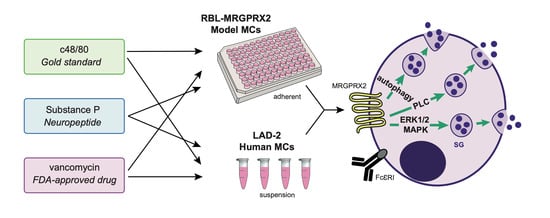
Authentic and Ectopically Expressed MRGPRX2 Elicit Similar Mechanisms to Stimulate Degranulation of Mast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Cell Culture
2.3. Cell Transfection
2.4. Isolation of Mouse Peritoneal MCs
2.5. Mast Cell Activation
2.6. β-Hexosaminidase Release Assay
2.7. Histamine Release Assay
2.8. Flow Cytometry Analysis
2.9. Immunostaining and Laser Confocal Microscopy Analysis
2.10. Western Blot Analyses
2.11. RNA Purification and Quantitative Real-Time PCR
2.12. Statistical Analysis
3. Results
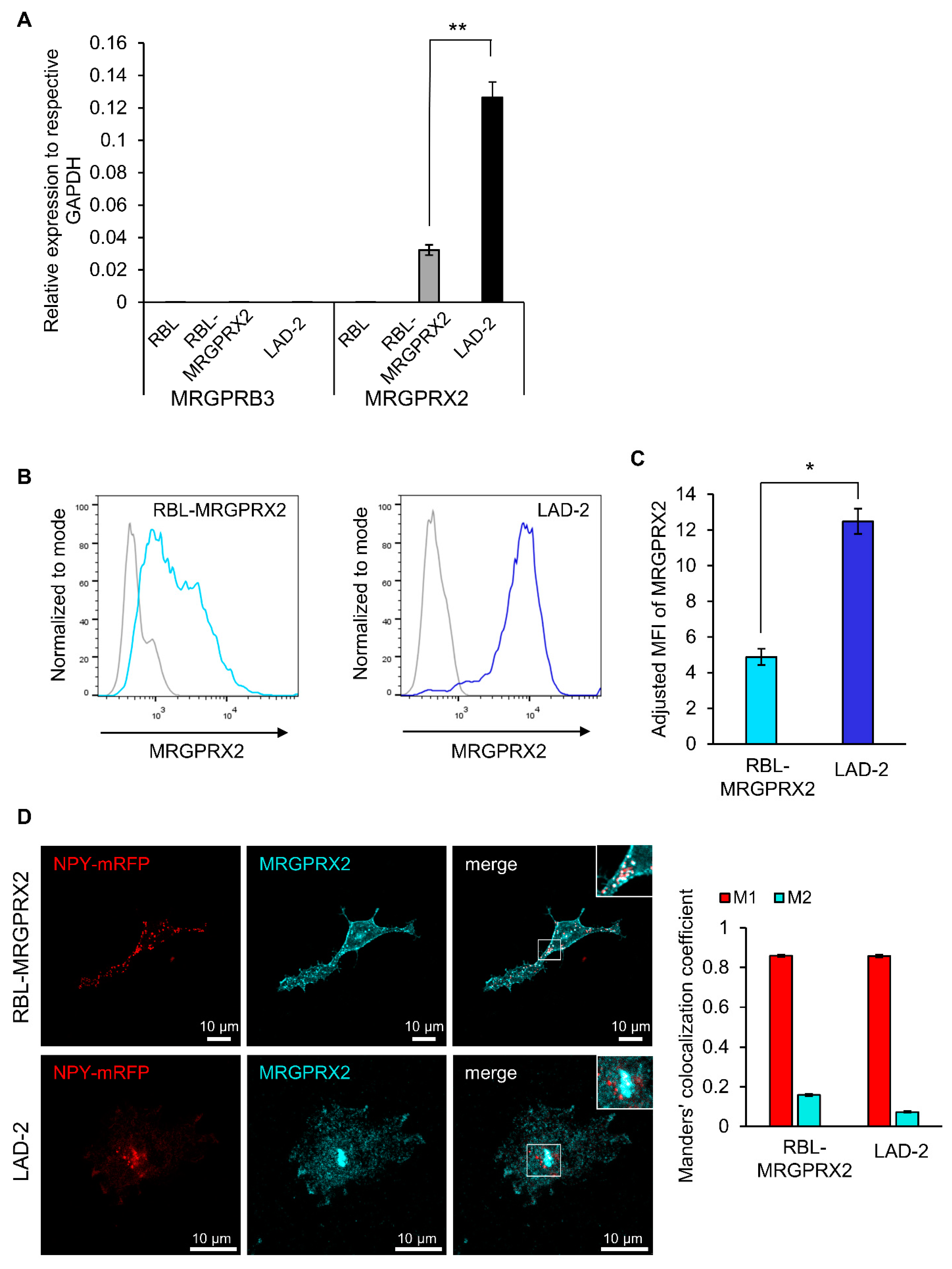
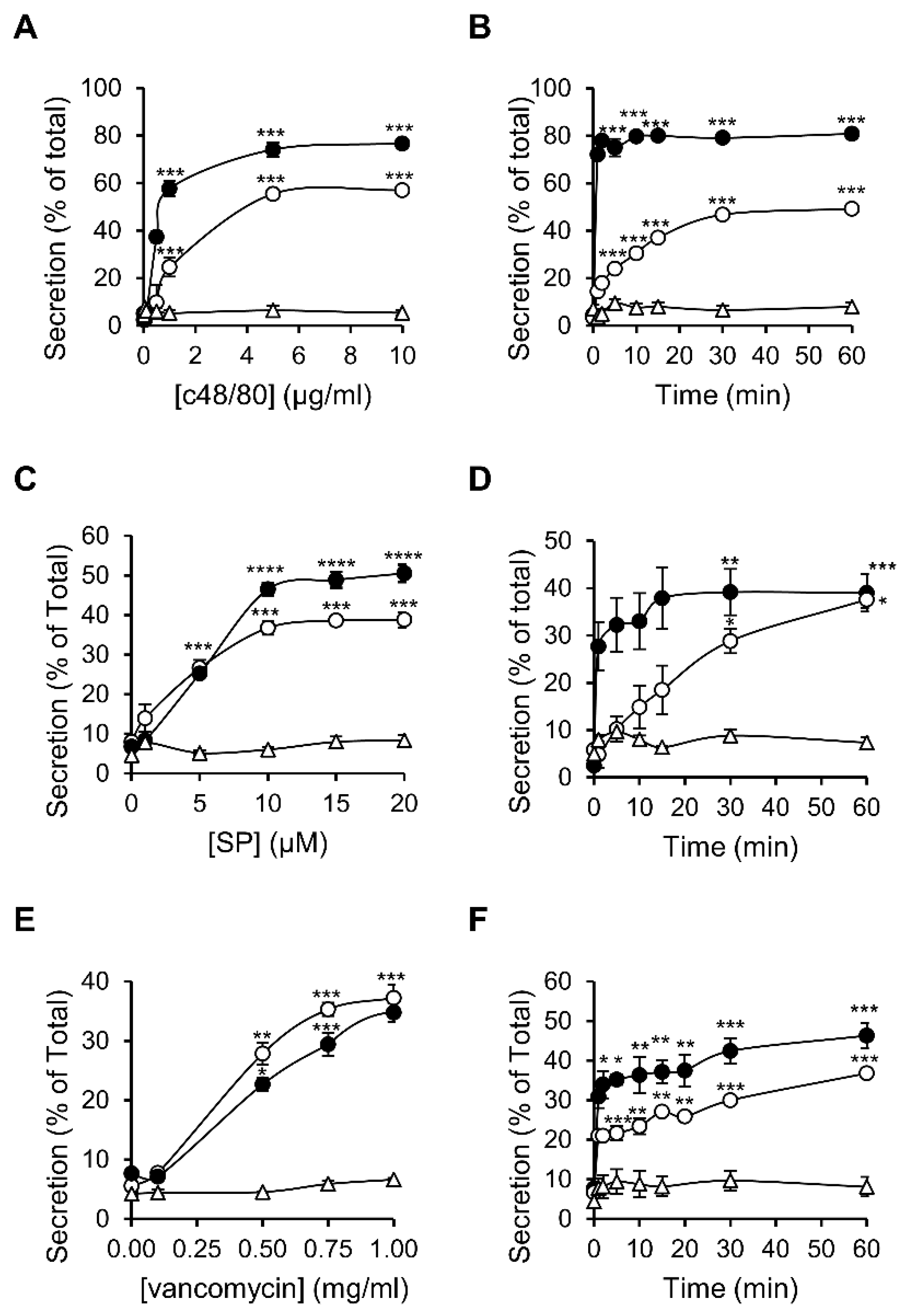
3.1. Dose and Time Dependence of Endogenous and Ectopic MRGPRX2 Responses
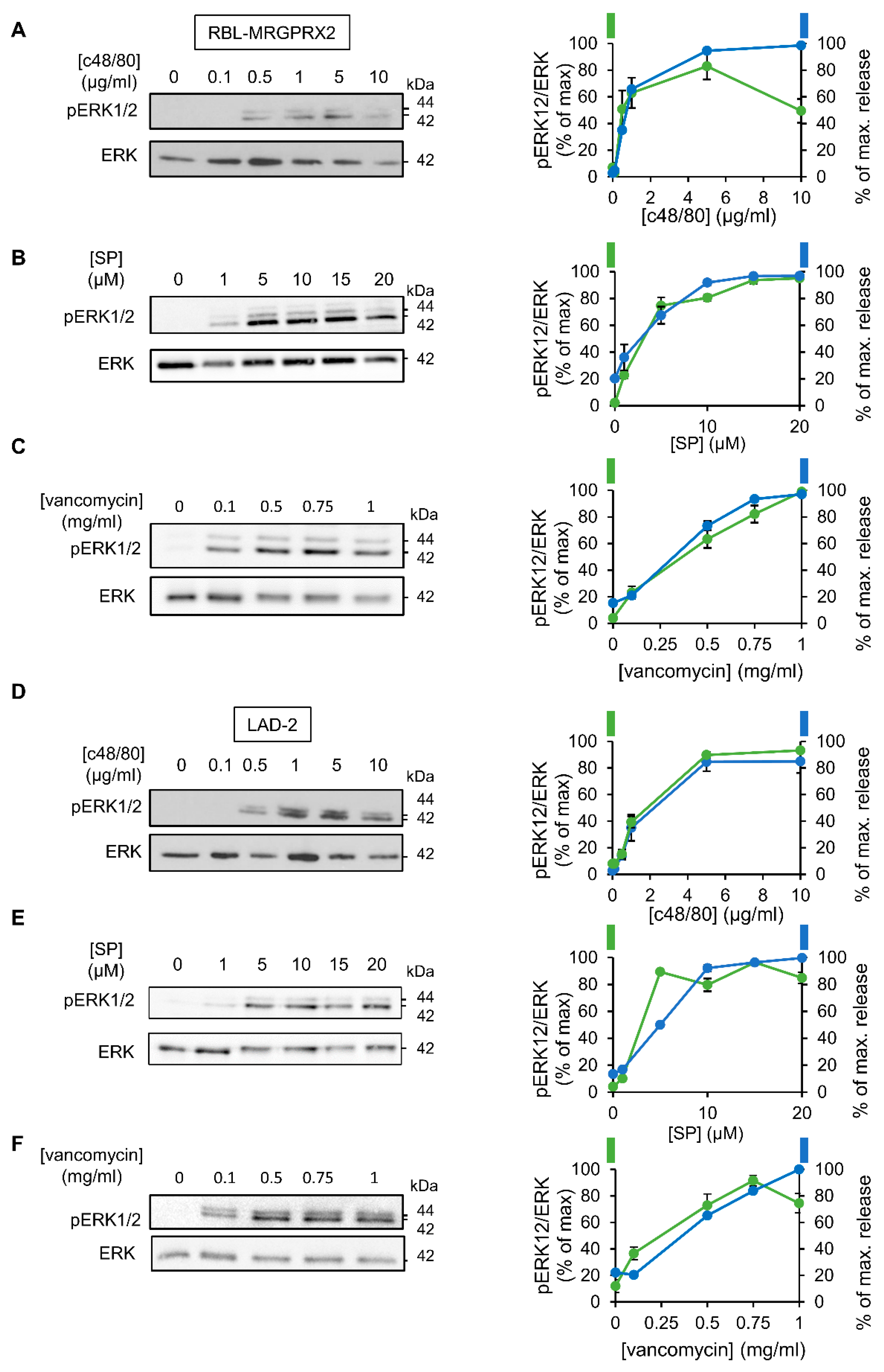
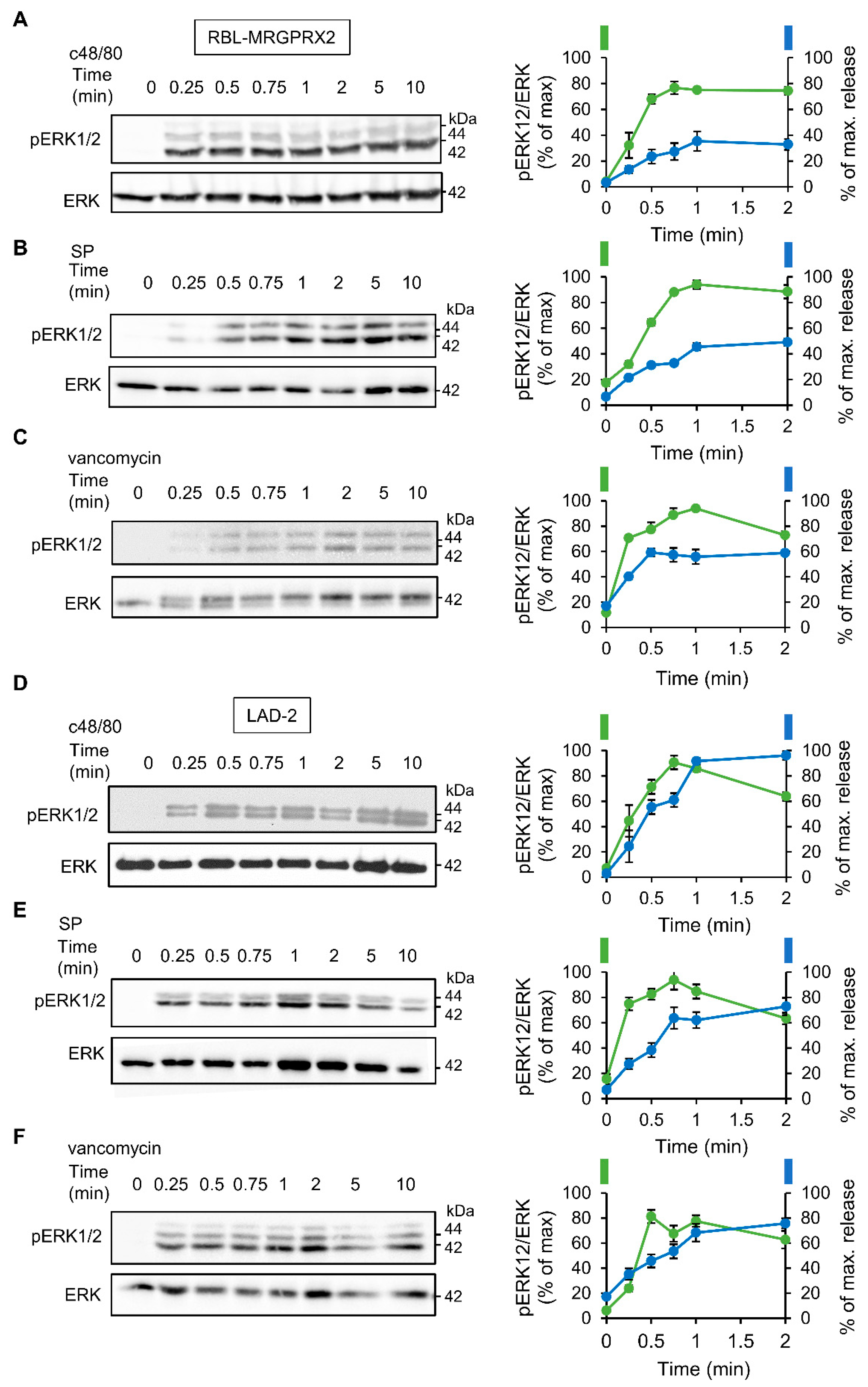
3.2. ERK1/2 Signaling Is Linked with Secretion in LAD-2 and RBL-MRGPRX2 Cells
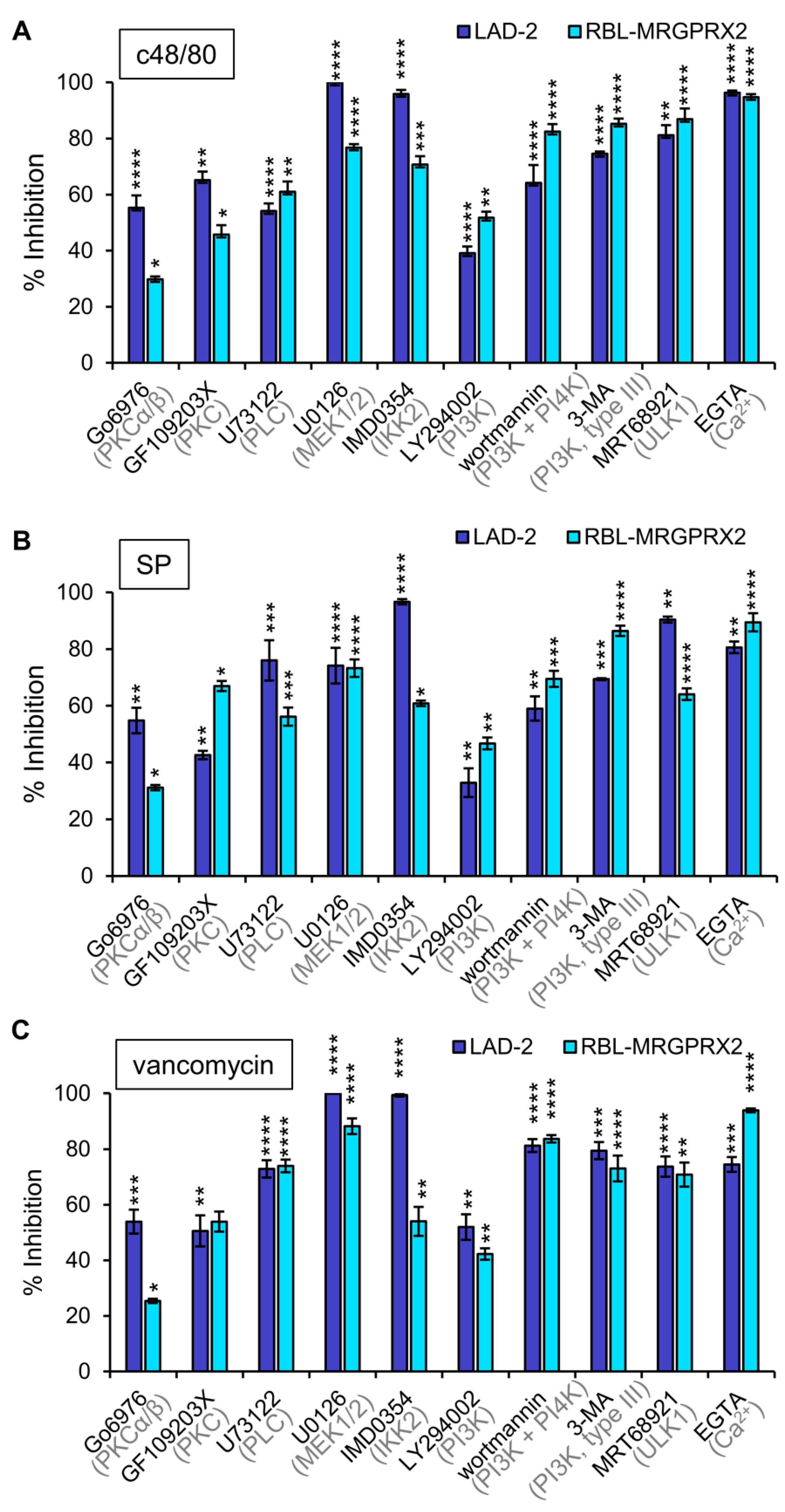
3.3. Inhibitor Profiling of MRGPRX2-Induced Secretion from LAD-2 and RBL-MRGPRX2 Cells
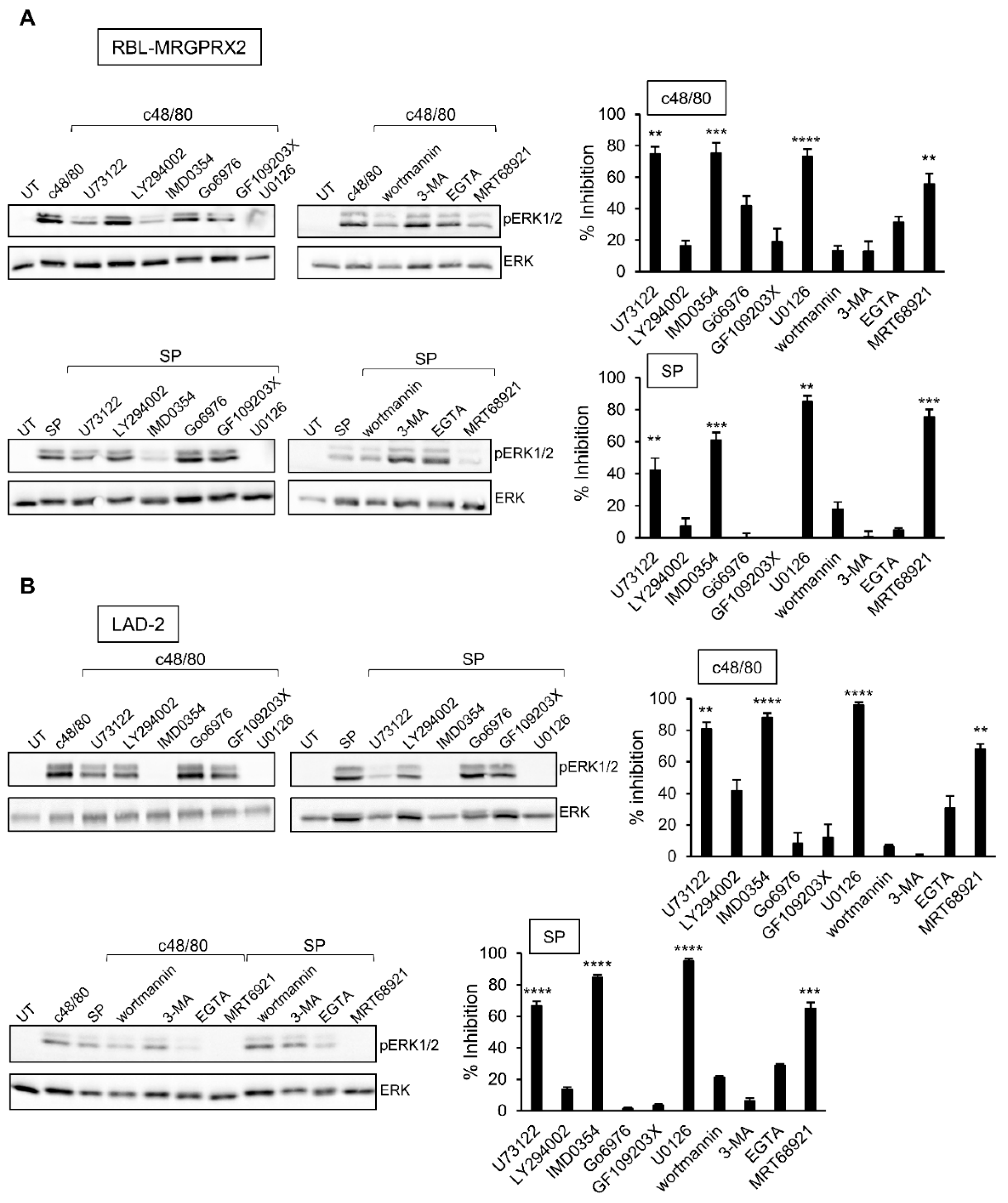
3.4. Inhibitor Profiling of MRGPRX2-Stimulated ERK1/2 Phosphorylation in LAD-2 and RBL-MRGPRX2 Cells
3.5. Secretion from Murine Peritoneal MCs Is Resistant to Inhibitors That Abrogate MRGPRX2-stimulated Secretion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shelburne, C.P.; Abraham, S.N. The mast cell in innate and adaptive immunity. Adv. Exp. Med. Biol. 2011, 716, 162–185. [Google Scholar] [CrossRef] [PubMed]
- Agier, J.; Pastwinska, J.; Brzezinska-Blaszczyk, E. An overview of mast cell pattern recognition receptors. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2018. [Google Scholar] [CrossRef]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gupta, K.; Lee, D.; Bayir, A.K.; Ahn, H.; Ali, H. beta-Defensins activate human mast cells via Mas-related gene X2. J. Immunol. 2013, 191, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Lagunoff, D.; Martin, T.W.; Read, G. Agents that release histamine from mast cells. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Ferry, X.; Brehin, S.; Kamel, R.; Landry, Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides 2002, 23, 1507–1515. [Google Scholar] [CrossRef]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Mousli, M.; Bronner, C.; Bueb, J.L.; Tschirhart, E.; Gies, J.P.; Landry, Y. Activation of rat peritoneal mast cells by substance P and mastoparan. J. Pharmacol. Exp. Ther. 1989, 250, 329–335. [Google Scholar] [PubMed]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Alysandratos, K.-D.; Asadi, S.; Angelidou, A.; Zhang, B.; Sismanopoulos, N.; Yang, H.; Critchfield, A.; Theoharides, T.C. Neurotensin and CRH interactions augment human mast cell activation. PLoS ONE 2012, 7, e48934. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. The role of mast cells and basophils in inflammation. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2000, 30, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.B.; Austen, K.F. Enzymes of the mast cell granule. J. Investig. Dermatol. 1980, 74, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.R.; Galli, S.J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature 1990, 346, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Kulinski, J.M.; Muñoz-Cano, R.; Olivera, A. Sphingosine-1-phosphate and other lipid mediators generated by mast cells as critical players in allergy and mast cell function. Eur. J. Pharmacol. 2016, 778, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Kandere, K. Mast cell involvement in neurogenic inflammation. In Migraine: A Neuroinflammatory Disease? Spierings, E.L.H., Sánchez del Río, M., Eds.; Birkhäuser Basel: Basel, Switzerland, 2002; pp. 115–132. [Google Scholar] [CrossRef]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef]
- Ali, H. Mas-related G protein coupled receptor-X2: A potential new target for modulating mast cell-mediated allergic and inflammatory diseases. J. Immunobiol. 2016, 1, 115. [Google Scholar] [CrossRef]
- Tatemoto, K.; Nozaki, Y.; Tsuda, R.; Konno, S.; Tomura, K.; Furuno, M.; Ogasawara, H.; Edamura, K.; Takagi, H.; Iwamura, H.; et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem. Biophys. Res. Commun. 2006, 349, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Babina, M. The pseudo-allergic/neurogenic route of mast cell activation via MRGPRX2: Discovery, functional programs, regulation, relevance to disease, and relation with allergic stimulation. Itch 2020, 5, e32. [Google Scholar] [CrossRef]
- Subramanian, H.; Gupta, K.; Guo, Q.; Price, R.; Ali, H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: Resistance to receptor phosphorylation, desensitization, and internalization. J. Biol. Chem. 2011, 286, 44739–44749. [Google Scholar] [CrossRef]
- Zylka, M.J.; Dong, X.; Southwell, A.L.; Anderson, D.J. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc. Natl. Acad. Sci. USA 2003, 100, 10043–10048. [Google Scholar] [CrossRef]
- van der Kleij, H.P.; Ma, D.; Redegeld, F.A.; Kraneveld, A.D.; Nijkamp, F.P.; Bienenstock, J. Functional expression of neurokinin 1 receptors on mast cells induced by IL-4 and stem cell factor. J. Immunol. 2003, 171, 2074–2079. [Google Scholar] [CrossRef]
- Subramanian, H.; Kashem, S.W.; Collington, S.J.; Qu, H.; Lambris, J.D.; Ali, H. PMX-53 as a dual CD88 antagonist and an agonist for Mas-related gene 2 (MrgX2) in human mast cells. Mol. Pharmacol. 2011, 79, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, M.B.; Groot, A.J.; Dastych, J.; Knol, E.F. TNF Trafficking to Human Mast Cell Granules: Mature Chain-Dependent Endocytosis. J. Immunol. 2007, 178, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.; Krier-Burris, R.A.; Lazki-Hagenbach, P.; Gorzalczany, Y.; Mei, Y.; Ji, P.; Bochner, B.S.; Sagi-Eisenberg, R. Mammalian diaphanous-related formin 1 (mDia1) coordinates mast cell migration and secretion through its actin-nucleating activity. J. Allergy Clin. Immunol. 2019, 144, 1074–1090. [Google Scholar] [CrossRef] [PubMed]
- Shefler, I.; Taube, Z.; Medalia, O.; Sagi-Eisenberg, R. Basic secretagogues activate protein tyrosine phosphorylation and release of arachidonic acid in mast cells via a novel protein kinase C and phosphatidylinositol 3-kinase-dependent mechanism. Eur. J. Immunol. 1998, 28, 3468–3478. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- BOLTE, S.; CORDELIÈRES, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2009, 38, D792–D799. [Google Scholar] [CrossRef] [PubMed]
- Spandidos, A.; Wang, X.; Wang, H.; Dragnev, S.; Thurber, T.; Seed, B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genom. 2008, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Seed, B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003, 31, e154. [Google Scholar] [CrossRef] [PubMed]
- Sahid, M.N.A.; Liu, S.; Mogi, M.; Maeyama, K. Tachykinin-1 receptor antagonism suppresses substance-P- and compound 48/80-induced mast cell activation from rat mast cells expressing functional mas-related GPCR B3. Inflamm. Res. 2020, 69, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kiseljak-Vassiliades, K.; Xu, M.; Mills, T.S.; Smith, E.E.; Silveira, L.J.; Lillehei, K.O.; Kerr, J.M.; Kleinschmidt-DeMasters, B.K.; Wierman, M.E. Differential somatostatin receptor (SSTR) 1-5 expression and downstream effectors in histologic subtypes of growth hormone pituitary tumors. Mol. Cell. Endocrinol. 2015, 417, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Song, J.; Seo, Y.; Koh, E.M.; Kim, S.-H.; Jung, K.J. Inhibitory Effects of AF-343, a Mixture of Cassia tora L., Ulmus pumila L., and Taraxacum officinale, on Compound 48/80-Mediated Allergic Responses in RBL-2H3 Cells. Molecules 2020, 25, 2434. [Google Scholar] [CrossRef]
- Petrosino, S.; Schiano Moriello, A.; Verde, R.; Allarà, M.; Imperatore, R.; Ligresti, A.; Mahmoud, A.M.; Peritore, A.F.; Iannotti, F.A.; Di Marzo, V. Palmitoylethanolamide counteracts substance P-induced mast cell activation in vitro by stimulating diacylglycerol lipase activity. J. NeuroInflamm. 2019, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Azouz, N.P.; Matsui, T.; Fukuda, M.; Sagi-Eisenberg, R. Decoding the regulation of mast cell exocytosis by networks of Rab GTPases. J. Immunol. 2012, 189, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Baumgart, T.; Hammond, S.; Holowka, D.; Baird, B. Differential targeting of secretory lysosomes and recycling endosomes in mast cells revealed by patterned antigen arrays. J. Cell Sci. 2007, 120, 3147–3154. [Google Scholar] [CrossRef]
- Azouz, N.P.; Zur, N.; Efergan, A.; Ohbayashi, N.; Fukuda, M.; Amihai, D.; Hammel, I.; Rothenberg, M.E.; Sagi-Eisenberg, R. Rab5 Is a Novel Regulator of Mast Cell Secretory Granules: Impact on Size, Cargo, and Exocytosis. J. Immunol. 2014, 192, 4043–4053. [Google Scholar] [CrossRef]
- Paton, W.D. Compound 48/80: A potent histamine liberator. Br. J. Pharmacol. Chemother. 1951, 6, 499–508. [Google Scholar] [CrossRef]
- Green, D.P.; Limjunyawong, N.; Gour, N.; Pundir, P.; Dong, X. A Mast-Cell-Specific Receptor Mediates Neurogenic Inflammation and Pain. Neuron 2019, 101, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Navinés-Ferrer, A.; Serrano-Candelas, E.; Lafuente, A.; Muñoz-Cano, R.; Martín, M.; Gastaminza, G. MRGPRX2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci. Rep. 2018, 8, 11628. [Google Scholar] [CrossRef] [PubMed]
- Beaven, M.A.; Ozawa, K. Role of calcium, protein kinase C and MAP kinase in the activation of mast cells. Allergol. Int. 1996, 45, 73–84. [Google Scholar] [CrossRef]
- Jabril-Cuenod, B.; Zhang, C.; Scharenberg, A.M.; Paolini, R.; Numerof, R.; Beaven, M.A.; Kinet, J.P. Syk-dependent phosphorylation of Shc. A potential link between FcepsilonRI and the Ras/mitogen-activated protein kinase signaling pathway through SOS and Grb2. J. Biol. Chem. 1996, 271, 16268–16272. [Google Scholar] [CrossRef] [PubMed]
- Santini, F.; Beaven, M.A. Tyrosine phosphorylation of a mitogen-activated protein kinase-like protein occurs at a late step in exocytosis. Studies with tyrosine phosphatase inhibitors and various secretagogues in rat RBL-2H3 cells. J. Biol. Chem. 1993, 268, 22716–22722. [Google Scholar] [CrossRef]
- Zhang, C.; Baumgartner, R.A.; Yamada, K.; Beaven, M.A. Mitogen-activated protein (MAP) kinase regulates production of tumor necrosis factor-alpha and release of arachidonic acid in mast cells. Indications of communication between p38 and p42 MAP kinases. J. Biol. Chem. 1997, 272, 13397–13402. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Rådinger, M.; Gilfillan, A.M. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008, 29, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Power, M.R.; Li, B.; Lin, T.-J. Inhibition of IKK down-regulates antigen + IgE-induced TNF production by mast cells: A role for the IKK-IκB-NF-κB pathway in IgE-dependent mast cell activation. J. Leukoc. Biol. 2005, 77, 975–983. [Google Scholar] [CrossRef]
- Hwang, S.L.; Lu, Y.; Li, X.; Kim, Y.D.; Cho, Y.S.; Jahng, Y.; Son, J.K.; Lee, Y.J.; Kang, W.; Taketomi, Y.; et al. ERK1/2 antagonize AMPK-dependent regulation of FcεRI-mediated mast cell activation and anaphylaxis. J. Allergy Clin. Immunol. 2014, 134, 714–721. [Google Scholar] [CrossRef]
- Shefler, I.; Seger, R.; Sagi-Eisenberg, R. Gi-mediated activation of mitogen-activated protein kinase (MAPK) pathway by receptor mimetic basic secretagogues of connective tissue-type mast cells: Bifurcation of arachidonic acid-induced release upstream of MAPK. J. Pharmacol. Exp. Ther. 1999, 289, 1654–1661. [Google Scholar]
- Lobingier, B.T.; von Zastrow, M. When trafficking and signaling mix: How subcellular location shapes G protein-coupled receptor activation of heterotrimeric G proteins. Traffic 2019, 20, 130–136. [Google Scholar] [CrossRef]
- Kimata, M.; Inagaki, N.; Kato, T.; Miura, T.; Serizawa, I.; Nagai, H. Roles of mitogen-activated protein kinase pathways for mediator release from human cultured mast cells. Biochem. Pharmacol. 2000, 60, 589–594. [Google Scholar] [CrossRef]
- Hu Frisk, J.M.; Kjellén, L.; Melo, F.R.; Öhrvik, H.; Pejler, G. Mitogen-Activated Protein Kinase Signaling Regulates Proteoglycan Composition of Mast Cell Secretory Granules. Front. Immunol. 2018, 9, 1670. [Google Scholar] [CrossRef] [PubMed]
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216. [Google Scholar] [CrossRef] [PubMed]
- Chahdi, A.; Mousli, M.; Landry, Y. Substance P-related inhibitors of mast cell exocytosis act on G-proteins or on the cell surface. Eur. J. Pharmacol. 1998, 341, 329–335. [Google Scholar] [CrossRef]
- Suzuki, K.; Verma, I.M. Phosphorylation of SNAP-23 by IkappaB kinase 2 regulates mast cell degranulation. Cell 2008, 134, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Roget, K.; Ben-Addi, A.; Mambole-Dema, A.; Gantke, T.; Yang, H.-T.; Janzen, J.; Morrice, N.; Abbott, D.; Ley, S.C. IκB kinase 2 regulates TPL-2 activation of extracellular signal-regulated kinases 1 and 2 by direct phosphorylation of TPL-2 serine 400. Mol. Cell. Biol. 2012, 32, 4684–4690. [Google Scholar] [CrossRef] [PubMed]
- Ushio, H.; Ueno, T.; Kojima, Y.; Komatsu, M.; Tanaka, S.; Yamamoto, A.; Ichimura, Y.; Ezaki, J.; Nishida, K.; Komazawa-Sakon, S.; et al. Crucial role for autophagy in degranulation of mast cells. J. Allergy Clin. Immunol. 2011, 127, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Ushio, H. An unexpected role for autophagy in degranulation of mast cells. Autophagy 2011, 7, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, N.; Athonvarangkul, D.; Mishall, P.; Sahu, S.; Singh, R. Autophagy proteins regulate ERK phosphorylation. Nat. Commun. 2013, 4, 2799. [Google Scholar] [CrossRef] [PubMed]


| Inhibitor | Target | Company | Cat. No. |
|---|---|---|---|
| Go6976 | PKCα/β1, PKD | A.G. Scientific (San Diego, CA, USA) | G-1017 |
| GF109203X | PKC | A.G. Scientific | G-1063 |
| U73122 | PLC (+PLD) | TOCRIS (Minneapolis, MN, USA) | 1268 |
| LY294002 | PI3K | TOCRIS | 1130 |
| wortmannin | PI3K + PI4K | A.G. Scientific | W-1022 |
| U0126 | MEK1, MEK2 | A.G. Scientific | U-1026 |
| MRT68921 | ULK1/2 | TOCRIS | 5780 |
| IMD0354 | IKKβ | Cayman Chemical (Ann Arbor, MI, USA) | 17290 |
| 3-MA | PI3K type III | Sigma-Aldrich | M9281 |
| EGTA | Ca2+ chelator | Sigma-Aldrich | E4378 |
| hMRGPRX2 (PrimerBank ID40255006c1) [32,33,34] | fw 5′-CTGGTAGGAAACGGGTTTGTG-3′ rv 5′-GCTGAGGACGTAGACAGAGAAG-3′ |
| rMRGPRB3 [35] | fw 5′-CCCCTGGAATGTTCTTTTGTGTAG-3′ rv 5′-ACAGTGAAAAATGCAGGAACTTGG-3′ |
| hGAPDH (cat #: HP205798, sequence from Origene, ordered from Hy Laboratories Ltd., Rehovot, Israel) | fw 5′-GTCTCCTCTGACTTCAACAGCG-3′ rv 5′-ACCACCCTGTTGCTGTAGCCAA-3′ |
| rGAPDH [36] | fw 5′-TGGAGTCTACTGGCGTCT-3′ rv 5′-TGTCATATTTVTCGTGGT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazki-Hagenbach, P.; Ali, H.; Sagi-Eisenberg, R. Authentic and Ectopically Expressed MRGPRX2 Elicit Similar Mechanisms to Stimulate Degranulation of Mast Cells. Cells 2021, 10, 376. https://doi.org/10.3390/cells10020376
Lazki-Hagenbach P, Ali H, Sagi-Eisenberg R. Authentic and Ectopically Expressed MRGPRX2 Elicit Similar Mechanisms to Stimulate Degranulation of Mast Cells. Cells. 2021; 10(2):376. https://doi.org/10.3390/cells10020376
Chicago/Turabian StyleLazki-Hagenbach, Pia, Hydar Ali, and Ronit Sagi-Eisenberg. 2021. "Authentic and Ectopically Expressed MRGPRX2 Elicit Similar Mechanisms to Stimulate Degranulation of Mast Cells" Cells 10, no. 2: 376. https://doi.org/10.3390/cells10020376
APA StyleLazki-Hagenbach, P., Ali, H., & Sagi-Eisenberg, R. (2021). Authentic and Ectopically Expressed MRGPRX2 Elicit Similar Mechanisms to Stimulate Degranulation of Mast Cells. Cells, 10(2), 376. https://doi.org/10.3390/cells10020376