Primary Human Trabecular Meshwork Model for Pseudoexfoliation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of TM Cells
2.2. TGF Treatment and Cell Viability Assays
2.3. Epithelial Mesenchymal Transition (EMT) and Morphological Changes
2.4. Gelatin Zymography Analysis for MMP9
2.5. RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
2.6. Immunoblotting
2.7. Immunofluorescence Assay for Fibrillar Aggregates
2.8. Statistics
3. Results
3.1. Continued Exposure of 10 ng/mL of TGF—β1 Causes Time Dependent Loss of Cell Viability and Cell Death in HTM Cells
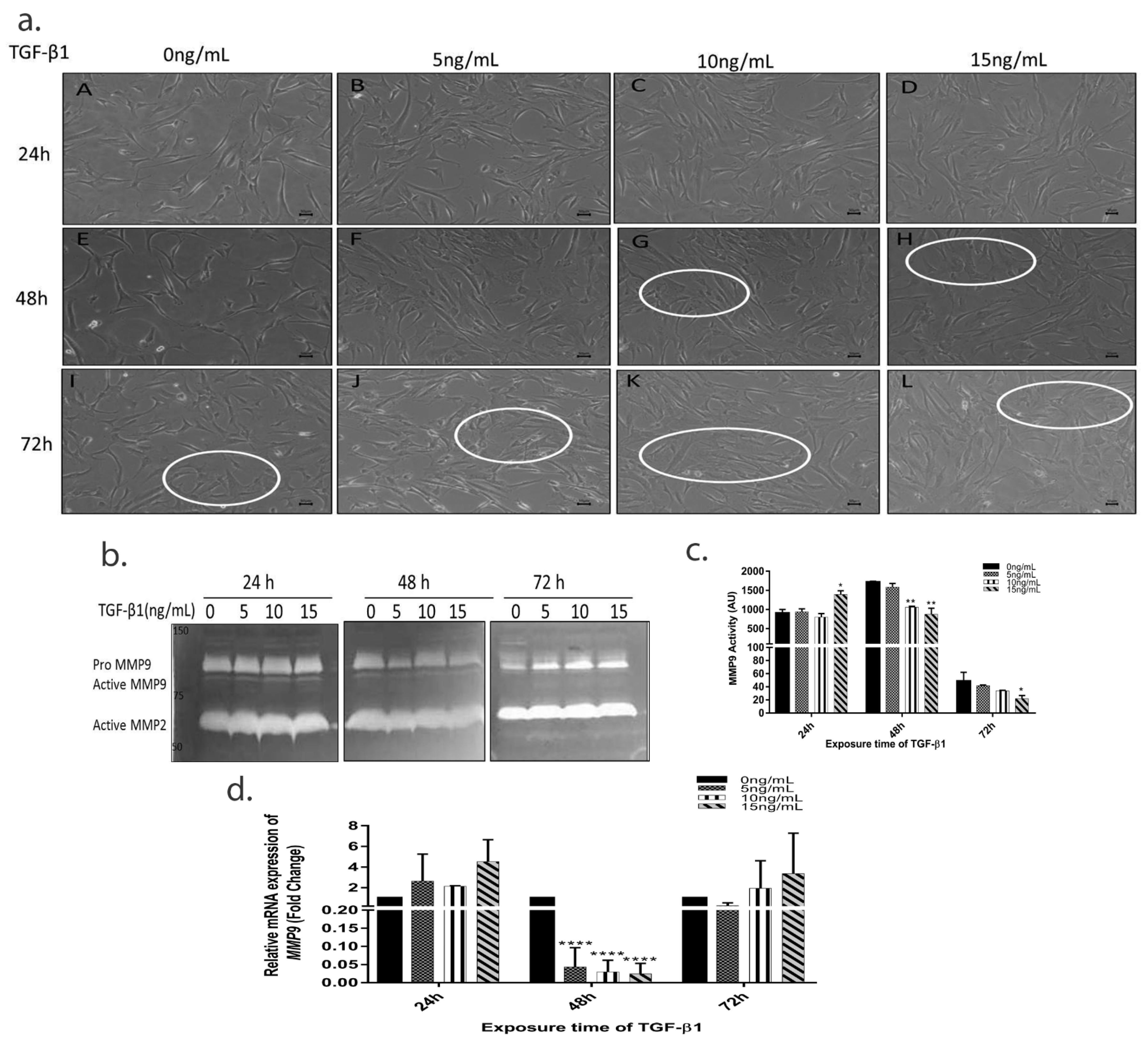
3.2. TGF-β1 Induces Time Dependent EMT in HTM Cells
3.3. TGF-β1 Causes a Time-Dependent Reduction in MMP9 Enzyme Activity
3.4. TGF-β1 Induced Expression of Pro-Fibrotic and ER Stress Markers
3.5. Continued TGF-β1 Exposure Induces Fibrillar Aggregate Formation in HTM Cells In Vitro
3.6. TGF-β1 Induces Downstream Changes on HTM Cells and ECM Using Both Smad and Non-Smad Signalling Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, R.K. The molecular pathophysiology of pseudoexfoliation glaucoma. Curr. Opin. Ophthalmol. 2008, 19, 95–101. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Naumann, G.O. Trabecular meshwork in pseudoexfoliation syndrome with and without open-angle glaucoma. A morphometric, ultrastructural study. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1750–1764. [Google Scholar]
- Zenkel, M.; Schlötzer-Schrehardt, U. The composition of exfoliation material and the cells involved in its production. J. Glaucoma 2014, 23 (Suppl. S1), S12–S14. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.K.; Ariue, B.; Alban, R.F.; Shaw, B.; Cho, S.H. PAI-1 promotes extracellular matrix deposition in the airways of a murine asthma model. Biochem. Biophys. Res. Commun. 2002, 294, 1155–1160. [Google Scholar] [CrossRef]
- Trantow, C.M.; Mao, M.; Petersen, G.E.; Alward, E.M.; Alward, W.L.; Fingert, J.H.; Anderson, M.G. Lyst mutation in mice recapitulates iris defects of human exfoliation syndrome. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1205–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Schlötzer-Schrehardt, U.; Ritch, R.; Call, M.; Chu, F.B.; Dong, F.; Rice, T.; Zhang, J.; Kao, W.W.-Y. Transient expression of Wnt5a elicits ocular features of pseudoexfoliation syndrome in mice. PLoS ONE 2019, 14, e0212569. [Google Scholar] [CrossRef] [Green Version]
- Kara, S.; Yildirim, N.; Ozer, A.; Colak, O.; Sahin, A. Matrix metalloproteinase-2, tissue inhibitor of matrix metalloproteinase-2, and transforming growth factor beta 1 in the aqueous humor and serum of patients with pseudoexfoliation syndrome. Clin. Ophthalmol. 2014, 8, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Schlötzer-Schrehardt, U.; Zenkel, M.; Küchle, M.; Sakai, L.Y.; Naumann, G.O. Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp. Eye Res. 2001, 73, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Vania, M.; Cheung, C.M.; Ang, M.; Chee, S.P.; Yang, H.; Li, J.; Wong, T.T. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol. Vis. 2012, 18, 431–438. [Google Scholar] [PubMed]
- Garweg, J.G.; Zandi, S.; Pfister, I.B.; Skowronska, M.; Gerhardt, C. Comparison of cytokine profiles in the aqueous humor of eyes with pseudoexfoliation syndrome and glaucoma. PLoS ONE 2017, 12, e0182571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauldie, J.; Kolb, M.; Sime, P.J. A new direction in the pathogenesis of idiopathic pulmonary fibrosis? Respir. Res. 2002, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Nieto, N. Oxidative-stress and IL-6 mediate the fibrogenic effects of Kupffer cells on stellate cells. Hepatology 2006, 44, 1487–1501. [Google Scholar] [CrossRef]
- Zenkel, M.; Lewczuk, P.; Jünemann, A.; Kruse, F.E.; Naumann, G.O.; Schlötzer-Schrehardt, U. Proinflammatory cytokines are involved in the initiation of the abnormal matrix process in pseudoexfoliation syndrome/glaucoma. Am. J. Pathol. 2010, 176, 2868–2879. [Google Scholar] [CrossRef] [PubMed]
- Sahay, P.; Reddy, S.; Prusty, B.K.; Modak, R.; Rao, A. TGFβ1, MMPs and cytokines profiles in ocular surface: Possible tear biomarkers for pseudoexfoliation. PLoS ONE 2021, 16, e0249759. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Chakraborty, M.; Roy, A.; Sahay, P.; Pradhan, A.; Raj, N. Differential miRNA Expression: Signature for Glaucoma in Pseudoexfoliation. Clin. Ophthalmol. 2020, 14, 3025–3038. [Google Scholar] [CrossRef]
- Waduthanthri, K.D.; Montemagno, C.; Çetinel, S. Establishment of human trabecular meshwork cell cultures using nontransplantable corneoscleral rims. Turk. J. Biol. Turk Biyol. 2019, 43, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Sahay, P.; Chakraborty, M.; Prusty, B.K.; Srinivasan, S.; Jhingan, G.D.; Mishra, P.; Modak, R.; Suar, M. Switch to Autophagy the Key Mechanism for Trabecular Meshwork Death in Severe Glaucoma. Clin. Ophthalmol. 2021, 15, 3027–3039. [Google Scholar] [CrossRef]
- Tamm, E.R.; Braunger, B.M.; Fuchshofer, R. Intraocular Pressure and the Mechanisms Involved in Resistance of the Aqueous Humor Flow in the Trabecular Meshwork Outflow Pathways. Prog. Mol. Biol. Transl. Sci. 2015, 134, 301–314. [Google Scholar]
- Aires, I.D.; Ambrósio, A.F.; Santiago, A.R. Modeling Human Glaucoma: Lessons from the in vitro Models. Ophthalmic Res. 2017, 57, 77–86. [Google Scholar] [CrossRef]
- Evangelho, K.; Mastronardi, C.A.; de-la-Torre, A. Experimental Models of Glaucoma: A Powerful Translational Tool for the Future Development of New Therapies for Glaucoma in Humans-A Review of the Literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef] [Green Version]
- Fink, A.L. Protein aggregation: Folding aggregates, inclusion bodies and amyloid. Fold. Des. 1998, 3, R9–R23. [Google Scholar] [CrossRef] [Green Version]
- Fernando, R.I.; Castillo, M.D.; Litzinger, M.; Hamilton, D.H.; Palena, C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011, 71, 5296–5306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palena, C.; Hamilton, D.H.; Fernando, R.I. Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol. 2012, 8, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef]
- Takahashi, E.; Inoue, T.; Fujimoto, T.; Kojima, S.; Tanihara, H. Epithelial mesenchymal transition-like phenomenon in trabecular meshwork cells. Exp. Eye Res. 2014, 118, 72–79. [Google Scholar] [CrossRef]
- Stone, R.C.; Pastar, I.; Ojeh, N.; Chen, V.; Liu, S.; Garzon, K.I.; Tomic-Canic, M. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 495–506. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Chartoumpekis, D.V.; Kyriazopoulou, V.; Zaravinos, A. EMT Factors and Metabolic Pathways in Cancer. Front. Oncol. 2020, 10, 499. [Google Scholar] [CrossRef]
- Lenna, S.; Trojanowska, M. The role of endoplasmic reticulum stress and the unfolded protein response in fibrosis. Curr. Opin. Rheumatol. 2012, 24, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, J.; Wang, M.; Zheng, D.; Liu, Y. Endoplasmic reticulum stress regulates epithelial-mesenchymal transition in human lens epithelial cells. Mol. Med. Rep. 2020, 21, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| α-SMA | GAAGGAGATCACGGCCCTA | ACATCTGCTGGAAGGTGGAC |
| COL6a2 | AGAGCTGTCCTTCGTGTTCCT | CTGTCATAGTCCTTCTCGTGGAA |
| FBLN5 | CCTGTTCCGCTGTGAGTG | ACTGATGCACGTGGTTGG |
| FN1 | CCACCCCCATAAGGCATAGG | GTAGGGGTCAAAGCACGAGTCATC |
| PAI-1 | GGCCATTACTACGACATCCTG | GGTCATGTTGCCTTTCCAGT |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| XBP1 | TCATGGCCTTGTAGTTGAGA | GGCATTTGAAGAACATGACTGG |
| CCT4 | TGCTTTTGCAGATGCTATGG | GGACAACCAGTTCCTCCAAA |
| IL-6 | CAAATTCGGTACATCCTCGACGGC | GGTTCAGGTTGTTTTCTGCCAGTGC |
| IL-8 | CCACCGGAAGGAACCATCTCAC | GGCAAAACTGCACCTTCACACAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, M.; Sahay, P.; Rao, A. Primary Human Trabecular Meshwork Model for Pseudoexfoliation. Cells 2021, 10, 3448. https://doi.org/10.3390/cells10123448
Chakraborty M, Sahay P, Rao A. Primary Human Trabecular Meshwork Model for Pseudoexfoliation. Cells. 2021; 10(12):3448. https://doi.org/10.3390/cells10123448
Chicago/Turabian StyleChakraborty, Munmun, Prity Sahay, and Aparna Rao. 2021. "Primary Human Trabecular Meshwork Model for Pseudoexfoliation" Cells 10, no. 12: 3448. https://doi.org/10.3390/cells10123448
APA StyleChakraborty, M., Sahay, P., & Rao, A. (2021). Primary Human Trabecular Meshwork Model for Pseudoexfoliation. Cells, 10(12), 3448. https://doi.org/10.3390/cells10123448






