Human Chorionic Gonadotropin-Mediated Induction of Breast Cancer Cell Proliferation and Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Cell Lines
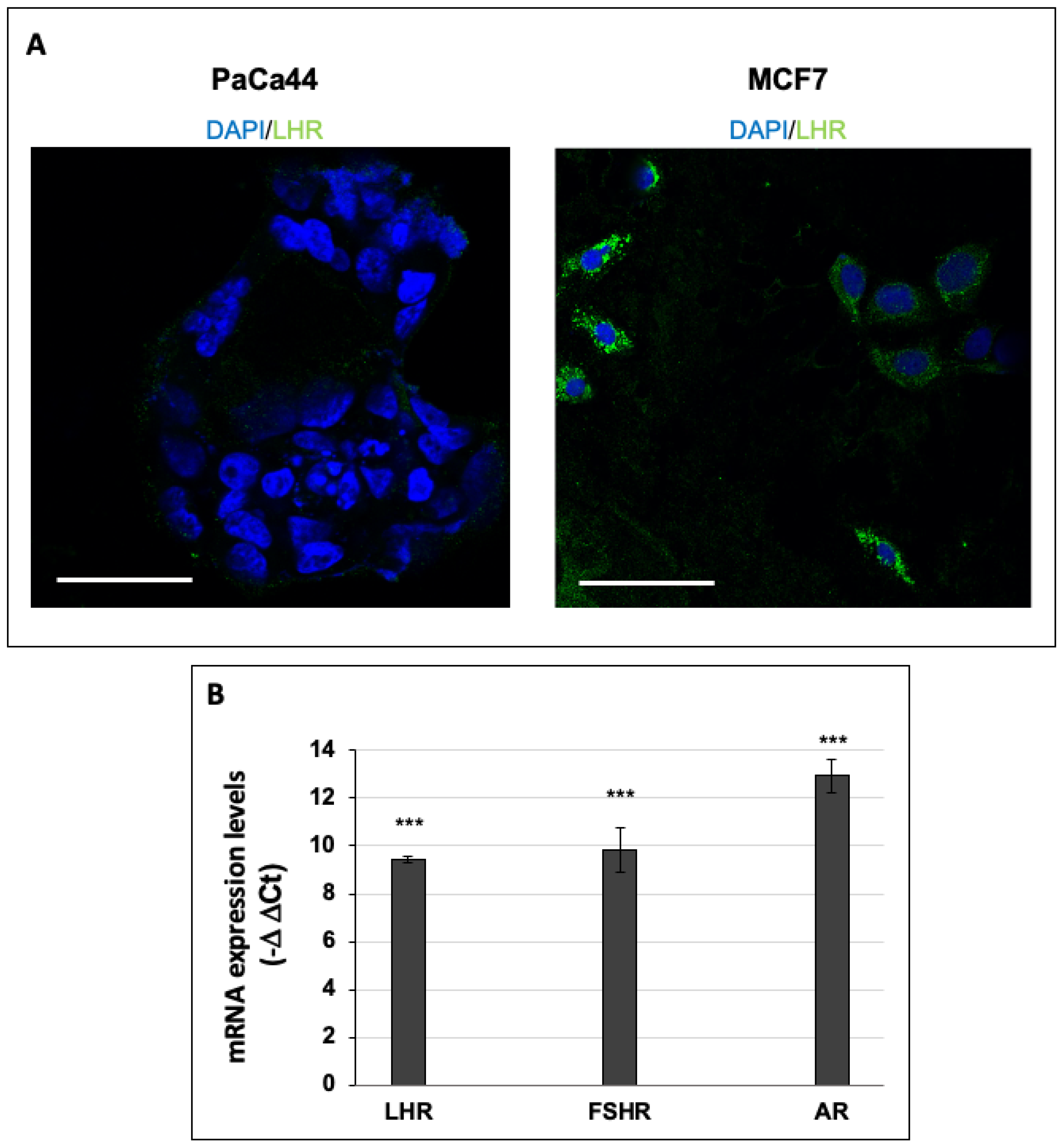
2.3. Immunofluorescence Analysis of LHR-Positive Cells
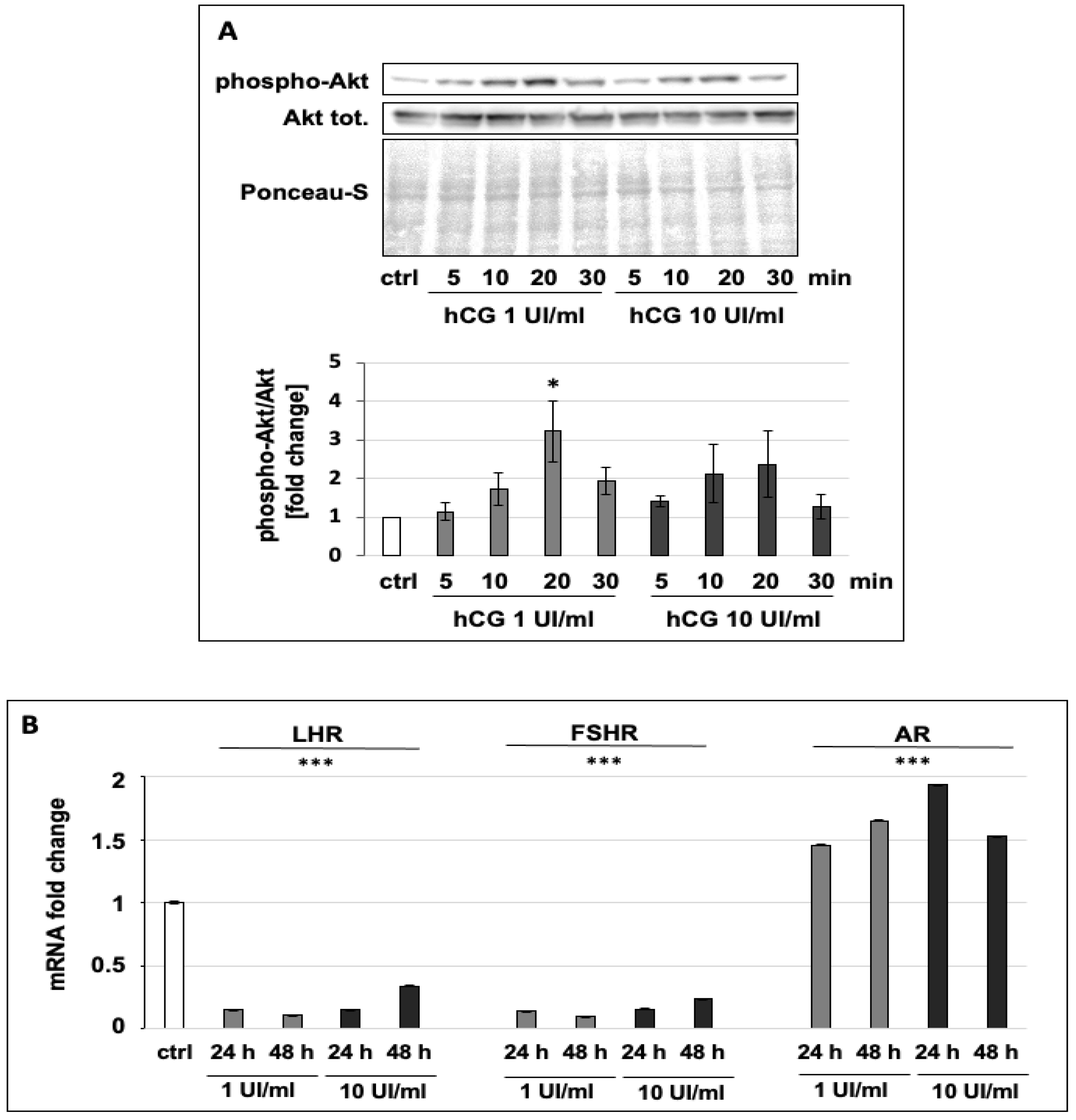
2.4. Immunoblot Analysis
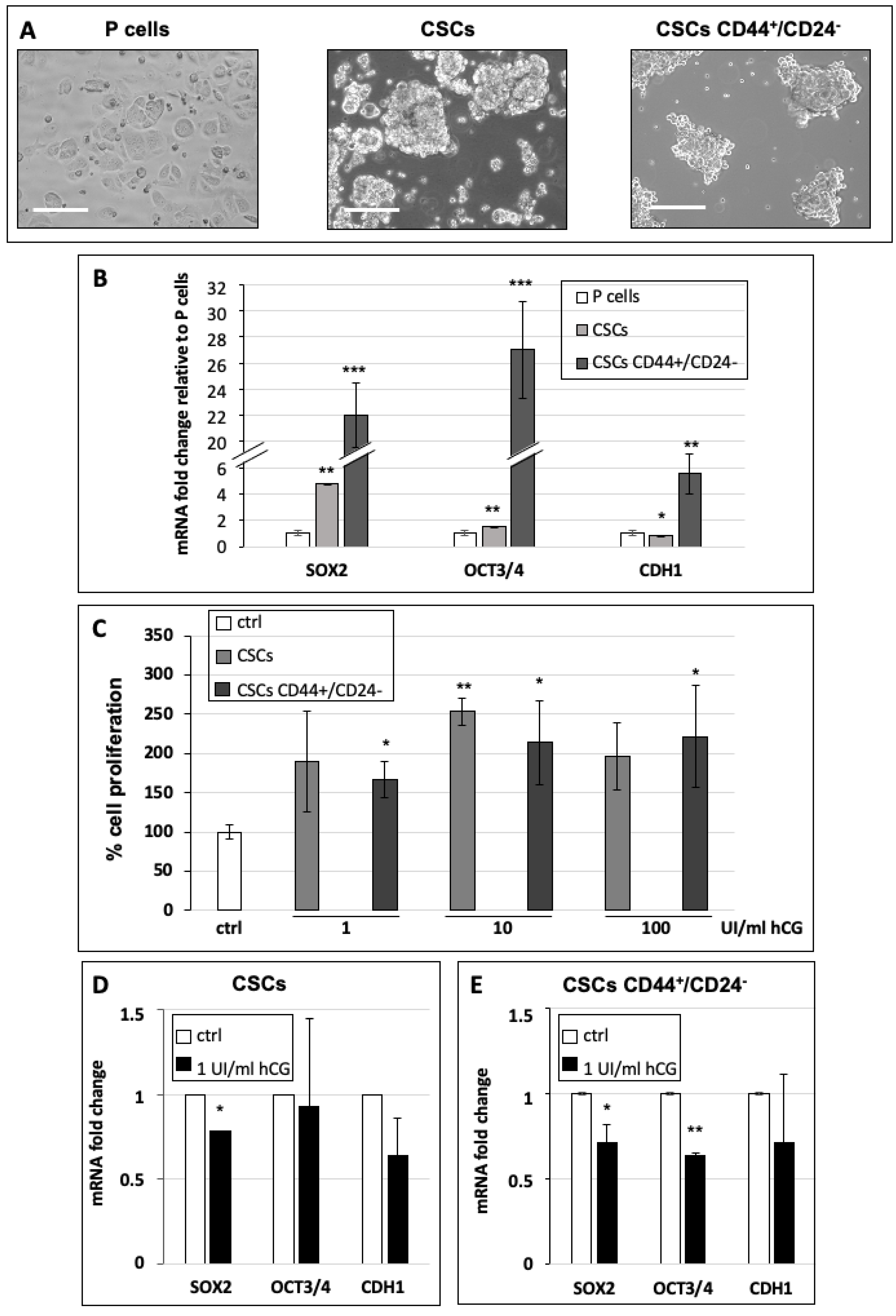
2.5. RNA Extraction and qPCR
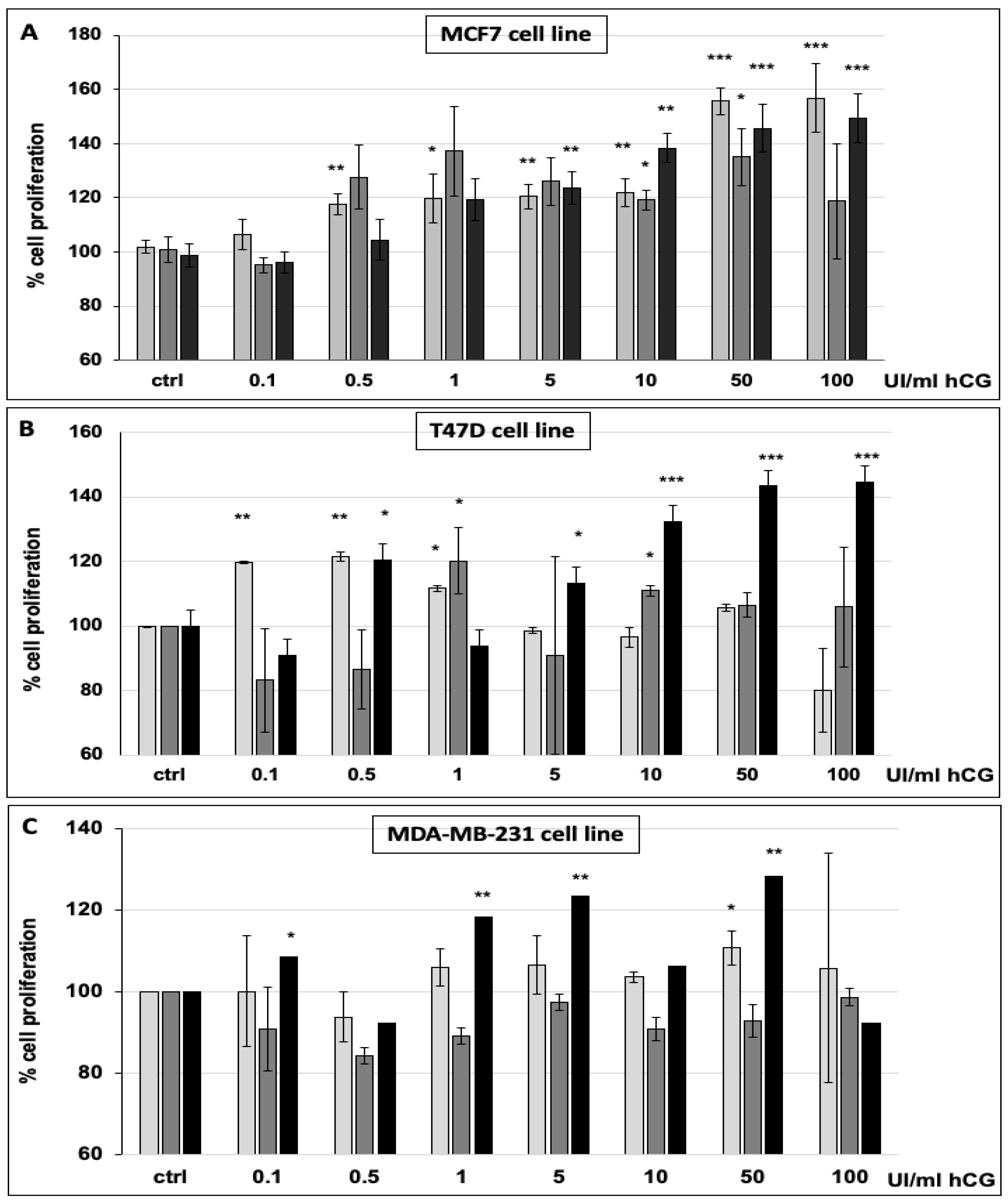
2.6. Cell Proliferation Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. hCG Stimulates Breast Cancer Cell Proliferation
3.2. hCG Increases the Phosphorylation of Akt and Regulates the Expression of Hormone Receptors
3.3. hCG Stimulates Breast Cancer Stem Cell Proliferation and Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Reimer, T.; Koczan, D.; Briese, V.; Friese, K.; Richter, D.; Thiesen, H.J.; Jeschke, U. Absolute Quantification of Human Chorionic Gonadotropin-Beta mRNA with TaqMan Detection. Mol. Biotechnol. 2000, 14, 47–58. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Treeck, O.; Ortmann, O. Human Chorionic Gonadotropin and Breast Cancer. Int. J. Mol. Sci. 2017, 18, 1587. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, N.; Camoglio, F. Pediatric-adolescent andrology: Single centre experience. Arch. Ital. Urol. Androl. 2020, 92. [Google Scholar] [CrossRef]
- Zampieri, N.; Murri, V.; Camoglio, F.S. Post-operative use of human chorionic gonadotrophin (u-hCG) inpatients treated for intrabdominal unilateral undescended testes. Am. J. Clin. Exp. Urol. 2018, 6, 133–137. [Google Scholar]
- Janssens, J.P.; Russo, J.; Russo, I.; Michiels, L.; Donders, G.; Verjans, M.; Riphagen, I.; Bossche, T.V.D.; Deleu, M.; Sieprath, P. Human chorionic gonadotropin (hCG) and prevention of breast cancer. Mol. Cell. Endocrinol. 2007, 269, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, G.; Schauer, I.G.; Yang, G.; Mercado-Uribe, I.; Yang, F.; Zhang, S.; He, Y.; Liu, J. Overexpression of the beta subunit of human chorionic gonadotropin promotes the transformation of human ovarian epithelial cells and ovarian tumorigenesis. Am. J. Pathol. 2011, 179, 1385–1393. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, T.; Xi, W.; Sun, X.; Zhou, L.; Guo, Y.; Zhao, C.; Bao, Y. Human chorionic gonadotropin promotes cell proliferation through the activation of c-Met in gastric cancer cells. Oncol. Lett. 2018, 16, 4271–4278. [Google Scholar] [CrossRef]
- Liao, X.H.; Wang, Y.; Wang, N.; Yan, T.-B.; Xing, W.-J.; Zheng, L.; Zhao, D.-W.; Li, Y.-Q.; Liu, L.-Y.; Sun, X.-G.; et al. Human chorionic gonadotropin decreases human breast cancer cell proliferation and promotes differentiation. IUBMB Life 2014, 66, 352–360. [Google Scholar] [CrossRef]
- Russo, I.H.; Koszalka, M.; Russo, J. Human chorionic gonadotropin and rat mammary cancer prevention. J. Natl. Cancer Inst. 1990, 82, 1286–1289. [Google Scholar] [CrossRef]
- Srivastava, P.; Russo, J.; Russo, I.H. Chorionic gonadotropin inhibits rat mammary carcinogenesis through acti-vation of programmed cell death. Carcinogenesis 1997, 18, 1799–1808. [Google Scholar] [CrossRef]
- Milose, J.C.; Filson, C.P.; Weizer, A.Z.; Hafez, K.S.; Montgomery, J.S. Role of biochemical markers in testicular cancer: Diagnosis, staging, and surveillance. Open Access J. Urol. 2011, 4, 1–8. [Google Scholar] [PubMed]
- Venyo, A.-G.; Herring, D.; Greenwood, H.; Maloney, F. The expression of Beta Human Chorionic Gonadotrophin (beta-HCG) in human urothelial carcinoma). Pan Afr. Med. J. 2011, 7, 20. [Google Scholar] [CrossRef]
- Sjöström, J.; Alfthan, H.; Joensuu, H.; Stenman, U.-H.; Lundin, J.; Hakamies-Blomqvist, L. Serum tumour markers CA 15-3, TPA, TPS, hCGbeta and TATI in the monitoring of chemotherapy response in metastatic breast cancer. Scand. J. Clin. Lab. Investig. 2001, 61, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Kuorelahti, A.; Rulli, S.; Huhtaniemi, I.; Poutanen, M. Human chorionic gonadotropin (hCG) up-regulates wnt5b and wnt7b in the mammary gland, and hCGbeta transgenic female mice present with mammary Gland tumors exhibiting characteristics of the Wnt/beta-catenin pathway activation. Endocrinology 2007, 148, 3694–3703. [Google Scholar] [CrossRef]
- Li, Z.; Du, L.; Li, C.; Wu, W. Human chorionic gonadotropin beta induces cell motility via ERK1/2 and MMP-2 ac-tivation in human glioblastoma U87MG cells. J. Neurooncol. 2013, 111, 237–244. [Google Scholar] [CrossRef]
- Wu, W.; Walker, A.M. Human chorionic gonadotropin beta (HCGbeta) down-regulates E-cadherin and promotes human prostate carcinoma cell migration and invasion. Cancer 2006, 106, 68–78. [Google Scholar] [CrossRef]
- Kuijper, T.M.; Ruigrok-Ritstier, K.; Verhoef-Post, M.; Piersma, D.; Bruysters, M.W.; Berns, E.M.; Themmen, A. (Axel) LH receptor gene expression is essentially absent in breast tumor tissue: Implications for treatment. Mol. Cell. Endocrinol. 2009, 302, 58–64. [Google Scholar] [CrossRef]
- Ambrosini, G.; Pozza, E.D.; Fanelli, G.; Di Carlo, C.; Vettori, A.; Cannino, G.; Cavallini, C.; Carmona-Carmona, C.A.; Brandi, J.; Rinalducci, S.; et al. Progressively De-Differentiated Pancreatic Cancer Cells Shift from Glycolysis to Oxidative Metabolism and Gain a Quiescent Stem State. Cells 2020, 9, 1572. [Google Scholar] [CrossRef]
- Pozza, E.D.; Dando, I.; Biondani, G.; Brandi, J.; Costanzo, C.; Zoratti, E.; Fassan, M.; Boschi, F.; Melisi, D.; Cecconi, D.; et al. Pancreatic ductal adenocarcinoma cell lines display a plastic ability to bi-directionally convert into cancer stem cells. Int. J. Oncol. 2014, 46, 1099–1108. [Google Scholar] [CrossRef]
- Wang, R.; Lv, Q.; Meng, W.; Tan, Q.; Zhang, S.; Mo, X.; Yang, X. Comparison of mammosphere formation from breast cancer cell lines and primary breast tumors. J. Thorac. Dis. 2014, 6, 829–837. [Google Scholar]
- Dando, I.; Pacchiana, R.; Pozza, E.D.; Cataldo, I.; Bruno, S.; Conti, P.; Cordani, M.; Grimaldi, A.; Butera, G.; Caraglia, M.; et al. UCP2 inhibition induces ROS/Akt/mTOR axis: Role of GAPDH nuclear translocation in genipin/everolimus anticancer synergism. Free Radic. Biol. Med. 2017, 113, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Bernales, S.; Jacobsen, B.M.; Cittelly, D.M.; Howe, E.N.; D’Amato, N.C.; Spoelstra, N.S.; Edgerton, S.M.; Jean, A.; Guerrero, J.; et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014, 16, R7. [Google Scholar] [CrossRef] [PubMed]
- Casarini, L.; Lispi, M.; Longobardi, S.; Milosa, F.; La Marca, A.; Tagliasacchi, D.; Pignatti, E.; Simoni, M. LH and hCG Action on the Same Receptor Results in Quantitatively and Qualitatively Different Intracellular Signalling. PLoS ONE 2012, 7, e46682. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Gulappa, T.; Menon, K.M.J. miR-122 Regulates LH Receptor Expression by Activating Sterol Response Element Binding Protein in Rat Ovaries. Endocrinology 2015, 156, 3370–3380. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chen, Y.; Yao, Y.; Zhang, H.; Wang, T. Increased invasion and tumorigenicity capacity of CD44+/CD24- breast cancer MCF7 cells in vitro and in nude mice. Cancer Cell Int. 2013, 13, 62. [Google Scholar] [CrossRef]
- Boukaidi, S.A.; Cooley, A.; Hardy, A.; Matthews, L.; Zelivianski, S.; Jeruss, J.S. Impact of infertility regimens on breast cancer cells: Follicle-stimulating hormone and luteinizing hormone lack a direct effect on breast cell proliferation in vitro. Fertil. Steril. 2012, 97, 440–444. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dando, I.; Carmona-Carmona, C.A.; Zampieri, N. Human Chorionic Gonadotropin-Mediated Induction of Breast Cancer Cell Proliferation and Differentiation. Cells 2021, 10, 264. https://doi.org/10.3390/cells10020264
Dando I, Carmona-Carmona CA, Zampieri N. Human Chorionic Gonadotropin-Mediated Induction of Breast Cancer Cell Proliferation and Differentiation. Cells. 2021; 10(2):264. https://doi.org/10.3390/cells10020264
Chicago/Turabian StyleDando, Ilaria, Cristian Andres Carmona-Carmona, and Nicola Zampieri. 2021. "Human Chorionic Gonadotropin-Mediated Induction of Breast Cancer Cell Proliferation and Differentiation" Cells 10, no. 2: 264. https://doi.org/10.3390/cells10020264
APA StyleDando, I., Carmona-Carmona, C. A., & Zampieri, N. (2021). Human Chorionic Gonadotropin-Mediated Induction of Breast Cancer Cell Proliferation and Differentiation. Cells, 10(2), 264. https://doi.org/10.3390/cells10020264







