vs. Other Infectious Pathogens. Cells. 2021; 10(2):438. https://doi.org/10.3390/cells10020438
Chicago/Turabian StyleHarb, Jean, Nicolas Mennesson, Cassandra Lepetit, Maeva Fourny, Margaux Louvois, Adrien Bosseboeuf, Sophie Allain-Maillet, Olivier Decaux, Caroline Moreau, Anne Tallet, and et al. 2021. "Comparison of Monoclonal Gammopathies Linked to Poliovirus or Coxsackievirus vs. Other Infectious Pathogens" Cells 10, no. 2: 438. https://doi.org/10.3390/cells10020438
APA StyleHarb, J., Mennesson, N., Lepetit, C., Fourny, M., Louvois, M., Bosseboeuf, A., Allain-Maillet, S., Decaux, O., Moreau, C., Tallet, A., Piver, E., Moreau, P., Salle, V., Bigot-Corbel, E., & Hermouet, S. (2021). Comparison of Monoclonal Gammopathies Linked to Poliovirus or Coxsackievirus vs. Other Infectious Pathogens. Cells, 10(2), 438. https://doi.org/10.3390/cells10020438








