Glycoengineering Human Neural and Adipose Stem Cells with Novel Thiol-Modified N-Acetylmannosamine (ManNAc) Analogs
Abstract
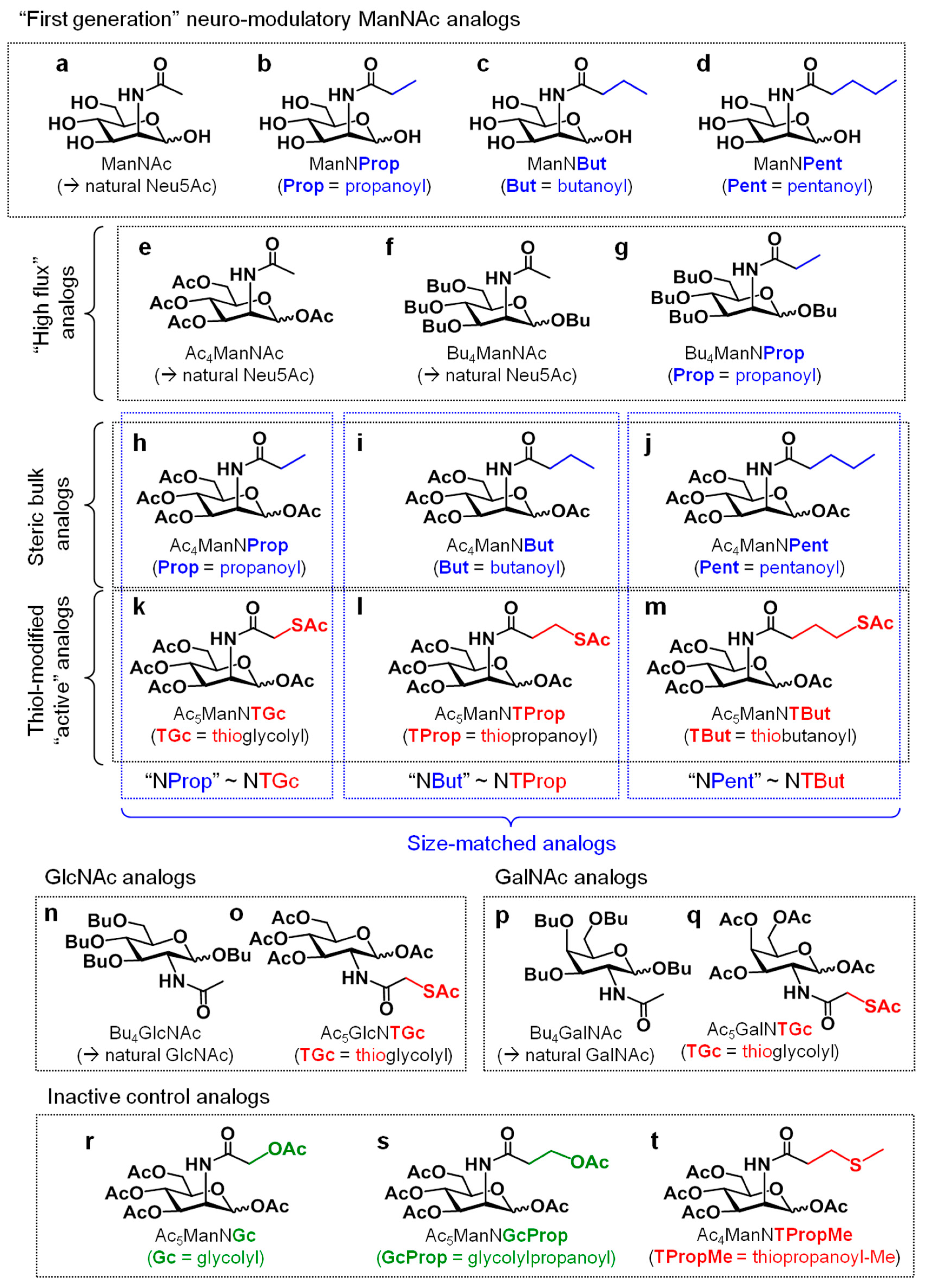
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Treatment of Cells with Sugar Analogs
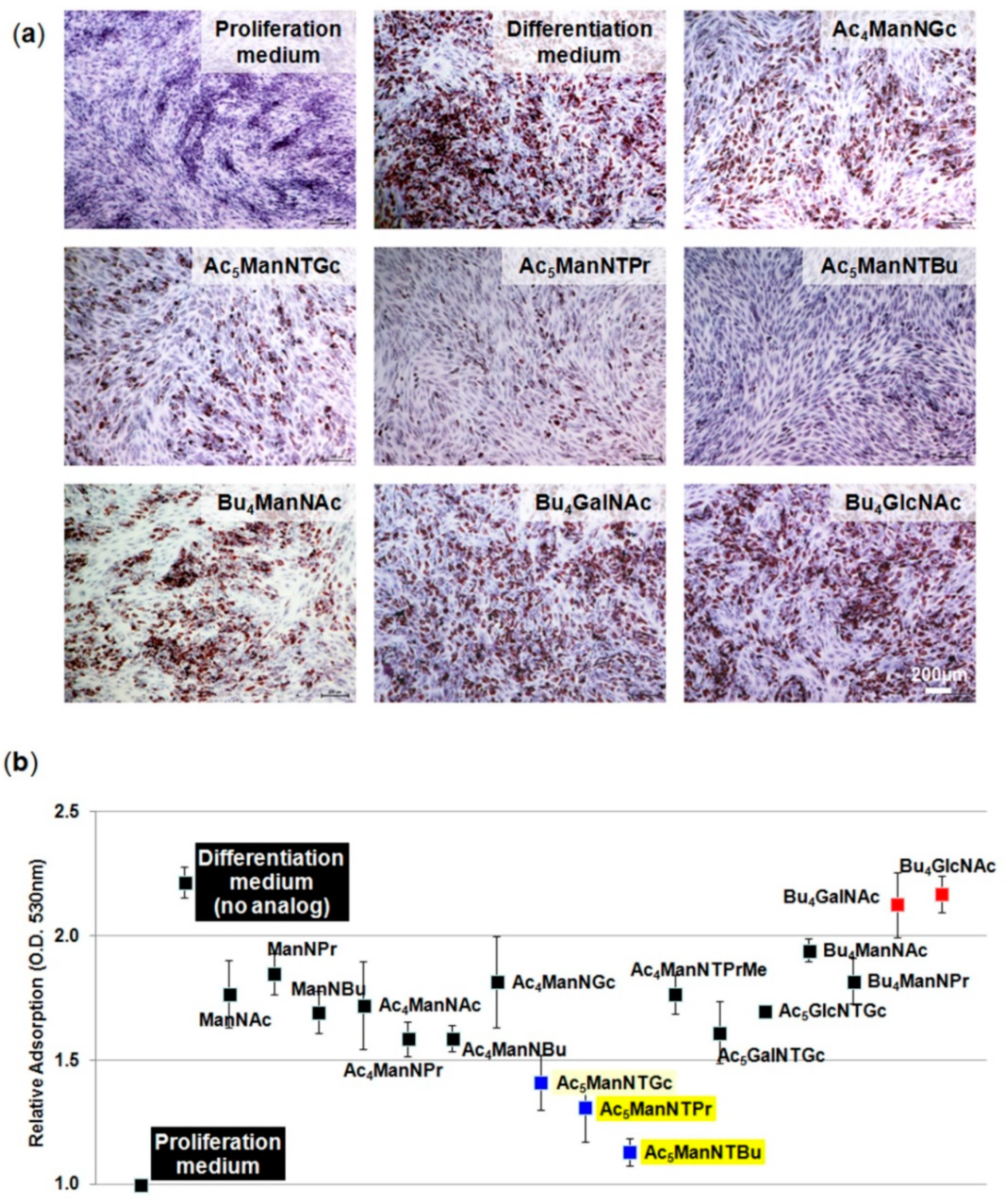
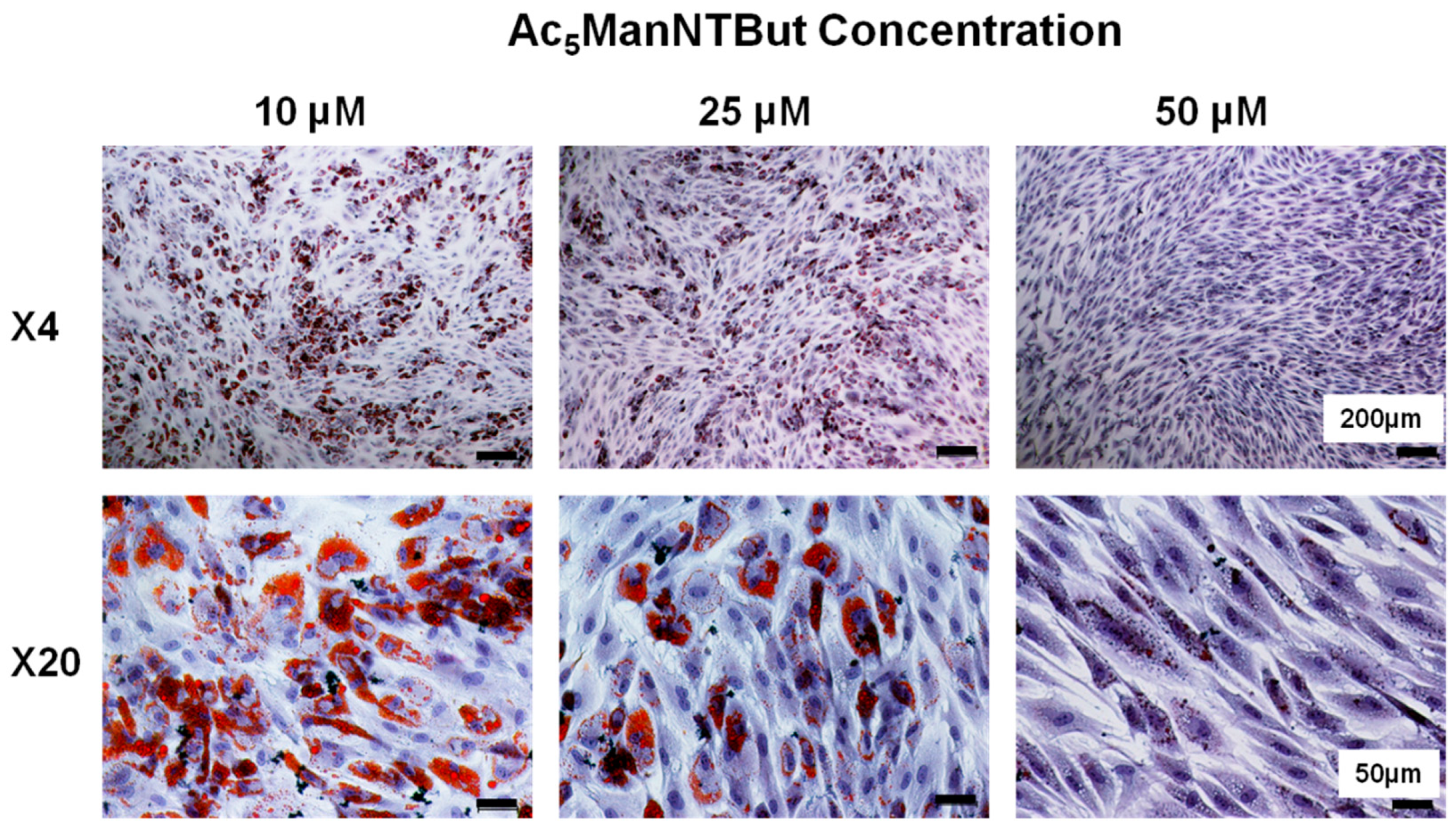
2.3. Oil Red O Staining
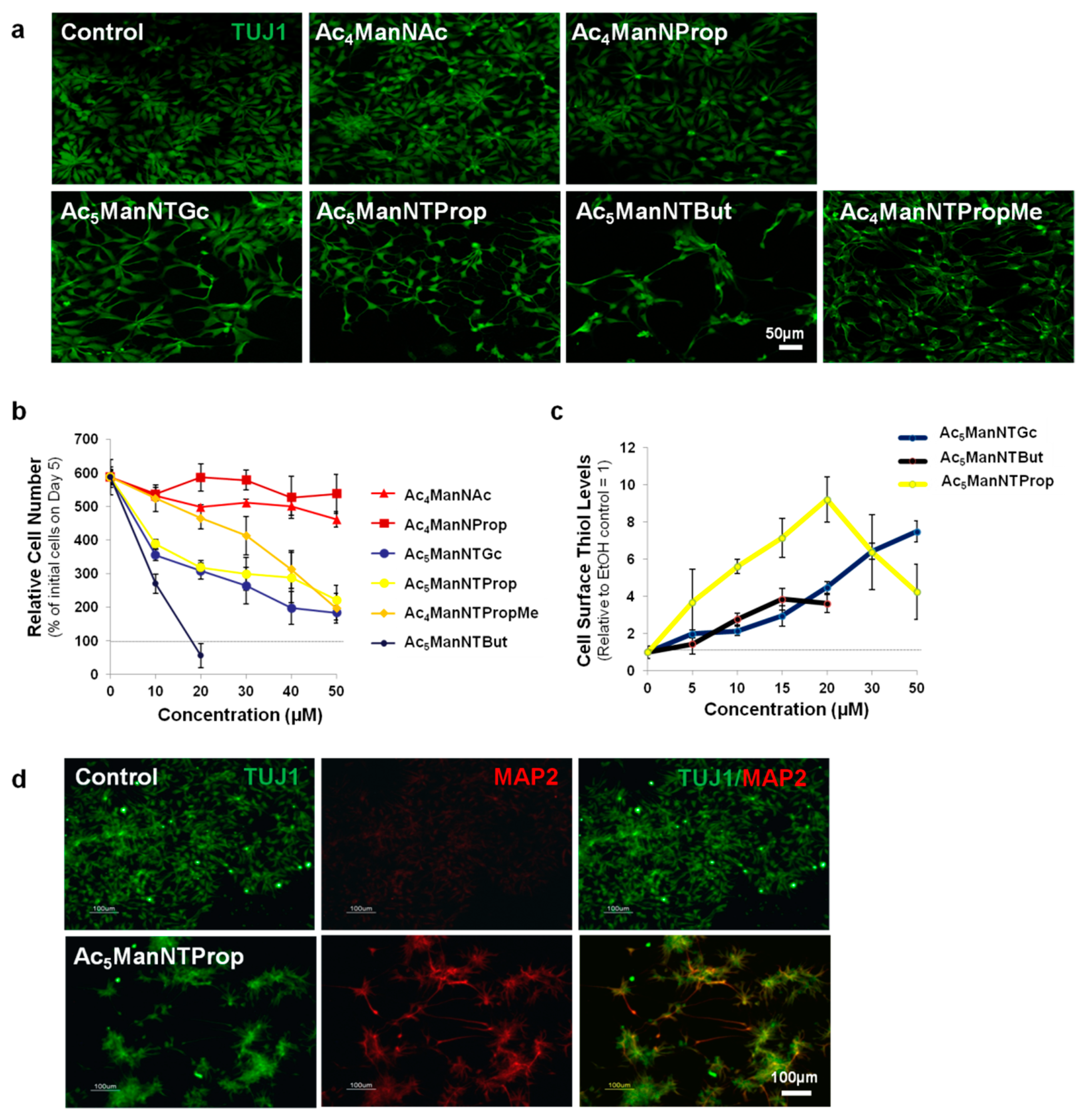
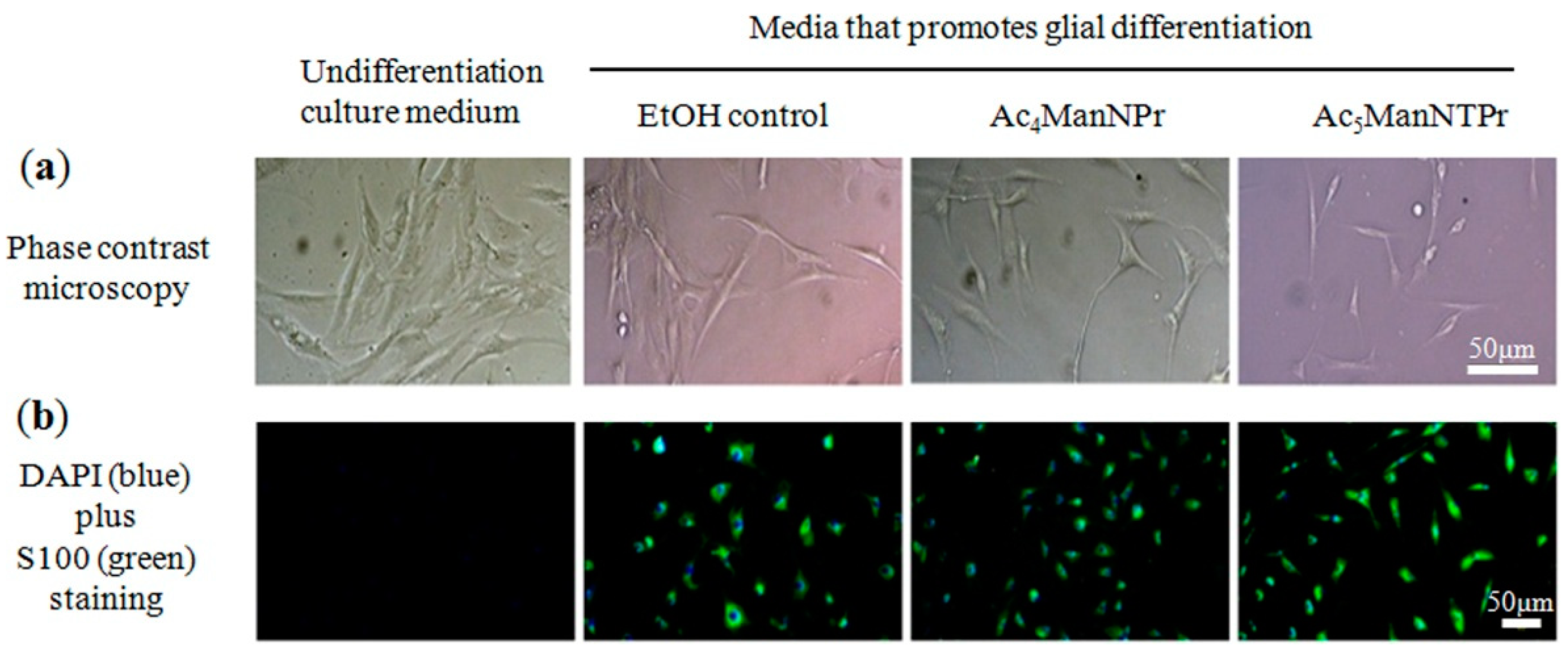
2.4. Immunofluorescence Staining
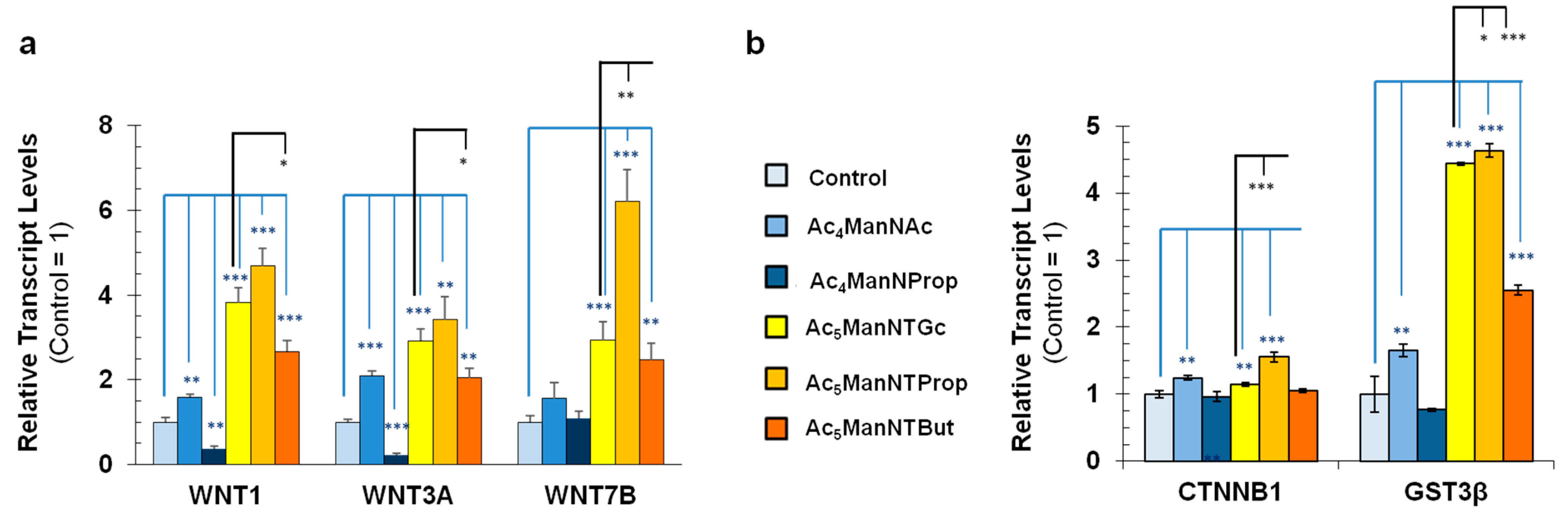
2.5. Reverse Transcriptase Polymerase Chain Reaction Analysis
2.6. Statistical Analysis
3. Results
3.1. Biological Responses of hNSCs Treated with Control and Thiol-Modified ManNAc Analogs
3.2. Impact of Thiol-Modified ManNAc Analogs on Wnt Signaling in hNSCs
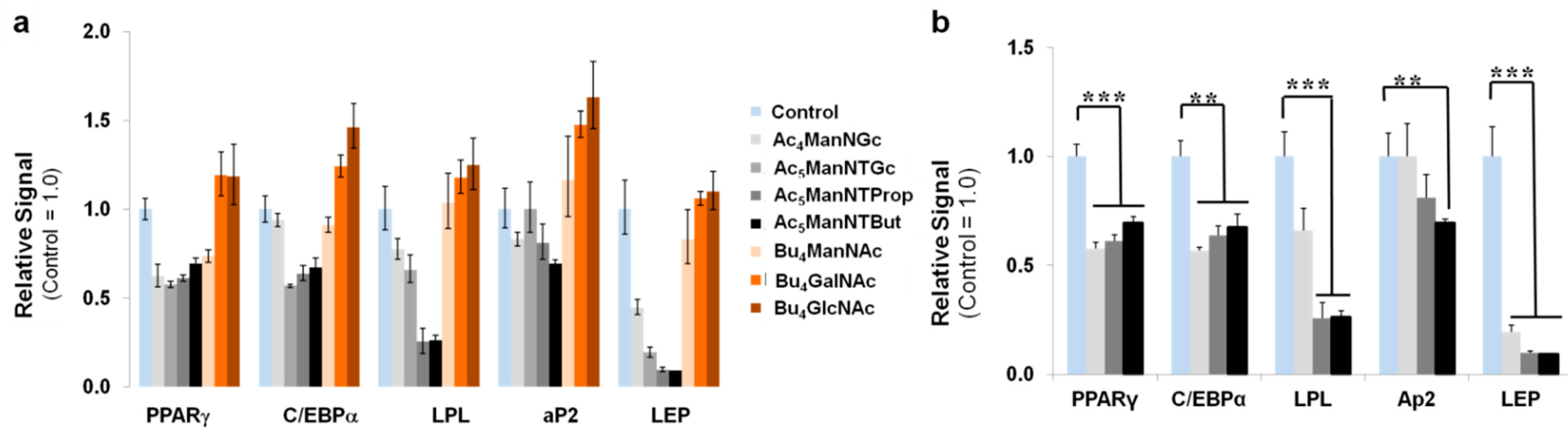
3.3. Thiol-Modified ManNAc Analogs Suppress Adipocyte Differentiation in hASCs
3.4. Glial Cell Differentiation of hASCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kayser, H.; Zeitler, R.; Kannicht, C.; Grunow, D.; Nuck, R.; Reutter, W. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J. Biol. Chem. 1992, 267, 16934–16938. [Google Scholar] [CrossRef]
- Du, J.; Meledeo, M.A.; Wang, Z.; Khanna, H.S.; Paruchuri, V.D.P.; Yarema, K.J. Metabolic glycoengineering: Sialic acid and beyond. Glycobiology 2009, 19, 1382–1401. [Google Scholar] [CrossRef] [PubMed]
- Agatemor, C.; Buettner, M.J.; Ariss, R.S.; Muthiah, K.; Saeui, C.T.; Yarema, K.J. Exploiting metabolic glycoengineering to advance healthcare. Nat. Rev. Chem. 2019, 3, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Agatemor, C.; Muthiah, K.; Ha, L.; Chai, J.; Osman, A.; Robertson, B.M.; Yarema, K.J. Chemistry, Molecular Sciences and Chemical Engineering; Barchi, J., Jr., Ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sampathkumar, S.-G.; Li, A.V.; Jones, M.B.; Sun, Z.; Yarema, K.J. Metabolic installation of thiols into sialic acid modulates adhesion and stem cell biology. Nat. Chem. Biol. 2006, 2, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Lvov, A.; Morin, T.J.; Kobertz, W.R. Chemical control of metabolically-engineered voltage-gated K+ channels. Bioorg. Med. Chem. Lett. 2011, 21, 5021–5024. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Che, P.-L.; Aich, U.; Tan, E.; Kim, H.J.; Sampathkumar, S.-G.; Yarema, K.J. Deciphering glycan linkages involved in Jurkat cell interactions with gold-coated nanofibers via sugar-displayed thiols. Bioorg. Med. Chem. Lett. 2011, 21, 4980–4984. [Google Scholar] [CrossRef]
- Sampathkumar, S.-G.; Li, A.V.; Yarema, K.J. Synthesis of non-natural ManNAc analogs for the expression of thiols on cell surface sialic acids. Nat. Protoc. 2006, 1, 2377–2385. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Fritz, T.A.; Taylor, W.H.; Esko, J.D. Disaccharide uptake and priming in animal cells: Inhibition of sialyl Lewis X by acetylated Gal-β1,4GalNAc β-onaphthalenemethanol. Proc. Natl. Acad. Sci. USA 1995, 92, 3323–3327. [Google Scholar] [CrossRef]
- Lemieux, G.A.; Yarema, K.J.; Jacobs, C.L.; Bertozzi, C.R. Exploiting differences in sialoside expression for selective targeting of MRI contrast reagents. J. Am. Chem. Soc. 1999, 121, 4278–4279. [Google Scholar] [CrossRef]
- Sampathkumar, S.-G.; Jones, M.B.; Yarema, K.J. Metabolic expression of thiol-derivatized sialic acids on the cell surface and their quantitative estimation by flow cytometry. Nat. Protoc. 2006, 1, 1840–1851. [Google Scholar] [CrossRef]
- Mathew, M.P.; Tan, E.; Shah, S.; Bhattacharya, R.; Meledeo, M.A.; Huang, J.; Espinoza, F.A.; Yarema, K.J. Extracellular and intracellular esterase processing of SCFA-hexosamine analogs: Implications for metabolic glycoengineering and drug delivery. Bioorg. Med. Chem. Lett. 2012, 22, 6929–6933. [Google Scholar] [CrossRef]
- Schmidt, C.; Stehling, P.; Schnitzer, J.; Reutter, W.; Horstkorte, R. Biochemical engineering of neural cell surfaces by the synthetic N-propanoyl-substituted neuraminic acid precursor. J. Biol. Chem. 1998, 273, 19146–19152. [Google Scholar] [CrossRef]
- Keppler, O.T.; Horstkorte, R.; Pawlita, M.; Schmidt, C.; Reutter, W. Biochemical engineering of the N-acyl side chain of sialic acid: Biological implications. Glycobiology 2001, 11, 11R–18R. [Google Scholar] [CrossRef] [PubMed]
- Büttner, B.; Kannicht, C.; Schmidt, C.; Löster, K.; Reutter, W.; Lee, H.-Y.; Nöhring, S.; Horstkorte, R. Biochemical engineering of cell surface sialic acids stimulates axonal growth. J. Neurosci. 2002, 22, 8869–8875. [Google Scholar] [CrossRef] [PubMed]
- Bork, K.; Reutter, W.; Gerardy-Schahn, R.; Horstkorte, R. The intracellular concentration of sialic acid regulates the polysialylation of the neural cell adhesion molecule. FEBS Lett. 2005, 579, 5079–5083. [Google Scholar] [CrossRef]
- Granell, A.E.; Palter, K.B.; Akan, I.; Aich, U.; Yarema, K.J.; Betenbaugh, M.J.; Thornhill, W.B.; Recio-Pinto, E. DmSAS is required for sialic acid biosynthesis in cultured Drosophila third instar larvae CNS neurons. ACS Chem. Biol. 2011, 6, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Wratil, P.R.; Horstkorte, R.; Reutter, W. Metabolic glycoengineering with N-acyl side chain modified mannosamines. Angew. Chem. Int. Ed. 2016, 55, 9482–9512. [Google Scholar] [CrossRef]
- Mahal, L.K.; Charter, N.W.; Angata, K.; Fukuda, M.; Koshland, D.E., Jr.; Bertozzi, C.R. A small-molecule modulator of poly-α2,8-sialic acid expression on cultured neurons and tumor cells. Science 2001, 294, 380–382. [Google Scholar] [CrossRef]
- Collins, B.E.; Fralich, T.J.; Itonori, S.; Ichikawa, Y.; Schnaar, R.L. Conversion of cellular sialic acid expression from N-acetyl- to N-glycolylneuraminic acid using a synthetic precursor, N-glycolylmannosamine pentaacetate: Inhibition of myelin-associated glycoprotein binding to neural cells. Glycobiology 2000, 10, 11–20. [Google Scholar] [CrossRef][Green Version]
- Kayser, H.; Geilen, C.C.; Paul, C.; Zeitler, R.; Reutter, W. Incorporation of N-acyl-2-amino-2-deoxy-hexoses into glycosphingolipids of the pheochromocytoma cell line PC 12. FEBS Lett. 1992, 301, 137–140. [Google Scholar] [CrossRef]
- Kayser, H.; Geilen, C.C.; Paul, C.; Zeitler, R.; Reutter, W. New amino sugar analogues are incorporated at different rates into glycoproteins of mouse organs. Experientia 1993, 49, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Che, P.-L.; Wang, Z.-Y.; Aich, U.; Yarema, K.J. Designing a binding interface for control of cancer cell adhesion via 3D topography and metabolic oligosaccharide engineering. Biomaterials 2011, 32, 5427–5437. [Google Scholar] [CrossRef]
- Du, J.; Tan, E.; Kim, H.J.; Zhang, A.; Bhattacharya, R.; Yarema, K.J. Comparative evaluation of chitosan, cellulose acetate, and polyethersulfone nanofiber scaffolds for neural differentiation. Carbohydr. Polym. 2014, 99, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Liu, X.Y.; Song, H.; Yarema, K.J.; Mao, H.-Q. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 2010, 31, 9031–9039. [Google Scholar] [CrossRef]
- Kim, E.J.; Sampathkumar, S.-G.; Jones, M.B.; Rhee, J.K.; Baskaran, G.; Yarema, K.J. Characterization of the metabolic flux and apoptotic effects of O-hydroxyl- and N-acetylmannosamine (ManNAc) analogs in Jurkat (human T-lymphoma-derived) cells. J. Biol. Chem. 2004, 279, 18342–18352. [Google Scholar] [CrossRef]
- Gilbert-Honick, J.; Ginn, B.; Zhang, Y.; Salehi, S.; Wagner, K.R.; Mao, H.Q.; Grayson, W.L. Adipose-derived stem/stromal cells on electrospun fibrin microfiber bundles enable moderate muscle reconstruction in a volumetric muscle loss model. Cell Transplant. 2018, 27, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.; Nahas, Z.; Kimmerling, K.A.; Rosson, G.D.; Elisseeff, J.H. An injectable adipose matrix for soft-tissue reconstruction. Plast. Reconstr. Surg. 2012, 129, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef]
- Sun, X.; Zhu., Y.; Yin, H.-Y.; Xu, F.; Xioa, B.; Jiang, W.-I.; Guon, W.-M.; Meng, H.-Y.; Lu, S.-B.; Wang, Y.; et al. Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: Potential advantage of cellular transient memory function. Stem Cell Res. Ther. 2018, 9, 133. [Google Scholar] [CrossRef]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef]
- Campbell, C.T.; Aich, U.; Weier, C.A.; Wang, J.J.; Choi, S.S.; Wen, M.M.; Maisel, K.; Sampathkumar, S.-G.; Yarema, K.J. Targeting pro-invasive oncogenes with short chain fatty acid-hexosamine analogues inhibits the mobility of metastatic MDA-MB-231 breast cancer cells. J. Med. Chem. 2008, 51, 8135–8147. [Google Scholar] [CrossRef] [PubMed]
- Elmouelhi, N.; Aich, U.; Paruchuri, V.D.P.; Meledeo, M.A.; Campbell, C.T.; Wang, J.J.; Srinivas, R.; Khanna, H.S.; Yarema, K.J. Hexosamine template. A platform for modulating gene expression and for sugar-based drug discovery. J. Med. Chem. 2009, 52, 2515–2530. [Google Scholar] [CrossRef] [PubMed]
- Almaraz, R.T.; Aich, U.; Khanna, H.S.; Tan, E.; Bhattacharya, R.; Shah, S.; Yarema, K.J. Metabolic oligosaccharide engineering with N-acyl functionalized ManNAc analogues: Cytotoxicity, metabolic flux, and glycan-display considerations. Biotechnol. Bioeng. 2012, 109, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Almaraz, R.T.; Tian, Y.; Bhattarcharya, R.; Tan, E.; Chen, S.-H.; Dallas, M.R.; Chen, L.; Zhang, Z.; Zhang, H.; Konstantopoulos, K.; et al. Metabolic flux increases glycoprotein sialylation: Implications for cell adhesion and cancer metastasis. Mol. Cell. Proteom. 2012, 11, M112.017558. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.P.; Tan, E.; Saeui, C.T.; Bovonratwet, P.; Sklar, S.; Bhattacharya, R.; Yarema, K.J. Metabolic flux-driven sialylation alters internalization, recycling, and drug sensitivity of the epidermal growth factor receptor (EGFR) in SW1990 pancreatic cancer cells. Oncotarget 2016, 7, 66491–66511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mathew, M.P.; Tan, E.; Labonte, J.W.; Shah, S.; Saeui, C.T.; Liu, L.; Bhattacharya, R.; Bovonratwet, P.; Gray, J.J.; Yarema, K. Glycoengineering of esterase activity through metabolic flux-based modulation of sialic acid. ChemBioChem 2017, 18, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Kirton, J.P.; Crofts, N.J.; George, S.J.; Brennan, K.; Canfield, A.E. Wnt/β-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: Potential relevance to vascular disease? Circ. Res. 2007, 101, 581–589. [Google Scholar] [CrossRef] [PubMed]
- D’Alimonte, I.; Lannutti, A.; Pipino, C.; Di Tomo, P.; Pierdomenico, L.; Cianci, E.; Antonucci, I.; Marchisio, M.; Romano, M.; Stuppia, L.; et al. Wnt signaling behaves as a “Master Regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. Rep. 2013, 9, 642–654. [Google Scholar] [CrossRef]
- Luchansky, S.J.; Yarema, K.J.; Takahashi, S.; Bertozzi, C.R. GlcNAc 2-epimerase can serve a catabolic role in sialic acid metabolism. J. Biol. Chem. 2003, 278, 8036–8042. [Google Scholar] [CrossRef]
- Mahal, L.K.; Yarema, K.J.; Bertozzi, C.R. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 1997, 276, 1125–1128. [Google Scholar] [CrossRef]
- Yarema, K.J.; Mahal, L.K.; Bruehl, R.E.; Rodriguez, E.C.; Bertozzi, C.R. Metabolic delivery of ketone groups to sialic acid residues. Application to cell surface glycoform engineering. J. Biol. Chem. 1998, 273, 31168–31179. [Google Scholar] [CrossRef] [PubMed]
- Saxon, E.; Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T.; Sampathkumar, S.-G.; Weier, C.; Yarema, K.J. Metabolic oligosaccharide engineering: Perspectives, applications, and future directions. Mol. Biosyst. 2007, 3, 187–194. [Google Scholar] [CrossRef]
- Aich, U.; Yarema, K.J. Glycoscience; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Heidelberg, Germany, 2008; pp. 2133–2190. [Google Scholar]
- Saxon, E.; Bertozzi, C.R. Chemical and biological strategies for engineering cell surface glycosylation. Annu. Rev. Cell Dev. Biol. 2001, 17, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Prescher, J.A.; Bertozzi, C.R. Chemistry in living systems. Nat. Chem. Biol. 2005, 1, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, C.R. A decade of bioorthogonal chemistry. Acc. Chem. Res. 2011, 44, 651–653. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kohler, J.J. Photoactivatable crosslinking sugars for capturing glycoprotein interactions. J. Am. Chem. Soc. 2008, 130, 3278–3279. [Google Scholar] [CrossRef] [PubMed]
- Okeley, N.M.; Toki, B.E.; Zhang, X.; Jeffrey, S.C.; Burke, P.J.; Alley, S.C.; Senter, P.D. Metabolic engineering of monoclonal antibody carbohydrates for antibody−drug conjugation. Bioconjugate Chem. 2013, 24, 1650–1655. [Google Scholar] [CrossRef]
- Rosso, S.B.; Inestrosa, N.C. WNT signaling in neuronal maturation and synaptogenesis. Front. Cell. Neurosci. 2013, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougal, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef]
- Bagchi, D.P.; Nishii, A.; Li, Z.; ElProposto, J.B.; Corsa, C.A.; Mori, H.; Hardij, J.; Learman, B.S.; Lumeng, C.N.; MacDougald, O.A. Wnt/β-catenin signaling regulates adipose tissue lipogenesis and adipocyte-specific tissue lipogenesis and adipocyte-specific loss is rigorously defended by neighboring stromal-vascular cells. Mol. Metab. 2020, 42, 101078. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Agatemor, C.; Saeui, C.T.; Bhattacharya, R.; Jia, X.; Yarema, K.J. Glycoengineering Human Neural and Adipose Stem Cells with Novel Thiol-Modified N-Acetylmannosamine (ManNAc) Analogs. Cells 2021, 10, 377. https://doi.org/10.3390/cells10020377
Du J, Agatemor C, Saeui CT, Bhattacharya R, Jia X, Yarema KJ. Glycoengineering Human Neural and Adipose Stem Cells with Novel Thiol-Modified N-Acetylmannosamine (ManNAc) Analogs. Cells. 2021; 10(2):377. https://doi.org/10.3390/cells10020377
Chicago/Turabian StyleDu, Jian, Christian Agatemor, Christopher T. Saeui, Rahul Bhattacharya, Xiaofeng Jia, and Kevin J. Yarema. 2021. "Glycoengineering Human Neural and Adipose Stem Cells with Novel Thiol-Modified N-Acetylmannosamine (ManNAc) Analogs" Cells 10, no. 2: 377. https://doi.org/10.3390/cells10020377
APA StyleDu, J., Agatemor, C., Saeui, C. T., Bhattacharya, R., Jia, X., & Yarema, K. J. (2021). Glycoengineering Human Neural and Adipose Stem Cells with Novel Thiol-Modified N-Acetylmannosamine (ManNAc) Analogs. Cells, 10(2), 377. https://doi.org/10.3390/cells10020377






